Abstract
Mice lacking p63, a single gene that encodes a group of transcription factors that either contain (TA) or lack (ΔN) a transactivation domain, fail to develop stratified epithelia as well as epithelial appendages and limbs. ΔNp63 isoforms are predominantly expressed during late embryonic and postnatal epidermal development, however, the function of these proteins remains elusive. Using an epidermal-specific inducible knockdown mouse model, we demonstrate that ΔNp63 proteins are essential for maintaining basement membrane integrity and terminal differentiation of keratinocytes. Furthermore, we have identified two ΔNp63α target genes that mediate these processes. We propose that ΔNp63α initially induces expression of the extracellular matrix component Fras1, which is required for maintaining the integrity of the epidermal–dermal interface at the basement membrane. Subsequently, induction of IκB kinase-α by ΔNp63α initiates epidermal terminal differentiation resulting in the formation of the spinous layer. Our data provide insights into the role of ΔNp63α in epidermal morphogenesis and homeostasis, and may contribute to our understanding of the pathogenic mechanisms underlying disorders caused by p63 mutations.
Keywords: IκB kinase-α
During epidermal morphogenesis, the sequential and coordinated action of transcription factors ultimately results in the formation of a mature epidermis that protects the organism from dehydration and environmental insults (reviewed in ref. 1). Two critical transitions that occur during normal epidermal morphogenesis are the commitment to stratification, when cells of the single-layered surface ectoderm are induced to become keratinocytes, and the commitment to terminal differentiation. The execution of the terminal differentiation program results in the migration of basal cells to the suprabasal cell layer and is associated with an increase in cellular adhesion, growth arrest, and the induction of biochemical markers of differentiation, such as keratin K1 (reviewed in ref. 2).
One gene that is critical for controlling epidermal morphogenesis is p63, a transcription factor that can be expressed as isoforms that contain (TA) or lack (ΔN) a transactivation domain (3). The critical role for p63 in regulating epidermal morphogenesis is illustrated by the phenotype of p63−/− mice, which fail to develop an epidermis, other stratified epithelia, and epithelial appendages (4, 5). The single layer of epithelial cells covering p63−/− mice at birth fails to provide barrier function, resulting in early postnatal lethality due to severe dehydration. However, despite the striking phenotype of p63−/− mice, the precise role of p63 in epidermal morphogenesis is controversial. Initial reports suggested a role for p63 in epithelial stem cell maintenance (5, 6). However, the finding that p63 is not enriched in epidermal stem cells challenges this notion (7–10). In contrast, we previously demonstrated that TAp63 is required for the commitment to stratification during epidermal morphogenesis, a process that is mediated, in part, by induction of AP-2γ (11, 12).
Whereas TAp63 isoforms function during the initial stages of epidermal morphogenesis, the role of ΔNp63 isoforms, the predominantly expressed p63 isoforms during later stages of epidermal morphogenesis (11, 13) as well as in mature epidermis (3, 14) has remained largely elusive. ΔNp63α is mainly expressed in keratinocytes of the proliferative basal layer, whereas its expression is down-regulated in postmitotic keratinocytes of the suprabasal differentiated layers of mature epidermis (3, 15). Based on this expression pattern and subsequent in vitro studies, it was proposed that ΔNp63α functions by maintaining the proliferative potential of basal keratinocytes while preventing their premature entry into terminal differentiation (16–18). This notion is further supported by recent findings demonstrating that down-regulating ΔNp63 in human primary keratinocytes causes hypoproliferation (19). The ability of ΔNp63 to maintain proliferation is accomplished in part by preventing expression of p21, a cyclin-dependent kinase inhibitor that is required for cell cycle exit during keratinocyte terminal differentiation (19–22). ΔNp63α inhibits p21 expression both through direct repression by binding to the p21 promoter (21) and by preventing Notch signaling, an upstream activator of p21 in the epidermis (22, 23).
In contrast to a role for ΔNp63α in maintaining basal keratinocytes, ΔNp63α also regulates the expression of genes required for keratinocyte terminal differentiation. For example, ΔNp63α directly induces p57Kip2 (24), a cyclin-dependent kinase inhibitor that is induced when keratinocytes undergo terminal differentiation (25). In addition, ΔNp63α directly represses the expression of genes required for cell cycle progression, including cyclin B2 and cdc2 (26). Finally, ΔNp63α synergizes with Notch to induce K1 expression in primary keratinocytes (22).
The contradictory in vitro findings summarized above warranted an in vivo analysis of the role of ΔNp63α in epidermal development and differentiation. To this end, we have generated mice in which ΔNp63 expression can be down-regulated specifically in the epidermis. Surprisingly, we found that down-regulating ΔNp63 isoforms caused severe epidermal defects, including impaired terminal differentiation and impaired basement membrane formation, culminating in the development of severe skin erosions. Furthermore, we found that these defects were caused in part by a failure of ΔNp63α to induce expression of two previously uncharacterized ΔNp63α target genes, Fras1 and IκB kinase-α (Ikkα), which are required for maintaining basement membrane integrity and terminal differentiation, respectively.
Results and Discussion
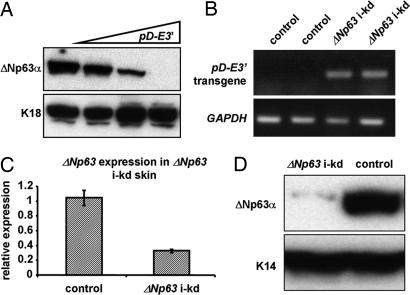
To determine the role of ΔNp63 in epidermal development and differentiation, we generated an inducible epidermal-specific ΔNp63 knockdown (i-kd) mouse model. To this end, we cloned an inverted repeat corresponding to exon 3′ (E3′), the only exon unique to ΔNp63, into the pDECAP vector (27) (pD-E3′). The resulting E3′ dsRNA transcript was predicted to be processed into ΔNp63-specific siRNAs in the nucleus. Indeed, cotransfection of cells with a ΔNp63α expression construct and increasing amounts of pD-E3′ resulted in a dose-dependent decrease in ΔNp63α protein expression (Fig. 1A). Because E3′ dsRNA could efficiently down-regulate ΔNp63α expression in vitro, we placed the pD-E3′ construct under control of our previously described RU486-regulatable gene-switch system (11). By using this system, expression of pD-E3′ could be induced in the basal layer of the epidermis by topical application of the progesterone antagonist RU486 (Fig. 1B). In three independent transgenic lines, topical application of RU486 for 4 days led to a down-regulation of endogenous ΔNp63, but not TAp63, expression in the skin [Fig. 1 C and D and supporting information (SI) Fig. 5].
Fig. 1.
In vitro ΔNp63 knockdown. Mouse exon 3′, the only exon unique to ΔNp63, was subcloned into pDECAP as an inverted repeat (pD-E3′). (A) Western blot analysis of Ptk2 cells transfected with a ΔNp63α expression construct (1 μg) and increasing amounts of pD-E3′ (1, 3, and 5 μg) by using an anti-p63 (mAb4A4) antibody. Anti-K18 was used as a loading control. The pD-E3′ construct was placed under control of an RU486 regulatable system to generate ΔNp63 inducible-knockdown (i-kd) mice. (B) RT-PCR for the pD-E3′ transgene on RNA isolated from newborn ΔNp63 i-kd and control skin after 4 days of RU486 treatment. GAPDH was amplified as an internal control. (C) Real-time RT-PCR and (D) Western blot analysis for ΔNp63/ΔNp63α using RNA or protein isolated from the skin of newborn ΔNp63 i-kd and control littermates that were treated with RU486 for 4 days.
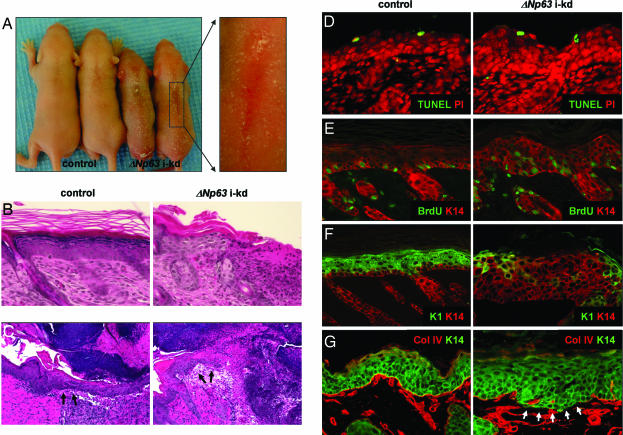
The mouse line with the most efficient ΔNp63 knockdown (J5107) was subsequently used for all experiments and is referred to as ΔNp63 i-kd. Importantly, ΔNp63α is the predominantly expressed ΔNp63 isoform in mature skin, whereas ΔNp63β and ΔNp63γ are not detectable (3, 14), therefore, down-regulating ΔNp63 expression allowed us to analyze the role of ΔNp63α in epidermal morphogenesis and homeostasis. Surprisingly, topical treatment of newborn ΔNp63 i-kd mice with RU486 for 4 days led to severe skin fragility characterized by multiple skin erosions (Fig. 2A). Histologically, these erosions appeared as areas of the skin where the epidermis was completely missing (Fig. 2B). Furthermore, ΔNp63 i-kd mice displayed an impaired ability to heal full-thickness skin wounds. Whereas full-thickness wounds completely healed after 96 h in adult control mice, wounds in ΔNp63 i-kd mice failed to heal and showed only marginal formation of a migrating epithelial tongue (Fig. 2C). Despite the skin fragility, TUNEL analysis demonstrated that the apoptotic index was unchanged in ΔNp63 i-kd skin (Fig. 2D). Down-regulating ΔNp63 also resulted in a failure of keratinocytes to undergo terminal differentiation. First, using a BrdU incorporation assay, we found that suprabasal keratinocytes in ΔNp63 i-kd epidermis failed to withdraw from the cell cycle (Fig. 2E). Second, down-regulating ΔNp63 resulted in a failure of suprabasal keratinocytes to initiate expression of markers of terminal differentiation, including K1 (Fig. 2F), and loricrin and filaggrin [see SI Fig. 6]. In addition, persistent down-regulation of ΔNp63, achieved by chronically treating adult ΔNp63 i-kd mice with RU486, resulted in basement membrane abnormalities as demonstrated by discontinuous staining for collagen IV, a marker for the basement membrane (Fig. 2G and SI Fig. 6). The impaired terminal differentiation and the basement membrane abnormalities may have contributed to the observed skin fragility of ΔNp63 i-kd mice by failing to provide the epidermis with adequate structural stability.
Fig. 2.
In vivo ΔNp63 knockdown. (A) Gross appearance of 5-day-old ΔNp63 i-kd and control mice after 4 days of topical RU486 treatment. (B) Sections of back skin of newborn ΔNp63 i-kd mice treated for 4 days with RU486 were stained with H&E. Original magnification: ×200. (C) Sections of full-thickness wounds induced on the heads of adult ΔNp63 i-kd and control mice taken 96 h after wounding. Arrows indicate the migrating epithelial tongue. Original magnification: ×100. (D) TUNEL analysis on sections of back skin of newborn ΔNp63 i-kd mice treated for 4 days with RU486. Propidium iodide was used as a nuclear counterstain. Original magnification: ×200. (E) After 4 days of RU486 treatment, newborn mice were injected with BrdU. BrdU incorporation was visualized by immunofluorescence with an anti-BrdU antibody (green). Anti-K14 antibody (red) was used to highlight the epithelial component of the skin. Original magnification: ×200. (F) Immunofluorescence using anti-K1 (green) and anti-K14 (red) antibodies on sections of back skin of newborn ΔNp63 i-kd mice after 4 days of RU486 treatment. Original magnification: ×200. (G) Immunofluorescence using anti-collagen IV (red) and anti-K14 (green) antibodies on sections of back skin of adult ΔNp63 i-kd mice that were treated for 21 days with RU486. Original magnification: ×200.
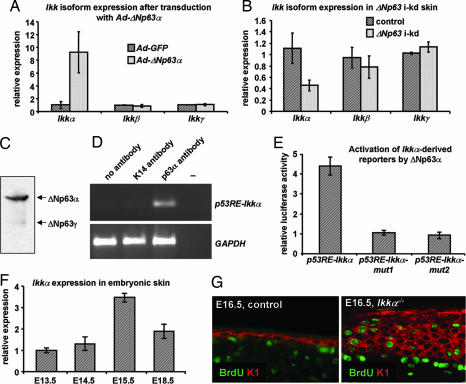
Based on these and published data, we propose that ΔNp63α induces the expression of genes that control basement membrane formation and terminal differentiation, including genes required for cell cycle withdrawal and for the induction of markers of terminal differentiation. To identify in vivo target genes of ΔNp63 that control these processes, we mimicked the switch from TA- to ΔNp63 isoforms that occurs during epidermal development. Primary keratinocytes from inducible TAp63α mice (11) were treated with RU486 to induce TAp63α expression (TAp63:ΔNp63 ≈ 5:1) and cells were transduced with adenoviruses expressing ΔNp63α (Ad-ΔNp63α) or GFP (Ad-GFP), following RU486 withdrawal (ΔNp63:TAp63 ≈ 50:1). Using microarrays (Affymetrix, Santa Clara, CA) and real-time RT-PCR validation, we found that Ikkα, but not Ikkβ or Ikkγ, mRNA was up-regulated ≈9-fold 12 h after transduction with Ad-ΔNp63α (Fig. 3A). In addition, Ikkα mRNA was decreased in ΔNp63 i-kd epidermis (Fig. 3B), further suggesting that ΔNp63α could regulate Ikkα expression. p63 regulates gene expression by interacting with degenerate p53 response elements (p53RE) (28, 29), one of which (p53RE-Ikkα) is present in the Ikkα promoter (SI Fig. 7). To determine whether ΔNp63α could interact with this element in the developing epidermis, we performed ChIP assays on WT skin isolated from embryonic day (E)15.5 embryos, the developmental stage when commitment to terminal differentiation occurs and when ΔNp63α is the predominantly expressed p63 isoform (Fig. 3C). Immunoprecipitation with a p63α antibody resulted in increased recovery of promoter fragments containing p53RE-Ikkα, demonstrating that ΔNp63α directly binds to the Ikkα promoter (Fig. 3D). Furthermore, ΔNp63α could transactivate a reporter construct containing p53RE-Ikkα, but not reporter constructs that harbor mutations in p53RE-Ikkα (p53RE-Ikkα-mut1 and p53RE-Ikkα-mut2) (Fig. 3E). A recent study, using in vitro experiments, suggested that induction of IKKα by ΔNp63α occurs through an indirect mechanism in Saos2 cells (30). However, our in vivo analyses clearly demonstrate that, when commitment to terminal differentiation occurs, ΔNp63α induces Ikkα expression by directly interacting with the Ikkα promoter. Although Ikkα+/− mice do not display an overt skin phenotype (31–33), the relatively modest reduction (≈60%) in Ikkα expression in ΔNp63 i-kd mice suggests that, in conjunction with reduced expression of other ΔNp63α target genes, reduced Ikkα expression has profound effects on epidermal biology. Alternatively, patchy transgene expression could have resulted in varying degrees of ΔNp63, and consequently Ikkα, down-regulation. A more efficient ΔNp63 knockdown in certain areas may also explain the focal nature of the skin erosions, which presumably developed in areas with the most efficient ΔNp63 down-regulation.
Fig. 3.
Ikkα is a direct transcriptional target of ΔNp63α. (A) Real-time RT-PCR analysis for Ikkα, Ikkβ, and Ikkγ on RNA isolated from primary keratinocytes after a switch in p63 isoform expression from TA- to ΔNp63. (B) Real-time RT-PCR analysis for Ikkα, Ikkβ, and Ikkγ on RNA isolated from newborn ΔNp63 i-kd and control skin after 4 days of topical RU486 treatment. (C) Western blot analysis of protein isolated from E15.5 wild-type skin using a p63 antibody that recognizes all p63 isoforms. (D) ChIP on E15.5 mouse skin using an anti-p63α antibody and primers surrounding p53RE-Ikkα. Anti-K14 antibody was used as a negative control. (E) Reporter constructs (0.5 μg) containing p53RE-Ikkα, p53RE-Ikkα-mut1 and p53RE-Ikkα-mut2 were transfected with or without a ΔNp63α expression vector (0.5 μg). (F) Real-time RT-PCR for Ikkα on RNA isolated from embryonic skin at various developmental stages. (G) One hour before dissecting E16.5 embryos, pregnant females were injected with BrdU. Immunofluorescence using anti-BrdU (green) and anti-K1 (red) antibodies was performed to detect the presence of proliferating cells. Original magnification: ×200. Error bars represent standard deviations of A, B, and F (three independent samples), or E (three independent experiments).
Interestingly, Ikkα−/− mice and p63−/− mice display similar developmental defects, although skin and appendage development arrest at a later stage in Ikkα−/− than in p63−/− mice (4, 5, 31–33). As proposed for p63−/− mice, the developmental defects affecting epithelial appendages in Ikkα−/− mice were demonstrated to be caused by an aborted epidermal differentiation program (34). To further define the role of IKKα in epidermal morphogenesis, we characterized the embryonic skin phenotype of Ikkα−/− mice and found that Ikkα−/− mice fail to develop a mature spinous layer. Instead, the intermediate cell layer, a transiently existing suprabasal cell layer, which contains K1-expressing proliferating cells (35, 36) (SI Fig. 8), persists as shown by the presence of proliferating cells in the suprabasal layers of E15.5 and E16.5 Ikkα−/− epidermis (Fig. 3G). Together with our finding that Ikkα expression levels peak at E15.5 (Fig. 3F), these data demonstrate that IKKα is required for maturation of intermediate cells into spinous cells. Unlike ΔNp63 i-kd epidermis, Ikkα−/− epidermis expresses certain genes required for epidermal maturation, including K1. Therefore, induction of additional ΔNp63α target genes, which remain to be identified, is likely to be required for maturation of the spinous layer.
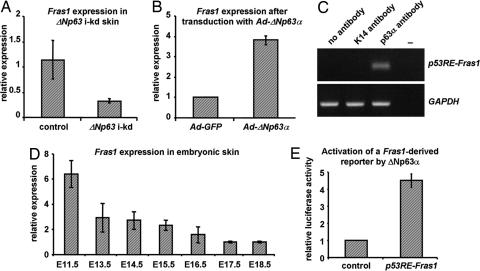
Furthermore, although Ikkα is a critical target gene of ΔNp63α, ΔNp63 i-kd epidermis not only fails to undergo terminal differentiation, it also fails to establish a functional basement membrane, suggesting that ΔNp63 induces the expression of genes required for basement membrane formation. To identify ΔNp63 target genes that function independent of IKKα, we performed microarray analyses on skin RNA isolated from ΔNp63 i-kd and control mice, and from Ikkα−/− and control mice. By performing a comparative analysis of the two sets of microarray data, we identified 51 genes that were specifically down-regulated in ΔNp63 i-kd skin. Among these was the gene encoding Fras1, a keratinocyte-produced extracellular matrix protein which forms a protein complex with its family members Frem1 (Fras1-related ECM protein 1) and Frem2 (Fras1-related ECM protein 2) (37). The absence of any one of these proteins results in a failure of this protein complex to form, causing a severe embryonic blistering phenotype in mice (37–40). Indeed, real-time RT-PCR analysis confirmed that Fras1 was down-regulated in ΔNp63 i-kd skin (Fig. 4A). In addition, we found that ΔNp63α could induce Fras1 expression in primary keratinocytes (Fig. 4B). To determine whether ΔNp63α could bind to the Fras1 promoter, we performed ChIP analysis on embryonic skin isolated at E11.5, a developmental stage when Fras1 is highly expressed (Fig. 4D). Immunoprecipitation demonstrated that ΔNp63α directly interacts with a region of the Fras1 promoter located ≈1500 bp 5′ from the translation start site (Fig. 4C). In addition, ΔNp63α could transactivate a reporter construct that contained this promoter region (Fig. 4E). Therefore, ΔNp63α controls basement membrane formation and maintenance by directly inducing Fras1 expression. The importance of Fras1 for maintaining skin integrity suggests that its down-regulation may have contributed to skin fragility in ΔNp63 i-kd mice.
Fig. 4.
Fras1 is a direct transcriptional target of ΔNp63α. Real-time RT-PCR analysis for Fras1 on (A) RNA isolated from newborn ΔNp63 i-kd and control skin treated for 4 days with RU486 and (B) RNA isolated from primary keratinocytes after a switch in p63 isoform expression from TA- to ΔNp63. (C) ChIP on E11.5 mouse skin using an anti-p63α antibody and primers surrounding p53RE-Fras1. Anti-K14 antibody was used as a negative control. (D) Real-time RT-PCR for Fras1 on RNA isolated from embryonic skin at various developmental stages. (E) A reporter construct (0.5 μg) containing p53RE-Fras1 was transfected with or without a ΔNp63α expression vector (0.5 μg). Error bars represent standard deviations of A, B, and D (three independent samples) or E (three independent experiments).
In summary, by down-regulating ΔNp63 expression in vivo, we demonstrate that ΔNp63α is required for the initiation of multiple pathways required for epidermal morphogenesis and homeostasis, including terminal differentiation and basement membrane integrity. Furthermore, we have identified two key genes that contribute to epidermal morphogenesis and are controlled by ΔNp63α. Initially, ΔNp63α induces expression of the extracellular matrix component Fras1, which is required for basement membrane integrity. During later stages of epidermal morphogenesis, ΔNp63α directly induces IKKα resulting in the formation of the spinous layer. In addition to providing insights into the regulation of epidermal morphogenesis, these findings may provide insight into the molecular etiology of ectodermal dysplasias that are caused by p63 mutations.
Materials and Methods
Cell Culture.
Primary keratinocytes were isolated from newborn mice and cultured as described (41). Ptk2 (rat kangaroo kidney) cells were grown and transfected as described (11). Cells were harvested for Western blot analysis 48 h after transfection.
Transgenic Mouse Lines.
To generate the inducible ΔNp63 knockdown transgene, exon 3′ of mouse ΔNp63 was cloned as an inverted repeat separated by a short spacer into pDECAP (pD-E3′) (27). The pD-E3′ construct was subcloned into the UAS-TK vector. Transgenic mice were generated by standard techniques. Founders were identified by tail-tip DNA PCR analysis using primers FW 5′-GGT CGA AGC GGA GTA CTG TC and RV 5′-CAC ACC TCC CCC TGA ACC T. Mice carrying the inducible pD-E3′ transgene were mated with K14.Glp65 mice (42) to generate ΔNp63 i-kd mice. To induce transgene expression, mice were treated daily with 1 mg/ml RU486 in 100% ethanol (Mifepristone; BioMol, Plymouth Meeting, PA). Transgene induction was confirmed by RT-PCR using primers FW 5′-CGC TTC GAG CAG ACA TGA TA and RV 5′-CCC CCT GAA CCT GAA ACA TA. GAPDH was amplified as a control by using primers FW 5′-AAG GTC GGT GTG AAC GGA TT and RV 5′-TGG TGG TGC AGG ATG CAT TG. Three independent transgenic lines, with varying ΔNp63 knockdown efficiencies, were established.
Wound Healing.
Six adult ΔNp63 i-kd and control mice were treated with 1 mg/ml RU486 for 7 days. On day 8, a 3-mm full-thickness wound was generated on the head by using a punch biopsy (Miltex, York, PA). After 96 h, wounds were excised and fixed in 10% neutral buffered formalin. Histological analysis was performed to confirm that all mice were in the same phase of the hair cycle.
Real-Time RT-PCR.
Relative gene expression levels were determined by real-time RT-PCR. RNA was isolated by using RNeasy kits (Qiagen, Valencia, CA), and cDNA was prepared by using the High-capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). Assays-on-Demand TaqMan probes for Ikkα, Ikkβ, Ikkγ, and Fras1 were obtained from Applied Biosystems. TaqMan primer and probe sequences for ΔNp63 were: FW 5′-GAA AAC AAT GCC CAG ACT CAA, RV 5′-TGT GCG TGG TCT GTG TTG, and 6FAM-TGA GCC ACA GTA CAC GAA CCT GGG and for TAp63: FW 5′-TGT ATC CGC ATG CAA GAC T, RV 5′-CTG TGT TGT AGG GGC TGG TGG AC, and 6FAM-ACC TCA GTG ACC CCA TGT GGC C. TaqMan Universal PCR Master Mix (Applied Biosystems) was used for PCR amplification of cDNA with the Opticon2 System (MJ Research, Tokyo, Japan). Each mRNA was normalized to the level of 18S RNA in each sample, and the relative level of each mRNA was determined by the comparative CT method.
Western Blot Analysis.
Protein was extracted from whole skin or transfected cells and subjected to Western blotting using a mouse anti-p63 (4A4) antibody (3). After incubation with an HRP-conjugated anti-mouse secondary antibody (Sigma, St. Louis, MO), protein bands were visualized by using SuperSignal West Pico Substrate (Pierce, Rockford, IL). Antibodies against K18 (Sigma) and K14 (41) were used as loading controls.
ChIP.
ChIP was performed on skins isolated from 60 E11.5 embryos or 20 E15.5 embryos. After homogenization, dissociated cells were fixed in 1% formaldehyde. After washing and sonication, chromatin was immunoprecipitated with 3 μg of either anti-p63α (H129; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-K14 antibodies (41). Immunoprecipitated samples were analyzed by PCR using primers surrounding p53RE-Ikkα: FW 5′-TCC TGG AAT CAC CCT GGA TTG and RV 5′-AAT AGG AAC CGA CGC ACG ATG or p53RE-Fras1: FW 5′-GTC TTA AGT TAC TCC TAG TCA GTG GTG and RV 5′-TTG GAT GGA ACC TGA GTC CT. GAPDH was amplified as control to confirm equal genomic DNA content in the immunoprecipitated samples by using primers FW: 5′-CCA ATG TGT CCG TCG TGG AT and RV: 5′-TGC TGT TGA AGT CGC AGG AG.
Reporter Gene Assays.
Fragments of the Ikkα and Fras1 promoters containing degenerate p53RE (p53RE-Ikkα and p53RE-Fras1 respectively) were cloned into pGL3-basic (Promega, Madison, WI) which was modified to include the SV40 minimal promoter or TATA box, respectively. Mutations in p53RE-Ikkα were generated by using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). In mutant 1, the core sequences (underlined in SI Fig. 7) were changed to GCCG and CCCG, whereas in mutant 2, the core sequences were changed to ATTT and AAAT. Ptk2 cells were cotransfected with reporter constructs (0.5 μg), a TAp63α or ΔNp63α expression construct (0.5 μg) and a pCMV-βgal plasmid (50 ng) as described (11). Cells were harvested 48 h later, and luciferase assays were performed by using the dual-light combined reporter gene assay system for detection of luciferase and β-galactosidase (Applied Biosystems). Average reporter gene activities and standard deviations were determined based on three independent experiments.
In Vivo BrdU Incorporation and Immunofluorescence.
Mice were injected i.p. with 250 μg/g BrdU (Sigma) in 0.9% sterile saline solution. After 1 h, tissue was fixed in 10% neutral buffered formalin. Primary antibodies used for immunofluorescence were FITC anti-BrdU (Becton Dickinson, Franklin Lakes, NJ), guinea pig anti-K14 (41), rabbit anti-K1 (41), and rabbit anti-collagen IV (Progen, Heidelberg, Germany). Secondary antibody conjugates used were Alexa-conjugated fluorochromes (Invitrogen, Carlsbad, CA).
TUNEL Analysis.
TUNEL analysis was performed by using the DeadEnd Fluorometric TUNEL System (Promega) according to the manufacturer's instructions. Sections were counterstained with propidium iodide (Sigma).
All experiments involving mice were performed under Institutional Animal Care and Use Committee approval (Protocol number AN-546).
Supplementary Material
Acknowledgments
We thank Dr. Frank McKeon (Harvard Medical School, Boston, MA) for providing mouse p63 expression constructs and the p63 antibody (mAb4A4), Dr. Shunsuke Ishii (RIKEN Tsukuba Institute, Ibaraki, Japan) for providing the pDECAP plasmid, and Dr. Peter Koch for his constructive comments on the manuscript. This work was supported by National Institutes of Health grants (to D.R.R. and M.K.), a research grant from the National Foundation for Ectodermal Dysplasias (to D.R.R. and M.I.K.), an award from the Centre de Recherches et d'Investigations Epidermiques et Sensorielles (to D.R.R.), a research professorship from the American Cancer Society (to M.K.), and grants from the Associazione Italiana per la Ricerca sul Cancro and European Commission Sixth Framework Programme (ECFP6) (to A.C.). B.M. was supported by a FIRC fellowship, and Y.S. was supported by a postdoctoral fellowship from the Japanese Society for the Promotion of Science.
Abbreviations
- Ad
adenovirus
- E3′
exon 3′
- IKKα
IκB kinase-alpha
- i-kd
inducible knockdown
- K
keratin
- pD
pDECAP
- p53RE
p53 response element
- TA
transactivation domain.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611376104/DC1.
References
- 1.Dai X, Segre JA. Curr Opin Genet Dev. 2004;14:485–491. doi: 10.1016/j.gde.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotto GP. Crit Rev Oral Biol Med. 1999;10:442–457. doi: 10.1177/10454411990100040201. [DOI] [PubMed] [Google Scholar]
- 3.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 4.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 5.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larderet G, Fortunel NO, Vaigot P, Cegalerba M, Maltere P, Zobiri O, Gidrol X, Waksman G, Martin MT. Stem Cells. 2006;24:965–974. doi: 10.1634/stemcells.2005-0196. [DOI] [PubMed] [Google Scholar]
- 9.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 11.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koster MI, Kim S, Huang J, Williams T, Roop DR. Dev Biol. 2006;289:253–261. doi: 10.1016/j.ydbio.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Laurikkala J, Mikkola ML, James M, Tummers M, Mills AA, Thesleff I. Development (Cambridge, UK) 2006;133:1553–1563. doi: 10.1242/dev.02325. [DOI] [PubMed] [Google Scholar]
- 14.Liefer KM, Koster MI, Wang XJ, Yang A, McKeon F, Roop DR. Cancer Res. 2000;60:4016–4020. [PubMed] [Google Scholar]
- 15.Parsa R, Yang A, McKeon F, Green H. J Invest Dermatol. 1999;113:1099–1105. doi: 10.1046/j.1523-1747.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 16.King KE, Ponnamperuma RM, Yamashita T, Tokino T, Lee LA, Young MF, Weinberg WC. Oncogene. 2003;22:3635–3644. doi: 10.1038/sj.onc.1206536. [DOI] [PubMed] [Google Scholar]
- 17.King KE, Ponnamperuma RM, Gerdes MJ, Tokino T, Yamashita T, Baker CC, Weinberg WC. Carcinogenesis. 2006;27:53–63. doi: 10.1093/carcin/bgi200. [DOI] [PubMed] [Google Scholar]
- 18.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, Haber DA. Mol Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 19.Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. Mol Cell Biol. 2003;23:2264–2276. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della GG, Koster MI, Zhang Z, Wang J, Tommasi DV, et al. Genes Dev. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beretta C, Chiarelli A, Testoni B, Mantovani R, Guerrini L. Cell Cycle. 2005;4:1625–1631. doi: 10.4161/cc.4.11.2135. [DOI] [PubMed] [Google Scholar]
- 25.Martinez LA, Chen Y, Fischer SM, Conti CJ. Oncogene. 1999;18:397–406. doi: 10.1038/sj.onc.1202300. [DOI] [PubMed] [Google Scholar]
- 26.Testoni B, Mantovani R. Nucleic Acids Res. 2006;34:928–938. doi: 10.1093/nar/gkj477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinagawa T, Ishii S. Genes Dev. 2003;17:1340–1345. doi: 10.1101/gad.1073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng X, Levine AJ, Lu H. Proc Natl Acad Sci USA. 1998;95:6681–6686. doi: 10.1073/pnas.95.12.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian J, Sun Y. Proc Natl Acad Sci USA. 1997;94:14753–14758. doi: 10.1073/pnas.94.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candi E, Terrinoni A, Rufini A, Chikh A, Lena AM, Suzuki Y, Sayan BS, Knight RA, Melino G. J Cell Sci. 2006;119:4617–4622. doi: 10.1242/jcs.03265. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC, Verma IM. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sil AK, Maeda S, Sano Y, Roop DR, Karin M. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- 35.Smart IH. Br J Dermatol. 1970;82:276–282. doi: 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- 36.Lechler T, Fuchs E. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiyozumi D, Sugimoto N, Sekiguchi K. Proc Natl Acad Sci USA. 2006;103:11981–11986. doi: 10.1073/pnas.0601011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vrontou S, Petrou P, Meyer BI, Galanopoulos VK, Imai K, Yanagi M, Chowdhury K, Scambler PJ, Chalepakis G. Nat Genet. 2003;34:209–214. doi: 10.1038/ng1168. [DOI] [PubMed] [Google Scholar]
- 39.Jadeja S, Smyth I, Pitera JE, Taylor MS, van Haelst M, Bentley E, McGregor L, Hopkins J, Chalepakis G, Philip N, et al. Nat Genet. 2005;37:520–525. doi: 10.1038/ng1549. [DOI] [PubMed] [Google Scholar]
- 40.Smyth I, Du X, Taylor MS, Justice MJ, Beutler B, Jackson IJ. Proc Natl Acad Sci USA. 2004;101:13560–13565. doi: 10.1073/pnas.0402760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao T, He W, Roop DR, Wang XJ. Genesis. 2002;32:189–190. doi: 10.1002/gene.10063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.