Abstract
Genetic deletion studies have shown that haploinsufficiency of Delta-like ligand (Dll) 4, a transmembrane ligand for the Notch family of receptors, results in major vascular defects and embryonic lethality. To better define the role of Dll4 during vascular growth and differentiation, we selected the postnatal retina as a model because its vasculature develops shortly after birth in a highly stereotypic manner, during which time it is accessible to experimental manipulation. We report that Dll4 expression is dynamically regulated by VEGF in the retinal vasculature, where it is most prominently expressed at the leading front of actively growing vessels. Deletion of a single Dll4 allele or pharmacologic inhibition of Dll4/Notch signaling by intraocular administration of either soluble Dll4-Fc or a blocking antibody against Dll4 all produced the same set of characteristic abnormalities in the developing retinal vasculature, most notably enhanced angiogenic sprouting and increased endothelial cell proliferation, resulting in the formation of a denser and more highly interconnected superficial capillary plexus. In a model of ischemic retinopathy, Dll4 blockade also enhanced angiogenic sprouting and regrowth of lost retinal vessels while suppressing ectopic pathological neovascularization. Our data demonstrate that Dll4 is induced by VEGF as a negative feedback regulator and acts to prevent overexuberant angiogenic sprouting, promoting the timely formation of a well differentiated vascular network.
Keywords: angiogenesis, retina, Notch, oxygen-induced retinopathy
Notch signaling pathways are evolutionarily conserved and play key roles in cell-fate determination and differentiation in many tissues during embryonic and postnatal development (1). Major components of the Notch pathway are expressed in the vasculature (2), and genetic deletion of certain Notch pathway components, including Notch1, Notch1/Notch4 (3, 4), Jagged1 (5), Delta-like ligand (Dll) 4 (6), Hey1/Hey2 (7), or presenilins (8, 9) results in embryonic lethality associated with vascular remodeling defects. Although most of these genes are expressed in multiple tissue and cell types, Dll4 is largely restricted to the vascular endothelium, suggesting that Dll4 is a key ligand for Notch receptors in the developing vasculature (6, 10, 11). During early embryonic development, genetic deletion of even a single Dll4 allele produces severe vascular abnormalities that result in embryonic lethality in most mouse strains (6, 12, 13). Indeed, of the many genes involved in vasculogenesis and angiogenesis, haploid insufficiency has been reported to result in major vascular defects and embryonic lethality only for Dll4 and VEGF-A (14, 15). Unfortunately, early embryonic lethality precludes most experimental manipulations, making it difficult to precisely understand the role of Dll4 during vascular development and in pathological settings. To overcome this limitation, we have studied the effects of Dll4 gene deletion in mice of the outbred ICR strain, in which haploinsufficiency produces only limited embryonic lethality (6, 12). We then compared the vascular phenotype observed in these mutant mice to that obtained in wild-type mice in which Dll4/Notch signaling was selectively inhibited by intravitreal injection of Dll4-Fc or a neutralizing antibody against the extracellular domain of Dll4. For these experiments, we selected the retina as a model system because the retinal vasculature develops postnatally in a stereotypic manner that is highly organized, temporally and spatially (16). Moreover, the murine model of oxygen-induced ischemic retinopathy (OIR) (17) is a well characterized model of pathological neovascularization associated with elevated expression of endogenous proangiogenic factors, including VEGF (18, 19), and thus relevant to pathological angiogenesis associated with diverse disease conditions (20). Finally, the retinal vasculature is readily accessible to experimental manipulations, including intravitreal microinjections of experimental agents. We report that during normal retinal vascular development, and in the OIR model, suppression of Dll4/Notch signaling markedly enhances angiogenic sprouting and promotes the formation of a denser primary capillary network. Consistent with this, we find that Dll4 expression is particularly prominent in the most active regions of vascular growth both during normal development and in the OIR model. We further demonstrate that Dll4 expression in these vessels is markedly suppressed by pharmacological inhibition of VEGF and that application of exogenous VEGF up-regulates Dll4 expression in normal retinal vessels. These data indicate that VEGF induces Dll4 expression as part of a negative regulatory loop, in which Dll4 acts as a potent endogenous inhibitor of vascular sprouting. Thus, by appropriately restraining VEGF-induced sprouting angiogenesis, Dll4 acts in concert with VEGF to promote the timely formation and differentiation of competent vascular networks.
Results
Dll4 Is Highly Expressed in Angiogenic Blood Vessels.
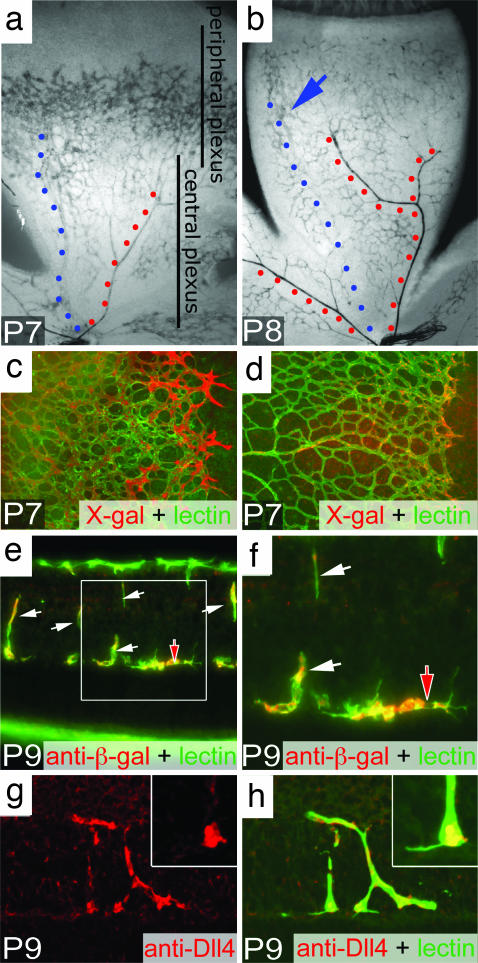
The retina of the mouse is avascular at birth. By the first postnatal day (P1), vascular “sprouts” emerge from the central vessels at the optic nerve head and begin to elaborate a primitive vascular plexus that rapidly extends across the retinal surface, reaching the peripheral margin of the retina by P8–P9. During this time, remodeling of the superficial plexus also is initiated, beginning centrally and proceeding peripherally. Beginning around P7, angiogenic sprouts originate from the maturing portions of the superficial vasculature and penetrate into the substance of the neural retina, forming the deep and intermediate capillary layers. The differentiation and maturation of all three vascular layers is essentially complete by the third postnatal week. The expression of Dll4 in the developing retina was first evaluated in ICR mice in which the entire Dll4 coding region was replaced with a lacZ reporter gene (Dll4+/lacZ mice) (6); expression of the reporter was detected by using an antibody against β-galactosidase or X-gal histochemistry. During the first postnatal week, Dll4 reporter expression was most prominent in the endothelial cells of actively growing capillaries at the leading front of the superficial vascular plexus (Fig. 1a). Lower levels of expression were noted in maturing capillaries located more centrally (Fig. 1a), as well as in newly forming veins and arteries. Consistent with the notion that Dll4 expression is dynamically regulated and most prominently associated with actively growing vessels, by P8/9, Dll4 was expressed at low to moderate levels throughout the capillaries of the superficial plexus, coincident with the cessation of angiogenic sprouting in this layer (Fig. 1b). Over this time, Dll4 expression was extinguished in the maturing segments of veins but increased in arteries (Fig. 1b), consistent with the pattern seen in the adult retina (6). Immunostaining with an antibody to the extracellular domain of Dll4 in wild-type mice confirmed the pattern of reporter gene expression, including the notably more prominent localization of Dll4 protein to endothelial cells at the actively growing capillary front (compare c and d in Fig. 1). Although Dll4 expression was down-regulated in the maturing superficial vasculature by P8–P9, at this time it was still prominent in the stalks (white arrows) and tips (red arrows) of angiogenic sprouts penetrating into the retina to form the deep and intermediate capillary layers. Again, the patterns of reporter expression (Fig. 1 e and f) and Dll4 antibody staining were consistent (Fig. 1 g and h). Interestingly, Dll4 immunostaining was strong but heterogeneous in tip cells, with little or no staining noted in filopodia (Fig. 1h Inset). These data indicate that Dll4 is most prominently expressed by endothelial cells of vessels undergoing active growth, including both tip and stalk cells, and at appreciably lower levels in maturing and fully differentiated capillaries.
Fig. 1.
Dll4 expression in the developing retinal vasculature. (a and b) X-gal staining of the developing superficial retinal vasculature in Dll4+/lacZ mice at P7 (a) and P8 (b). Note the highest level of Dll4 expression in arteries (red dots) and lower levels of expression in the forming vein (blue dots) and capillaries in the area proximal to the edge of the superficial plexus (blue arrow). (c and d) Dll4 reporter gene (c, X-gal staining, red pseudocolor) and Dll4 protein (d, red) expression in the leading front of the superficial retinal plexus in Dll4+/lacZ (c) or wild-type (d) mice at P7. Green, GS lectin. (e and f) Dll4 reporter gene expression in tips (red filled arrow) and stalks (white arrows) of growing deep retinal vessels at P9. GS lectin (green) and anti-β-gal (red) staining are shown. (g and h) Dll4 immunostaining (red) in tips (red filled arrows) and stalks (white arrows) of deep retinal sprouts in wild-type mice at P9. (Inset) Higher power view of sprout tip. Green, GS lectin. (Original magnifications: ×40 for a and b, ×100 for c and d, ×400 for e, and ×800 for f–h.)
Dll4 Single Allele Deletion Increases Angiogenic Sprouting.
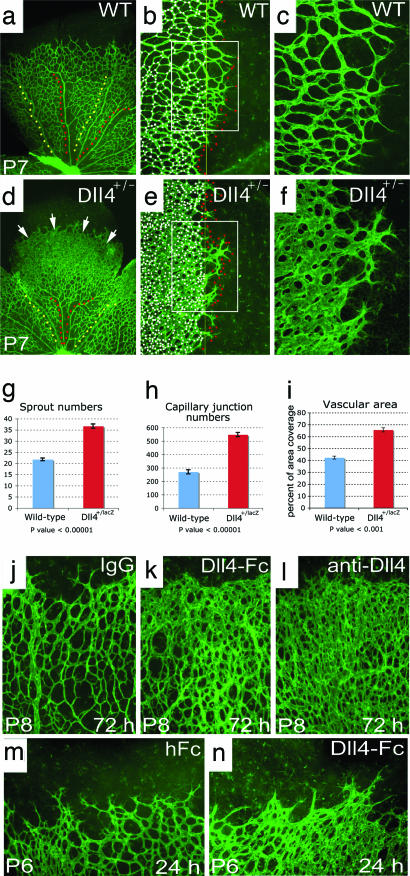
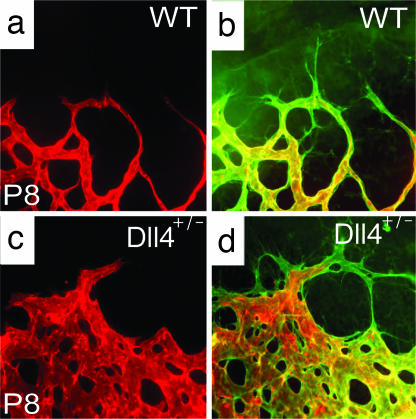
To determine whether the dynamic regulation of Dll4 in actively growing retinal vessels reflects an important role for Dll4 in angiogenesis, we next evaluated the effect of Dll4 gene deletion on the postnatal development of the retinal vasculature in Dll4+/lacZ pups. Heterozygous Dll4 deletion had its most striking effect on the elaboration of the primary retinal capillary plexus, which was much denser in Dll4+/lacZ mice than in wild-type littermates (Fig. 2 a and d). Moreover, maturation of peripheral regions of the plexus into a hierarchical vascular network was delayed, as evidenced by substantially shorter lengths of arteries and veins (Fig. 2 a and d). Higher power views show that the peripheral plexus in the retinas of Dll4+/lacZ mice consisted of capillaries that were larger in diameter, more highly interconnected, and hyperfused, so that in some areas the vessels coalesced to form a syncytium. In addition, there were many more sprouts and filopodia present at the growing vascular front (Fig. 2 b, c, e, and f); filopodia also were observed in more interior portions of the plexus at a higher than normal frequency (data not shown). The development of the primary retinal capillary plexus is guided by filopodia extending from endothelial cells located at the tips of growing vessels (21), such that contacts between processes extending from neighboring cells form a template for the elaboration and fusion of endothelial tubes, leading to formation of the interconnected primitive capillary network. Quantitative analyses revealed that, compared with wild-type controls, retinas of Dll4+/lacZ mice showed a >50% increase in the number of filopodia at the growing front of the superficial retinal vasculature (Fig. 2 b, e, and g) as well as a >2-fold increase in the number of capillary interconnections per unit area (Fig. 2 b, e, and h), resulting in a significant increase in the vascular coverage (Fig. 2i). Despite these marked morphologic changes, intravascular injection of fluoresceinated lectin completely filled the developing superficial vascular plexus, except for the filopodia/sprouts extending from the tip cells, in Dll4+/lacZ mice as in wild-type mice, indicating that all components of the developing vasculature had lumens and were functional (Fig. 2). Taken together, the above findings indicate that Dll4 is a potent endogenous inhibitor of vessel sprouting and filopodia extension, such that even a partial deficiency in Dll4 expression results in formation of a much denser and more highly interconnected plexus.
Fig. 2.
Retinal vascular abnormalities in Dll4+/lacZ mice. (a–f) GS lectin staining of the developing retinal vessels in wild-type (a–c) and Dll4+/lacZ (d–f) mice at P7. (a and d) Delayed capillary remodeling and formation of syncytium-like vascular plexus in retinas of Dll4+/lacZ mice. Red dots mark arteries, and yellow dots mark veins. White arrows indicate syncytium-like vascular plexus. (b and e) Increased sprouting and capillary network complexity in the superficial retinal vasculature of Dll4+/lacZ mice. Red dots mark tips of sprouts, and white dots mark intercapillary junctions. (c and f) Filopodia are substantially more numerous at the leading edge of the growing retinal vasculature in Dll4+/lacZ mice. (g–i) Quantitation of sprout (g) and capillary junction (h) numbers and vascular area (i) in ×100 microscopy fields. Error bars represent standard errors. (j–l) GS lectin staining of the developing retinal vasculature of wild-type mice 3 days after intravitreal injection of 0.5 μg of control IgG (j), Dll4-Fc (k), or anti-Dll4 antibody (l). Dll4-Fc or anti-Dll4 antibody administration to wild-type mice produced vascular abnormalities similar to those seen in Dll4+/lacZ mice. Note the very dense capillary network. (m and n) Intravitreal injection of 0.8 μg of Dll4-Fc (m) in wild-type mice at P5 induces formation of the syncytium-like vascular plexus within 24 h. (Original magnifications: ×40 for a and d, ×100 for j–n, and ×200 for c and f.)
Acute Pharmacological Inhibition of Dll4/Notch Interaction Stimulates Endothelial Proliferation and Angiogenesis.
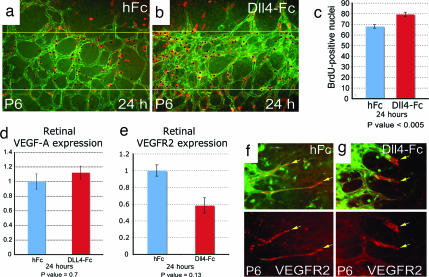
To confirm that the increased angiogenic sprouting observed during postnatal retinal development in Dll4+/lacZ mice was directly attributable to a local, intraretinal deficiency in Dll4/Notch signaling, and not secondary to an undetected systemic abnormality, we injected a soluble version of Dll4 [termed Dll4-Fc (22)] that acts as a blocker of Dll4/Notch interactions, or a neutralizing antibody specific for the extracellular domain of Dll4, into the vitreous of wild-type mice. Three days after intraocular administration of either Dll4 blocker, the retinal vessels exhibited morphologic changes that closely resembled those found in Dll4+/lacZ mice (Fig. 2 j–l). Moreover, characteristic morphologic changes occurred rapidly, being clearly evident within 24 h (Fig. 2 m and n). In addition to the morphologic evidence of acute increases in angiogenic sprouting and vascular area, BrdU labeling showed an increase in endothelial cell proliferation within 24 h of Dll4 blockade (Fig. 4 a–c); note that the observed ≈15% increase in the proliferation rate could yield an ≈50% increase in endothelial cell number within three to four doubling times. The observed increases in angiogenic sprouting and endothelial proliferation that occur after Dll4 blockade do not correlate with prominent acute increases in total VEGF-A or VEGFR2 mRNA levels within the retina (VEGFR2 levels actually show a modest decline) or an appreciable acute change in the distribution of VEGFR-2 (Fig. 4 d and g), suggesting that other molecular mediators are regulated by Dll4 blockade and account for the resulting increase in angiogenesis.
Fig. 4.
Effect of Dll4 pharmacological inhibition on the developing retinal vasculature and proliferation. (a and b) BrdU labeling of retinal vasculature in hFc-treated (a) and Dll4-Fc-treated (b) retinas. Counterstaining with anti-VE-Cadherin antibody is shown. (c) Quantification of BrdU-positive nuclei numbers per half of ×20 microscopy fields. (d and e) Real-time PCR analysis of VEGF-A (d) and VEGFR2 (e) gene expression in the retinas treated for 24 h with hFc or Dll4-Fc. Expression data were normalized to GAPDH. Error bars represent standard errors. (f and g) VEGFR2 antibody staining of the leading front of the growing retinal vasculature treated for 24 h with hFc (f) or Dll4-Fc (g). (Original magnifications: ×200 for a and b and ×400 for f and g.)
Dll4 Modulates Pathological Angiogenesis in the OIR Model.
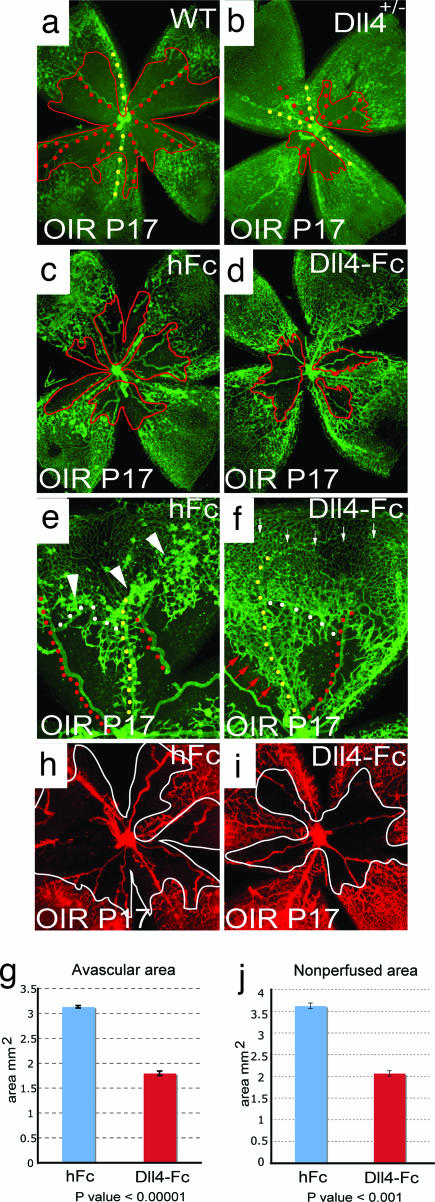
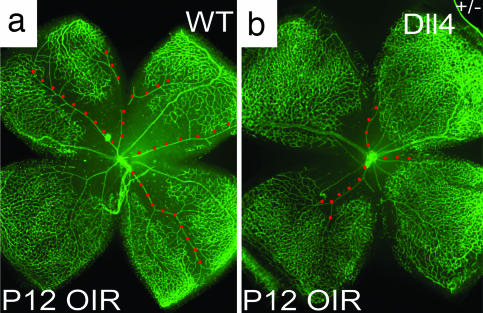
To determine whether Dll4/Notch signaling also plays a role in modulating pathologic angiogenesis, we used the OIR model. Exposure of mouse pups to hyperoxia at P7 results in a rapid obliteration of capillaries in the central retina. After return to room air at P12, the avascular zone becomes severely hypoxic, inducing high levels of VEGF, which in turn elicits extensive abnormal neovascularization, characterized by the ectopic growth of epiretinal vascular tufts into the vitreous (17), as well as subsequent regrowth of the lost superficial retinal vessels. When evaluated at P17, both the size of the residual avascular area and the development of epiretinal neovascular tufts were substantially reduced in Dll4+/lacZ mice compared with their wild-type littermates (Fig. 5 a and b). Interestingly, in evaluating animals at P12, we found that vasoobliteration was also reduced in Dll4+/lacZ mice (Fig. 6), indicating that developing vasculature in Dll4+/lacZ mice is less susceptible to oxygen-induced vessel pruning. Thus, it was formally possible that the reduction in pathological neovascularization observed at P17 in Dll4+/lacZ mice was secondary to a relative attenuation of the initial hypoxic insult. To address this possibility, we studied the effect of administration of Dll4-Fc on pathological retinal neovascularization in wild-type mice. In these experiments, Dll4-Fc or a control protein (hFc) was injected intravitreally at P13, 1 day after the animals were returned to room air, after vasoobliteration was complete. When the retinas were evaluated at P17, administration of Dll4-Fc had stimulated more extensive sprouting of new retinal vessels from capillaries and veins bordering the avascular zone, resulting in a more rapid regrowth of blood vessels into the central retina, where the vasculature had been depleted (Fig. 5 c–f; quantification shown in Fig. 5g). The majority of these newly forming vessels were well perfused, such that the nonperfused retinal area was decreased in Dll4-Fc-treated mice relative to controls (Fig. 5 h–j). Moreover, Dll4-Fc dramatically suppressed the ectopic growth of pathological neovascular tufts into the vitreous (Fig. 5 e and f), as well as the formation of abnormal arteriovenous shunts (Fig. 5 e and f, white dots). Thus, attenuation of Dll4/Notch signaling favored the extension of new vascular sprouts along the retinal surface and obtunded the formation of epiretinal neovascularization, resulting in a more rapid reformation of the superficial vascular plexus. These data indicate that Dll4 can act as a negative regulator of capillary sprouting during pathologic states, as well as during normal development.
Fig. 5.
Effect of Dll4 inhibition on normal retinal vascular development and neovascularization in OIR model. (a and b) GS lectin staining of OIR retinas at P17 from wild-type (a) and Dll4+/lacZ (b) mice. Note smaller avascular area (red line) and increased vessel density in Dll4+/lacZ retina. (c and d) GS lectin staining of OIR retinas at P17 from wild-type mice injected at P13 with 0.5 μg of hFc (c) or Dll4-Fc (d). Note the smaller avascular zone (red line) in the Dll4-Fc-treated retinas. (e and f) GS lectin staining of OIR retinas at P17 from wild-type mice injected at P13 with 0.5 μg of hFc (e) or Dll4-Fc (f). Note that most of sprouting (red arrows) occurs from veins (yellow dots) and capillaries. Also, note the reduced number of neovascular tufts (white arrowheads) and substantially denser capillary network (small white arrows) in Dll4-Fc-treated retinas. White dots indicate arteriovenous shunts whose appearance is reduced in Dll4-Fc-treated retinas. (g and j) Quantification of avascular (g) and nonperfused (j) area in hFc- and Dll4-Fc-treated P17 OIR retinas. Error bars represent standard errors. (h and i) Perfusion staining with L. esculentum lectin of the developing retinal vasculature in P17 OIR mice injected at P13 with 0.5 μg of hFc (h) or Dll4-Fc (i) at P17. (Original magnifications: ×20 for a–d and ×40 for e, f, h, and i.)
Fig. 6.
Reduced oxygen-induced vasoobliteration in the central retina in Dll4+/lacZ mice. Shown is GS lectin staining of the retinal vasculature in wild-type (a) and Dll4+/lacZ (b) OIR mice at P12. Note that most of the capillary pruning in wild-type retinas occurs in the vicinity of arteries (labeled with red dots). (Original magnification: ×20.)
VEGF Induces Dll4 Expression in Angiogenic Blood Vessels.
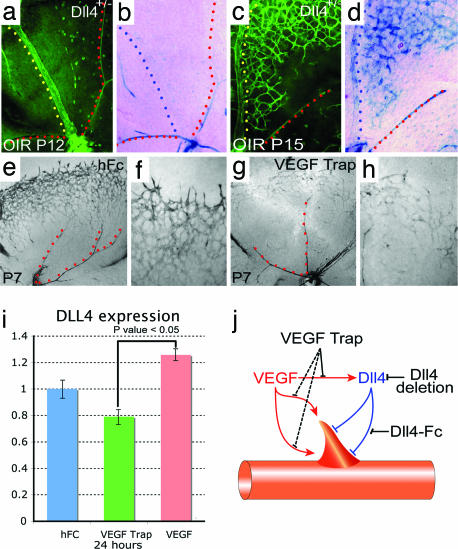
Interestingly, in the OIR model most of the angiogenic sprouting induced by Dll4-Fc originates from veins and capillaries bordering the central avascular zone. During normal retinal development, Dll4 expression is reduced in the capillaries and veins of the superficial retinal plexus by the beginning of the second postnatal week (see above). The fact that Dll4-Fc markedly enhanced angiogenic sprouting in the OIR model suggested that Dll4 expression might be reinduced, either directly by hypoxia, or secondary to the release of hypoxia-induced factors such as VEGF, recapitulating its development role as a negative regulator of sprouting angiogenesis. To explore this possibility, we evaluated Dll4 reporter gene expression in Dll4+/lacZ mice exposed to OIR. At P12, just after the period of hyperoxia, little or no Dll4 reporter expression was detected in veins, and levels remained low in surviving capillaries while arteries showed strong expression (Fig. 7 a and b), i.e., the pattern normally observed in the maturing retinal vasculature. However, 3 days later, at P15, Dll4 reporter expression was very high in veins, as well as in newly forming retinal capillaries and angiogenic sprouts (Fig. 7 c and d), consistent with the notion that the hypoxic insult had dramatically induced Dll4 expression.
Fig. 7.
VEGF induces Dll4 expression in angiogenic sprouts. (a–d) GS lectin (a and c) and X-gal staining (b and d) of P12 (a and b) and P15 (c and d) OIR retinas from Dll4+/lacZ mice. Note strong Dll4 gene reporter expression in the vein (yellow and blue dots) and angiogenic sprouts in OIR retinas at P15. (e–h) X-gal staining of retinal vasculature from P7 Dll4+/lacZ mice treated for 48 h with 300 ng of hFc (e and f) or VEGF Trap (g and h). Blocking VEGF markedly reduces Dll4 expression in the tip and stalk endothelial cells. (i) Real-time PCR analysis of Dll4 expression in the retinas treated for 24 h with hFc, VEGF Trap, or VEGF165. Dll4 expression data were normalized to GAPDH. Error bars represent standard errors. (j) Interaction between VEGF and Dll4 in angiogenic sprouting. VEGF initiates proliferation and blood vessel sprouting and guides growing vessels. At the same time VEGF induces expression of Dll4 that blocks excessive proliferation and sprouting. (Original magnifications: ×40 for e and g, ×100 for a–d, and ×200 for f and h.)
In the OIR model, Dll4 is induced in veins and capillaries adjacent to the zone of vasoobliteration, precisely where VEGF levels are most elevated (23). Similarly, during normal retinal vascular development, Dll4 expression is most pronounced at the growing front of the superficial vasculature proximate to the portions of the retina that have not yet been vascularized, again coincident with areas known to express the highest levels of VEGF (24). These observations suggested that endogenous VEGF might promote the expression of Dll4 in vascular endothelial cells during normal retinal development, as well as in conditions of pathological angiogenesis. Consonant with this suggestion, it has been reported previously that VEGF can up-regulate Dll4 expression in cultured endothelial cells (25). To test the hypothesis that endogenous VEGF is responsible for the dynamic regulation of Dll4 expression during normal vascular development, we injected a potent and selective blocker of VEGF-A, VEGF Trap (26) (a fusion protein comprising VEGF binding domains from human VEGF receptors 1 and 2 expressed in series with the Fc domain of human IgG), or a control protein (hFc) into the vitreous of Dll4+/lacZ mice on P5 and assessed Dll4 reporter expression on P7. VEGF blockade markedly inhibited Dll4 expression at the leading front of the growing superficial vascular plexus (Fig. 7 e–h) but had no appreciable effect on Dll4 expression in differentiated arteries. Quantitative PCR confirmed that intraocular administration of VEGF Trap reduced, whereas intravitreal injection of VEGF165 increased, expression of Dll4 in retinas within 24 h of treatment (Fig. 7i; residual Dll4 expression after VEGF Trap treatment may largely be accounted for by the remaining arterial expression depicted in Fig. 7g). Thus, Dll4 expression in retinal capillaries and veins is dynamically regulated by local expression of VEGF, whereas the increase in Dll4 expression in differentiated arteries appears to be independent of VEGF.
Discussion
Taken together, the above data provide evidence for the functional integration of the VEGF and Dll4 signaling pathways as key coordinated regulators that together control angiogenesis, blood vessel differentiation, and homeostasis. VEGF serves as the primary, and perhaps indispensable, initiator of angiogenic sprouting in normal and pathological conditions (27, 28). Coincidentally with initiating angiogenesis, VEGF also up-regulates Dll4 expression within the endothelium of angiogenic vessels, which we propose acts in feedback fashion as a “brake” or negative regulator of VEGF-induced angiogenesis, modulating this process to control overexuberant vascular sprouting and endothelial cell proliferation, thereby promoting the timely formation of a productive and well differentiated vascular network (Fig. 7j). The precise mechanisms by which Dll4/Notch signaling acts to constrain VEGF's actions remain to be determined, but, because both Dll4 and its Notch receptors are transmembrane proteins, it seems likely that negative regulation follows cell-to-cell contact between a ligand-bearing cell and a receptor-expressing cell. Although tip cells are major carriers of Dll4 and its negative signal, it seems unlikely that Dll4/Notch-mediated inhibition of angiogenesis is limited to tip cells and their immediate neighbors, because Dll4 is expressed more widely in the developing retinal vasculature, and inhibition of endogenous Dll4/Notch signaling also increased proliferation throughout the expanding superficial retinal plexus, in addition to enhancing the number and activity of filopodia-bearing tip cells.
Some of the vascular abnormalities described here in the retina resemble those observed in the developing yolk sacs of Dll4+/lacZ and Notch1/4 mutant embryos (4, 6), suggesting that Dll4 plays an analogous role as a negative regulator of angiogenesis in many developing vascular beds. Similarly, it is likely that Dll4 is involved in the modulation of diverse forms of pathological angiogenesis. For example, VEGF also induces Dll4 as a negative feedback regulator of vascular sprouting during tumor angiogenesis (22). Here, blockade of Dll4/Notch signaling was found to retard tumor growth by enhancing the chaotic, nonproductive vascular sprouting characteristic of tumor angiogenesis. Thus, pharmacological inhibition of Dll4 may have therapeutic applications in diverse diseases characterized by pathological angiogenesis.
Materials and Methods
Animals.
VelociGene technology was used to replace the entire Dll4 coding region with the β-galactosidase reporter gene in C57BL/6:129 hybrid mouse embryonic stem cells (17). Chimeric males were bred to ICR females. Dll4+/lacZ mice backcrossed for three generations to ICR (87.5% ICR) were used for this study. C57BL/6 mice (Taconic Farms, Germantown, NY) were used to study the effect of Dll4-Fc or neutralizing Dll4 antibody on normal vascular development and retinal neovascularization in OIR. Because of a recessive (rd/rd) mutation, the retinal photoreceptor cell layer starts to degenerate at P12 in ICR mice. Therefore, to obviate potential secondary effects of photoreceptor loss on the retinal vasculature, for evaluating later stages of retinal vascular development, and for all OIR experiments, male Dll4+/lacZ mice were bred to C57BL/6 females to produce Rd/rd offspring, which do not exhibit photoreceptor degeneration. All animal manipulations were approved by Institutional Animal Care and Use Committee and conformed to Association for Research in Vision and Ophthalmology guidelines for the use of animals.
Antibodies and Reagents.
Dll4-Fc comprises the extracellular domain of mouse Dll4 and the Fc part of human IgG. Dll4-Fc was expressed in CHO cells and affinity-purified by protein A chromatography. Dll4-Fc was shown to inhibit Notch signaling in vitro (22). Anti-Dll4 antibody was produced by immunization of rabbits with recombinant mDll4-hFc. The antiserum was partially purified by protein A chromatography before use. Other antibodies used for immunohistochemistry were rabbit polyclonal to β-gal (Invitrogen, Carlsbad, CA) and goat polyclonal to VEGFR2 (R & D Systems, Minneapolis, MN).
Real-Time PCR.
For retinal gene expression studies 5 μg of VEGF Trap, 1 μg of VEGF165, 5 μg of Dll4-Fc, or 5 μg of hFc was injected intravitreally at P5. Retinas were harvested 24 h after the injection, and retinal gene expression was analyzed by using the TaqMan (Applied Biosystems, Foster City, CA) real-time PCR chemistry and detection system, using primer pairs and labeled probes specific for Dll4, VEGF-A, and VEGFR2. The number of cycles necessary to reach the threshold for amplification of the cDNA was obtained and normalized to a housekeeping reference (GAPDH).
Histochemistry and Immunostaining.
Mouse pups were humanely killed between P5 and P17. Eyes were enucleated, and retinas were dissected, fixed overnight with 4% paraformaldehyde, stained with FITC-labeled Griffonia simplicifolia (GS) lectin I (Vector Laboratories, Burlingame, CA), and flat-mounted. In some cases, retinas were immunostained by using antibodies against Dll4 or β-gal before being stained with GS lectin. Alternatively, after 15 min of fixation in 4% paraformaldehyde, retinas were embedded in OCT media and frozen, and 20-μm sections were cut. Biotinylated secondary antibody and a streptavidin-HRP tyramide signal amplification system (Invitrogen, CA) were used for anti-β-gal and anti-Dll4 immunostaining. After X-gal staining, retinas were postfixed for 4 h in 4% paraformaldehyde and counterstained with GS lectin. To label patent blood vessels, 50 ml of Texas red-labeled Lycopersicon esculentum (LE) lectin (1 mg/ml; Vector Laboratories, CA) was injected into the left cardiac ventricle and allowed to circulate for 5 min. Proliferating cells were labeled by administration of BrdU (1 mg/kg i.p.) 20 h after intravitreal injection of hFc or Dll4-Fc. Retinas were harvested 4 h later and stained with ant-BrdU (Dako North America, Inc., Carpinteria, CA) and VE-Cadherin (BD PharMingen, San Diego, CA) antibodies. Images were taken by using a Nikon (Melville, NY) Eclipse or a Leica (Wetzlar, Germany) confocal microscope. Images were assembled into figures by using Photoshop and Illustrator software (Adobe Systems, San Jose, CA).
Postnatal Retinal Vascularization, OIR, and Intravitreal Microinjections.
Five- to 17-day-old pups were used to assess the effect of pharmacological inhibition of Dll4/Notch signaling. OIR was produced following the method developed by Smith et al. (17). Intravitreal microinjections (30–100 nl) were made between the equator and the corneal limbus by using a Drummond Scientific (Broomall, PA) nanoinjector equipped with a glass needle.
Quantification of Sprouting.
For each eye, vascular sprouts were counted in nine different ×100 images taken at the leading front of the developing retinal vasculature, and the mean number of sprouts per field per retina was calculated. The same images were used to quantify the mean numbers of intercapillary junctions per field per retina in the region immediately proximal to the edge of the superficial plexus (Fig. 2 b and e). Each point at which three capillary segments met was counted as one junction, intersections of four capillary segments were counted as two junctions, etc. Student's t test and two-way ANOVA were used to assess statistical significance.
Fig. 3.
Perfusion staining of developing retinal vasculature in Dll4 mice. (a–d) Perfusion staining with L. esculentum lectin (a and c) and counterstaining with GS lectin (green, b and d) of the developing retinal vasculature in wild-type (a and b) and Dll4+/lacZ (c and d) mice at P8. (Original magnification: ×630.)
Acknowledgments
We gratefully acknowledge Regeneron colleagues Jingtai Cao, Christopher Daly, Irene Noguera-Troise, and Yang Liu for valuable scientific input and discussions.
Abbreviations
- Dll
Delta-like ligand
- GS lectin
Griffonia simplicifolia lectin
- OIR
oxygen-induced ischemic retinopathy
- Pn
postnatal day n.
Footnotes
Conflict of interest statement: I.B.L., R.A.R., N.P., N.W.G., G.T., G.D.Y., and S.J.W. are employees of, and own stock or stock options in, Regeneron Pharmaceuticals.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Shawber CJ, Kitajewski J. BioEssays. 2004;26:225–234. doi: 10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- 3.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 4.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Circulation. 2005;111:1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 6.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, et al. Proc Natl Acad Sci USA. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, et al. Proc Natl Acad Sci USA. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima M, Yuasa S, Ueno M, Takakura N, Koseki H, Shirasawa T. Mech Dev. 2003;120:657–667. doi: 10.1016/s0925-4773(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 10.Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 11.Benedito R, Duarte A. Gene Expr Patterns. 2005;5:750–755. doi: 10.1016/j.modgep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 16.Gariano RF, Gardner TW. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 17.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 18.Neely KA, Gardner TW. Am J Pathol. 1998;153:665–670. doi: 10.1016/S0002-9440(10)65607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saint-Geniez M, D'Amore PA. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N, Kerbel RS. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 21.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 23.Donahue ML, Phelps DL, Watkins RH, LoMonaco MB, Horowitz S. Curr Eye Res. 1996;15:175–184. doi: 10.3109/02713689608997411. [DOI] [PubMed] [Google Scholar]
- 24.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et al. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]