Abstract
In adulthood, the action of androgens on seminiferous tubules is essential for full quantitatively normal spermatogenesis and fertility. In contrast, their role in the fetal testis, and particularly in fetal germ cell development, remains largely unknown. Using testicular feminized (Tfm) mice, we investigated the effects of a lack of functional androgen receptor (AR) on fetal germ cells, also named gonocytes. We demonstrated that endogenous androgens/AR physiologically control normal gonocyte proliferation. We observed an increase in the number of gonocytes at 17.5 days postconception resulting from an increase in proliferative activity in Tfm mice. In a reciprocal manner, gonocyte proliferation is decreased by the addition of DHT in fetal testis organotypic culture. Furthermore, the AR coregulator Hsp90α (mRNA and protein) specifically expressed in gonocytes was down-regulated in Tfm mice at 15.5 days postconception. To investigate whether these effects could result from direct action of androgens on gonocytes, we collected pure gonocyte preparations and detected AR transcripts therein. We used an original model harboring a reporter gene that specifically reflects AR activity by androgens and clearly demonstrated the presence of a functional AR protein in fetal germ cells. These data provide in vivo and in vitro evidence of a new control of endogenous androgens on gonocytes identified as direct target cells for androgens. Finally, our results focus on a new pathway in the fetal testis during the embryonic period, which is the most sensitive to antiandrogenic endocrine disruptors.
Keywords: androgen receptor, fetal testis, gonocytes, Tfm
During mammalian development, androgens produced by the fetal testis are the most important hormones controlling the masculinization of the reproductive tract and the genitalia (1). In the absence of production or action of androgens, genetic males are outwardly female in appearance. In the testis, the requirement for androgens for proper function has been demonstrated by studies of the murine testicular feminization (Tfm) mutation, androgen receptor-null mutants, and human testosterone insensitive syndrome (2–5). It is also established that from puberty onward, germ cells are the targets of androgens (spermatogenic arrest at the late spermatocyte/spermatid stage) with evidence pointing to an effect mediated by Sertoli cells (2, 6–8).
There are various lines of evidence that point to a role for androgens in fetal germ cell development. First, cases of androgen insensitivity and low androgen levels are associated with a high risk of testicular cancer (9–11), which may result from altered development of gonocytes. Second, there is general agreement that the decrease in sperm count (12, 13) and the increase in the incidence of testicular cancer (9, 14) observed in many countries result from endocrine disruptor (particularly antiandrogens) exposure during the fetal testis development (15). Recent investigations show that in utero exposure to an antiandrogenic disruptor (Vinclozolin) can influence development and induce reprogramming in the epigenetic imprinting of the fetal male germ line that promotes transgenerational disease states (16–18).
Androgen actions are mediated through the androgen receptor (AR), which belongs to the steroid receptor superfamily whose members function primarily as transcription factors to regulate the expression of target genes by binding to specific hormone-responsive elements (19). There is nearly universal agreement across species that AR is present in the adult testis in Sertoli cells, peritubular cells, and Leydig cells (20). In contrast, the localization of AR in the fetal testis remains more controversial. It is clear that AR is expressed in peritubular cells (21). Alternatively, the conventional view of the localization of AR in Sertoli cells is that it is present in testis only after birth (22, 23). However, Cupp et al. detected it earlier in gestation (24). The localization of AR in fetal germ cells remains even more controversial to the extent that there are reports of its presence (24, 25) and absence (21). A clear understanding of cellular localization of AR is necessary for an appropriate comprehension of the cellular and molecular mechanisms of androgens and antiandrogens.
New findings show that the male germ line is the most sensitive to antiandrogenic endocrine disruptor during the embryonic period. Until now, the effect of androgens on gonocytes during fetal development has never been investigated. To address these questions, we studied gametogenesis during fetal life in Tfm mice, which carry a naturally inactivating mutation of the androgen receptor (26, 27). We demonstrated an increase in the proliferation of gonocytes in Tfm mice leading to an increase in germ cell number per testis. These surprising inhibitory effects of androgens on germ cell proliferation raise the following question: do androgens regulate germ cells directly or through another cell type? In the present study, we detected AR transcripts in pure gonocyte extracts, showing that androgens may act directly, and demonstrated the presence of a functional AR protein in gonocytes by using an original model harboring an androgen-dependent reporter gene.
Results
Tfm Mice Study.
Morphometric studies.
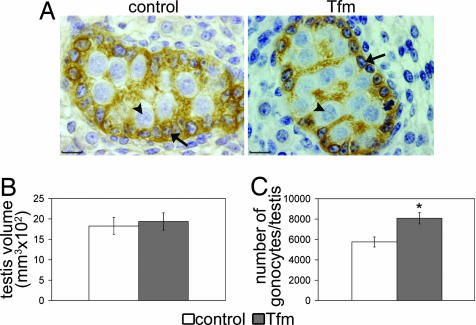
At 17.5 days postconception (dpc), testicular histology and volume were similar in Tfm mice and control littermates (respective volumes: 0.19 ± 0.02 vs. 0.18 ± 0.02 mm3, n = 5; Fig. 1 A and B). Tfm mice had 40% more gonocytes than control littermates (8,080 ± 558 in Tfm vs. 5,756 ± 493 in control, n = 5, P < 0.05; Fig. 1C), but the mean nuclear diameter was not significantly different (8.86 ± 0.47 vs. 7.94 ± 0.35 μm).
Fig. 1.
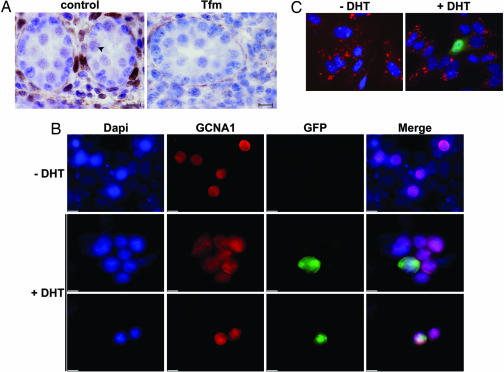
Morphometric analysis of 17.5 dpc fetal testes. (A) Histologic appearance of the testis in control and testicular feminized (Tfm) fetuses. Sertoli cells (arrows) were immunostained with anti-mullerian hormone and located at the periphery of the seminiferous cord, whereas gonocytes (arrowheads) were the large unstained cells in the center of the cord. (Scale bars, 10 μm.) (B and C) Testicular volume (B) and number of gonocytes per testis (C) in Tfm mice and their normal littermates. All results are shown as the mean ± SEM; n = 5. ∗, P < 0.05 in Student's t test.
Gonocyte proliferation.
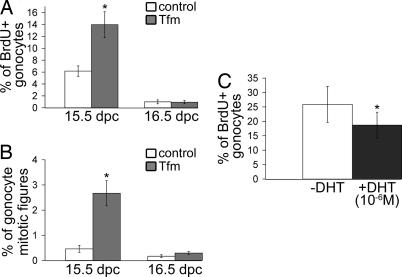
During fetal life, gonocytes develop in two successive phases: proliferation until 15.5 to 16.5 dpc and a quiescent phase until day 0 to 1 postpartum (28). To determine the cause of the increase of gonocyte number at 17.5 dpc in Tfm mice, 5-bromo-2′-deoxyuridine (BrdU) incorporation in gonocytes (Fig. 2A) and the mitotic figures (Fig. 2B) were analyzed from 15.5 to 17.5 dpc. Androgen receptor deficiency resulted in a significant increase (P < 0.05) in the percentage of BrdU-positive gonocytes at 15.5 dpc, but there was no significant difference at 16.5 dpc. Likewise, the percentage of gonocytes with mitotic figures was significantly higher (P < 0.05) in Tfm mice than in control littermates at 15.5 dpc but was not different at 16.5 dpc. Neither BrdU incorporation nor mitotic figures were observed in Tfm mice or control littermates on gestational day 17.5 (data not shown). In a reciprocal manner, gonocyte proliferation was analyzed in explanted 13.5 dpc testis (Fig. 2C), which was treated, or not, for 30 h with dihydrotestosterone (DHT). The number of BrdU-positive gonocytes in the DHT-treated testis was the mirror image of the situation in Tfm mice with a 27% decrease in their proliferation compared with paired controls (P < 0.05).
Fig. 2.
Effect of androgen receptor deficiency and dihydrotestosterone treatment on gonocyte proliferation. Pregnant females (15.5 or 16.5 dpc) were injected with BrdU 3 h before being killed. (A and B) The percentages of BrdU-positive gonocytes (A) and gonocyte mitotic figures (B) were determined from at least 1,000 counted gonocytes. (C) Testes were explanted at 13.5 dpc and treated (or not) for 30 h with dihydrotestosterone (10−6 M). The percentages of BrdU-positive gonocytes were determined from at least 1,000 counted gonocytes. Values are means ± SEM; ∗, P < 0.05 in Student's t test (A and B; n = 5) or in a paired Student's t test (C; n = 7).
Hsp90α expression in fetal Tfm gonocytes.
The AR signaling pathway comprises a multimeric cytosolic complex, including the chaperone protein Hsp90 (heat shock protein 90) (29). Two Hsp90-related proteins (Hsp90α and Hsp90β) have been identified in mouse testis. One of them, Hsp90α, is specifically expressed in gonocytes during fetal and neonatal life (30).
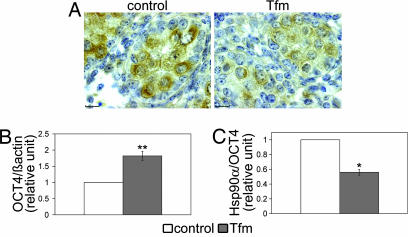
The possibility that AR deficiency might affect components of the AR cytosolic complex in gonocytes was examined by measuring Hsp90α mRNA and protein levels. Immunohistochemical analysis of Hsp90α clearly showed that lack of AR in Tfm mice resulted in a marked decrease in Hsp90α staining in the gonocytes (Fig. 3A). Hsp90α mRNA was analyzed in mRNA extracts from whole testes of control and Tfm mice at 15.5 dpc using real-time RT-PCR (Q-PCR). To determine whether Hsp90α expression was modified in Tfm mice, we first evaluated the expression of the gene coding for Oct-4. This gene is specifically expressed in gonocytes (31, 32) and did not present androgen response element sequences on the promoter. We used the AliBaba2 program (www.gene-regulation.com) to search for predicted transcription factor binding sites on the Oct-4 promoter sequence (accession no. AJ297528). Furthermore, Fig. 3B shows an increase in testicular Oct-4 mRNA expression in Tfm testes (n = 5, P < 0.01) correlated with the increase in gonocyte number. For this reason, we normalized Hsp90α mRNA levels using Oct-4 as an endogenous reference. In Fig. 3C, Q-PCR analysis shows that the Hsp90α mRNA level was 1.8-fold lower in Tfm mice than in control littermates (P < 0.05).
Fig. 3.
Effect of androgen receptor deficiency on heat shock protein 90α (Hsp90α) and mRNA levels in 15.5-dpc testes. (A) immunohistochemical staining of Hsp90α in control and testicular feminized (Tfm) testes. (Scale bars, 10 μm.) (B and C) Oct-4 (B) and Hsp90α (C) mRNA expression in 15.5-dpc testis from control and Tfm fetuses was measured by Q-PCR using β-actin or Oct-4, respectively, as reference. Results are expressed in relative units with the control animals having a value of 1. n = 5; ∗, P < 0.05; ∗∗, P < 0.01 in Student's t test.
Androgen Receptor Localization in Gonocytes.
mRNA localization.
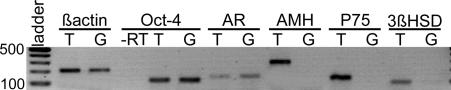
To determine whether the AR was localized on gonocytes, we used RT-PCR to search for its mRNA in gonocytes isolated by magnetic-activated cell sorting (MACS) from 15.5 dpc normal mice. An AR transcript was detected in two independent preparations of gonocytes (Fig. 4). As expected, an AR transcript was found in whole testicular tissue and Oct-4 followed the same expression pattern as the AR transcripts. Amplification of anti-mullerian hormone (AMH), P75, and 3β-hydroxysteroid dehydrogenase (3β-HSD) transcripts in whole testis extract, but never in gonocyte preparations, indicated that there was no contamination by somatic cells.
Fig. 4.
Detection of androgen receptor mRNA in testicular gonocytes at 15.5 dpc. mRNA expression of androgen receptor, Oct-4, AMH, P75, and 3β-HSD was examined by RT-PCR analysis in whole testicular tissue (T) and in magnetic-activated cell sorting isolated gonocytes (G). Results shown are representative of two independent experiments. −RT, control for genomic DNA contamination using Oct-4 primers on isolated gonocytes mRNA extracts.
Identification of a functional androgen receptor protein.
Prior studies were performed using conventional immunohistochemistry to examine the cell localization of AR protein in testis at 15.5 dpc. AR-immunoreactive staining was strong in testicular interstitial cells. AR was also positive in nuclei of gonocytes, but the signal intensity was weaker. Sertoli cells were AR-negative. No staining was seen with Tfm mouse negative controls (Fig. 5A).
Fig. 5.
Cell-specific expression of androgen receptor protein in 15.5-dpc testis. (A) Immunohistochemical staining of androgen receptor in control and testicular feminized (Tfm) testes. The arrowhead indicates an example of a stained gonocyte. No signal was detected in Tfm testes. (Scale bars, 10 μm.) (B) Enriched gonocyte cultures were transfected with the ARE2-TATA-GFP-NLS vector and cultivated with or without dihydrotestosterone (DHT) (10−6 M) for 30 h. GCNA1, a gonocyte marker, and GFP were detected by immunostaining (red and green, respectively). Green gonocytes were present only after DHT treatment indicating the presence of a functional androgen receptor protein. (C) Five-day-old Sertoli cell cultures served as positive control. Cells were transfected with ARE2-TATA-GFP-NLS vector and cultivated without or with DHT (10−6 M). Anti-mullerian hormone, a cytoplasmic marker of Sertoli cells, and GFP were detected by immunostaining (red and green, respectively). As expected, green Sertoli cells were present only after DHT treatment indicating the presence of a functional androgen receptor protein. All of the slides in B and C were counterstained with 4′-6 diaminido-2-phenylindole (DAPI) to visualize nuclei (blue). (Scale bars, 10 μm.)
By using cultures of isolated cells enriched in gonocytes from 15.5 dpc testes, we investigated the capacity of gonocytes to respond to DHT treatment. Gonocyte cultures were transfected with the ARE2-TATA-GFP-NLS vector and treated or not with DHT (10−6 M). After 30 h of induction, GFP was detected in the nucleus of some germ cell nuclear antigen 1-positive cells, a specific marker of gonocytes (33), whereas without DHT, we never observed any germ cell nuclear antigen 1-GFP-positive cells (Fig. 5B). The cultures contained a majority of Sertoli cells, but we never observed any GFP-positive Sertoli cell after DHT treatment (data not shown) in keeping with the reported absence of AR on Sertoli cells at 15.5 dpc (22). As a positive control, we transfected Sertoli cells from 5-days-postpartum animals known to express a functional AR (22, 34). Immunofluorescence showed that DHT treatment led to nuclear accumulation of GFP in AMH-positive cells, whereas without DHT, no GFP-positive cell was observed (Fig. 5C).
Discussion
A role for androgens in controlling fetal germ cell development has never been seriously considered in the past, perhaps because Sertoli cells and germ cells were not generally known to express AR during fetal life (21). The present results demonstrate that endogenous androgens inhibit fetal germ cell development, because gonocyte proliferation was increased in Tfm mice at 15.5 dpc compared with control mice.
Our results show that fetal germ cells have a critical period of sensitivity to androgens during their proliferation phase. We found no difference between Tfm and control mice in the mitotic index of gonocytes during the quiescent phase. Interestingly, this correlates with a recent study, which showed that an exposure of pregnant rat to antiandrogens only during the 8 to 15 dpc period affected later adult spermatogenesis (17), whereas the same treatment between 15 and 20 dpc had no effect (35, 36). However, we cannot rule out the possibility that the migration and proliferation of primordial germ cells, which occur before 11.5 dpc, could be also affected.
Interestingly, we demonstrated that AR deficiency leads to an increase in gonocyte number during fetal life. This role of androgen has never been studied during the fetal period in the different androgen or AR transgenic models. In the adult, there is mounting evidence that androgens and AR control the proliferation of several cell types in different tissues (37–41). However, the effect of androgens on the fetal testis evidenced here cannot be compared with the adult testis because it involves a completely different mechanism. Indeed, it cannot be direct on adult spermatogonia because these cells so far have been found to lack AR.
In other respects, the effects of androgens on germ cell development that we describe here are unlikely to result from androgen action on the hypothalamus–pituitary–gonadotropic axis, because gonadotropic hormone secretion occurs after the critical period of androgen action. Furthermore, no change in the number of gonocytes was observed in vivo in fetal rats after decapitation (42), in mice with inactivation of the follicle-stimulating hormone receptor (C.R., unpublished data) or in vitro in response to gonadotropin treatment of cultivated rat fetal testis (43). Furthermore, the decrease in gonocyte proliferation in organotypic culture in the presence of DHT clearly demonstrated a direct action of androgens on the fetal testis.
Our results demonstrate that AR mRNA and a functional AR are expressed in fetal germ cells in which, in all likelihood, it serves to transduce the effect of androgens. This result was observed on both 15.5 dpc and 17.5 dpc (data not shown), and is an important finding because it shows that the gonocyte is a direct target of endogenous androgens and of environmental endocrine disruptors acting as antiandrogens. Discrepancies in immunohistochemical localization of the AR in gonocytes in fetal mouse testis (25, 34) may be the result of the tissue and cell type dependence of the in vivo interactions of AR and coregulators, which results in the presence of different epitopes. Furthermore, we observed down-regulation of Hsp90α (an AR coregulator) mRNA and protein in Tfm mice. Similar results were observed in the uterus after treatment with flutamide, an antiandrogen (44). Because Hsp90α is exclusively expressed on gonocytes (30), this provides a further demonstration that androgens may act directly on fetal germ cells through the AR.
The mechanism of action of androgens in gonocytes remains to be elucidated. Androgens action in testis can be in part mediated through their conversion in estradiol by aromatase (45), which can be regulated by androgens. Even if aromatase regulation has not been investigated in the testis of Tfm mouse, Tfm rats exhibited lower levels of aromatase activity in brain (46). Furthermore, the androgen metabolite, 5α-androstane-3β, 17β-Diol, can modulate the estrogen receptor β (ERβ)-mediated gene transcription (47). ERβ is normally expressed in fetal mouse gonocytes (48). However, the lack of effect on fetal gonocytes of ERβ knockout mouse (49) suggests the ERβ pathway cannot control the number of gonocyte in Tfm mice during fetal life. This finding agrees with the present data showing that DHT, a nonaromatizable androgen, is efficient to inhibit gonocyte proliferation in vitro.
Our demonstration that androgens may act directly on the gonocytes does not exclude other mechanisms of action. We cannot exclude that androgen action on the gonocytes may be initiated by peritubular cells (8). It is well established that endogenous androgens play a physiological role in the development of Sertoli cells, probably through peritubular cells. In Tfm mice, like in ARKO mice, a decrease in the number of Sertoli cells was observed just after birth (39, 50). In Tfm mice, we did not detect changes in the percentage of Sertoli cells that incorporated BrdU at 15.5 dpc (data not shown). One may therefore hypothesize that the effect of androgens on Sertoli cell proliferation only becomes visible from late fetal life onward. This could explain the apparent discrepancy between an increase in the number of fetal germ cells in Tfm mice (our results) and decreased spermatogenesis in adults treated in utero with an antiandrogen (35, 36), because it is currently accepted that the number of germ cells supported by a single Sertoli cell is limited (51). Another explanation could be that the absence of androgen action on gonocytes, besides quantitative changes, also induces qualitative changes in the form of enhanced differentiation and less stem cell formation among the gonocytes.
In conclusion, our study demonstrates that endogenous androgens physiologically control germ cell growth in the male mouse fetus. It also points to a mechanism of androgen action on gonocytes. Elucidation of this pathway in the fetal testis will clarify not only fetal testis physiology, but also the effects of environmental antiandrogens that act during fetal life. Our observations open new perspectives for future investigations of how germ cell reprogramming gives rise to transgenerational effects.
Materials and Methods
Mice and Genotyping.
Androgen-insensitive Tfm mice (C57BL/6J-Aw-J.Cg-EdaTa-6J+/+ArTfm, Tfm/Y) (The Jackson Laboratory, Bar Harbor, ME) were genotyped by real-time Taqman allelic discrimination (ABI Prism 7000; Applied Biosystems, Foster City, CA). PCR primers (forward 5′-ACG AGG CAG CAG CAT ACC A-3′ and reverse 5′-TAG TCC AAT GGG TTC TCC AGC TT-3′), and two internal probes, one specific to the wild-type sequence associated with a VIC fluorochrome (5′-CGC ACC CCC CGC C-3′) and the other one specific to the muted sequence associated with a FAM fluorochrome (5′-CCG CAC CCC CGC C-3′), were used in genotyping. All mice were housed and bred according to National Institutes of Health guidelines and maintained on a constant 12-h light/12-h dark cycle.
Immunohistochemistry.
Sections of 15.5 and 17.5 dpc mouse testes fixed in Bouin's fluid or in histochoice (Electron Microscopy Science) were prepared by standard procedures and immunostained for AMH, Hsp90α, or AR as previously described (30, 49). To unmask the AR and Hsp90α protein, sections were microwaved in a 0.01 M citrate buffer solution (pH 6.0) for 5 min at 900 W and 3 min at 620 W. Slides were incubated with the rabbit polyclonal anti-AMH antibody (1/500; Santa Cruz Biotechnology, Santa Cruz, CA), with the rabbit polyclonal anti-Hsp90α antibody (Stressgen; 1:1,000), or with the rabbit polyclonal anti-AR antibody (sc-816; 1/500; Santa Cruz Biotechnology). Tfm sections were used as negative controls for AR staining. The results obtained for animals born from four different mothers were compared.
Cell Counting and Measurement of Testicular Volume.
Germ cells were counted in one of 20 serial sections of testes from Tfm and control littermate fetuses removed at 17.5 dpc and stained for AMH as described in ref. 49. The gonocytes were identified in the seminiferous cords as the large round cells unstained by AMH. Gonocytes were counted as described in ref. 49. Testicular volume was determined by measuring the areas of each section with a computerized video densitometer (Histolab; Microvision Instruments) and added them together. The resulting value was multiplied by 20 and by the section thickness (5 μm).
Measurement of Bromodeoxyuridine Incorporation Index.
Three hours before being killed, pregnant females were injected intraperitoneally with BrdU (Sigma–Aldrich, St. Louis, MO) at dose of 50 mg/kg body weight. Testes were fixed for 30 min at room temperature with 4% buffered formaldehyde and immunostained for both BrdU and AMH. Briefly, sections were permeabilized by heating the slides (immersed in 0.01 M citrate buffer, pH 6.0) in a microwave for 5 min at 900 W and 3 min at 620 W, washed and incubated with an anti-BrdU antibody (Roche, Gipf-Oberfrick, Switzerland; 1/200) for 1 h at 37°C, and the primary antibody was then revealed as described in ref. 49. AMH immunostaining was revealed with VIP reagent (Vector Laboratories, Burlingame, CA). The percentage of proliferating gonocytes was determined by counting at least 1,000 gonocytes on the sections.
Organotypic Cultures.
Testes were explanted at 13.5 dpc and cultivated as previously described (52) on DMEM-HAM F12 (1:1) without phenol red (Invitrogen, Carlsbad, CA) for 30 h. One testis was cultivated with DHT (10−6 M) and the contralateral testis without DHT. BrdU (0.01 mg/ml) was added to the medium 3 h before the end of the culture. The testes were fixed for 30 min at room temperature with 4% buffered formaldehyde and immunostained for both BrdU and AMH, as previously described.
Cell Isolation and Transfection.
Briefly, testes from 60 mouse fetuses (15.5 dpc) were isolated as described by Wang et al. (30). The cell pellet was resuspended in DMEM-HAM F12 (1:1) without phenol red (Invitrogen) and kept for 2 h in a 92-mm culture plate in 5% CO2 atmosphere at 37°C. Nonadherent cells were plated in a laminin-coated Lab-Tek chamber (Costar, Cambridge, MA) at a cell density of 5.105 cells per well in DMEM-HAM F12 without phenol red (Invitrogen) supplemented with amino acids (1×; reference 11130-036; Invitrogen) and gentamicin (0.04 mg/ml; Sigma–Aldrich). After 12 h of culture, cells were transiently transfected with p-ARE2-TATA-GFP-NLS plasmid using the LyoVec method (Invivogen) with or without 10−6 M DHT (Sigma–Aldrich) in the presence of 10% stripped FBS (a gift from N. Di Clemente, Institut National de la Santé et de la Recherche Médicale U493, Clamart, France). GFP induction was analyzed after 30 h of culture.
Purified Sertoli cells were isolated from 5-days-postpartum testis mice as described (53) to serve as a positive control.
Plasmid Construction.
A minimum promoter (ARE2-TATA) with two complementary 100-pb oligonucleotides (5′-CGa tta atA TAG TAC GTG ATG TTC TAG GCC TAG TAC GTG ATG TTC TCA GCT TAG GGT ATA TAA GCA GAG CTG GTT TAG TGA ACC GTC AGA TCg cta gcG C-3′) containing two ARE sequences (54), a TATA box, and a unique restriction site for NheI and AseI were synthesized (Invitrogen). ARE2-TATA-eGFP-NLS vector was constructed by replacing the cytomegalovirus promoter of CMV-eGFP-NLS vector, a gift from Y. Saintigny (Commissariat à l'Énergie Atomique, Fontenay-aux-Roses, France) by the inducible ARE2-TATA promoter.
Immunocytofluorescence.
Cells were fixed in Bouin's fluid for 15 min, permeabilized for 10 min with 0.5% Triton X-100, and incubated overnight at 4°C with a rat anti-GCNA1 antibody (1:100) provided by G. Enders (Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS) and a mouse anti-GFP antibody (1:250; BD Biosciences, Franklin Lakes, NJ). After washes in PBS, cells were incubated with an Alexa 594-conjugated donkey anti-rat IgM (Invitrogen; 1:200) and an Alexa 488-conjugated donkey anti-mouse IgG (Molecular Probes; 1:200). Fluorescent images were captured using an Olympus AX70 epifluorescent microscope equipped with a charge-coupled camera (Princeton Instruments, Trenton, NJ) and IPLab software (Scanalytics, Fairfax, VA).
Magnetic-Activated Cell Sorting.
Cells were isolated from testis from 60 mouse fetuses (15.5 dpc) according to the protocol of Wang et al. (30). The cell pellet was resuspended in DMEM-HAM F12 (1:1) without phenol red (Invitrogen) and kept for 2 h in a 92-mm culture plate (Nunc, Naperville, IL) and for 1 h on laminin-coated surfaces (six-well plate; Nunc) in a 5% CO2 incubator at 37°C to allow adhesion of Sertoli and myoid cells. Nonadherent cells were collected and further purified by magnetic-activated cell sorting as previously described (55) with minor modifications. Briefly, the cells were centrifuged at 400 g for 15 min and the pellet (≈106 cells) was suspended in 80 μl of incubation buffer (PBS-BSA 0.5%, EDTA 2 mM) and labeled with a rabbit anti-P75 antibody (1/1,000; Chemicon International, Temecula, CA) and 8 μl of biotin conjugated anti-CD71 antibody (BD Biosciences) for 10 min at 4°C. Cells were washed and resuspended with antibiotin antibody microbeads and anti-rabbit microbeads (Miltenyi Biotech, Auburn, CA) according to the manufacturer's procedure. Cells were separated using two MS columns (Miltenyi Biotech). The P75− CD71− fraction was labeled with 0.5 μg/106 cells of rat anti-CD9 antibody (BD Biosciences), then with an anti-rat antibody microbeads (Miltenyi Biotech), and cells were selected by retention on two LC columns (Miltenyi Biotech). The purity of the P75−CD71−/CD9+ fraction was assessed after 35 cycles in a PCR thermocycler (ABI PRISM 7000 Sequence Detector System; Applied Biosystems) using the following primers: P75 forward 5′-GTA GCC TGC CCC TGA CCA A-3′ and reverse 5′-GCC TCG TGG GTA AAG GAG TCT-3′ (56); AMH forward 5′-TCC TAC ATC TGG CTG AAG TGA TAT GGG AGC-3′ and reverse 5′-CTC AGG GTG GCA CCT TCT CTG CTT GGT TGA-3′; Oct-4 forward 5′-CCC GGA AGA GAA AGC GAA CT-3′ and reverse 5′-CAA GCT GAT TGG CGA TGT A-3′. For the detection of the 3β-HSD, we used the primers forward 5′-CTC AGT TCT TAG GCT TCA GCA ATT AC-3′, reverse 5′-CCA AAG GCA GGA TAT GAT TTA GGA-3′, and the internal probe 5′-TTT CAC TTA GAA CTT AGT ATT GCT TTT-3′ (57). AR expression in pure gonocytes was tested with the TaqMan gene expression assay Mm 00442688-m1.
RNA Isolation and Reverse Transcription.
mRNA was extracted from 15.5 dpc testes or magnetic-activated cell sorting isolated gonocytes with the μMACS mRNA isolation kit (Miltenyi Biotec). They were then reverse transcribed using the QuantiTect reverse transcription kit (Qiagen, Valencia, CA)
Real-Time Quantitative PCR.
Q-PCR was performed on an ABI PRISM 7000 Sequence Detector System using a SYBR Green PCR Master Mix (Applied Biosystems) and specific primers for the Hsp90α gene: forward 5′-GTT TTC CAA AGT CCC GAG AAC A-3′ and reverse 5′-AAA GCA GAA TGT GAT TGG GCA-3′. The primers and probes used for Oct-4 and β-actin were TaqMan gene expression assays, respectively, Mm00658129-gH and Mm00607939-S1 and were amplified with the TaqMan PCR Master Mix (Applied Biosystems). Q-PCR was performed and analyzed as previously described (58). The amounts of the various genes were normalized to the endogenous reference (β-actin and Oct-4). For each treatment, the mRNA levels were determined in samples from three different litters.
Statistical Analysis.
The results are presented as means ± SEM. The statistical significance of the difference between the mean values for two different genotypes was evaluated using Student's unpaired t test or Student's paired t test.
Acknowledgments
We thank Dr. G. Enders for providing the anti-GCNA1 antibody; Dr. Y. Saintigny for providing the GFP-NLS plasmid; and C. Joubert, V. Neuville, and S. Leblay (Commissariat à l'Énergie Atomique) for caring for the animals. Jorge Merlet holds a fellowship from the Ministère de l'Education Nationale de la Recherche et de la Technologie (2004–2007). This work was partly supported by “Ministère de l'Ecologie et du Developpement Durable” Grant PNRPE 2005-2008.
Abbreviations
- AMH
anti-mullerian hormone
- AR
androgen receptor
- DHT
dihydrotestosterone
- dpc
days postconception
- ERβ
estrogen receptor β
- Hsp90
heat shock protein
- Tfm
testicular feminized.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Jost A, Vigier B, Prepin J, Perchellet JP. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 2.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, et al. Proc Natl Acad Sci USA. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPhaul MJ. Mol Cell Endocrinol. 2002;198:61–67. doi: 10.1016/s0303-7207(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 4.O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. J Cell Sci. 2002;115:3491–3496. doi: 10.1242/jcs.115.17.3491. [DOI] [PubMed] [Google Scholar]
- 5.Young CY, Johnson MP, Prescott JL, Tindall DJ. Endocrinology. 1989;124:771–775. doi: 10.1210/endo-124-2-771. [DOI] [PubMed] [Google Scholar]
- 6.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Proc Natl Acad Sci USA. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, et al. Proc Natl Acad Sci USA. 2006;103:17718–17723. doi: 10.1073/pnas.0608556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 10.Fisher JS. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 11.Slowikowska-Hilczer J, Walczak-Jedrzejowska R, Kula K. Folia Histochem Cytobiol. 2001;39:67–72. [PubMed] [Google Scholar]
- 12.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Br Med J. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swan S, Elkin E, Fenster L. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adami HO, Bergstrom R, Mohner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Ziegler H, Rahu M, et al. Int J Cancer. 1994;59:33–38. doi: 10.1002/ijc.2910590108. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe RM, Irvine DS. Br Med J. 2004;328:447–451. doi: 10.1136/bmj.328.7437.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anway MD, Skinner MK. Endocrinology. 2006;147:S43–49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 17.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang HS, Anway MD, Rekow SS, Skinner MK. Endocrinology. 2006;147:5524–5541. doi: 10.1210/en.2006-0987. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Takeyama K, Sato T, Kato S. J Biochem (Tokyo) 2005;138:105–110. doi: 10.1093/jb/mvi118. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. J Androl. 2002;23:870–881. [PubMed] [Google Scholar]
- 21.Williams K, McKinnell C, Saunders PT, Walker M, Fisher JS, Turner KJ, Atanassova N, Sharpe M. Hum Reprod Update. 2001;7:236–247. doi: 10.1093/humupd/7.3.236. [DOI] [PubMed] [Google Scholar]
- 22.You L, Sar M. Endocrine. 1998;9:253–261. doi: 10.1385/ENDO:9:3:253. [DOI] [PubMed] [Google Scholar]
- 23.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 24.Cupp AS, Skinner MK. Reprod Toxicol. 2001;15:317–326. doi: 10.1016/s0890-6238(01)00124-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Kudo A, Kawakami H, Hirano H. Anat Rec. 1996;245:509–518. doi: 10.1002/(SICI)1097-0185(199607)245:3<509::AID-AR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. Mol Endocrinol. 1991;5:573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- 27.Gaspar ML, Meo T, Bourgarel P, Guenet JL, Tosi M. Proc Natl Acad Sci USA. 1991;88:8606–8610. doi: 10.1073/pnas.88.19.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG. J Reprod Fertil. 1991;93:233–243. doi: 10.1530/jrf.0.0930233. [DOI] [PubMed] [Google Scholar]
- 29.Pratt WB, Toft DO. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Thuillier R, Culty M. Biol Reprod. 2004;71:1652–1664. doi: 10.1095/biolreprod.104.030205. [DOI] [PubMed] [Google Scholar]
- 31.Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 32.Scholer HR, Dressler GR, Balling R, Rohdewohld H, Gruss P. EMBO J. 1990;9:2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enders GC, May JJ., II Dev Biol. 1994;163:331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- 34.Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Endocrinology. 1994;135:1227–1234. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- 35.Cupp AS, Uzumcu M, Suzuki H, Dirks K, Phillips B, Skinner MK. J Androl. 2003;24:736–745. doi: 10.1002/j.1939-4640.2003.tb02736.x. [DOI] [PubMed] [Google Scholar]
- 36.Uzumcu M, Suzuki H, Skinner MK. Reprod Toxicol. 2004;18:765–774. doi: 10.1016/j.reprotox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Compston JE. Physiol Rev. 2001;81:419–447. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- 38.Thomson AA. Reproduction. 2001;121:187–195. doi: 10.1530/rep.0.1210187. [DOI] [PubMed] [Google Scholar]
- 39.Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G. Endocrinology. 2005;146:2674–2683. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- 40.Walker WH, Cheng J. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 41.Culig Z, Steiner H, Bartsch G, Hobisch A. Endocr Relat Cancer. 2005;12:229–244. doi: 10.1677/erc.1.00775a. [DOI] [PubMed] [Google Scholar]
- 42.Migrenne S, Racine C, Guillou F, Habert R. Endocrinology. 2003;144:2617–2622. doi: 10.1210/en.2002-0011. [DOI] [PubMed] [Google Scholar]
- 43.Boulogne B, Olaso R, Levacher C, Durand P, Habert R. Int J Androl. 1999;22:356–365. doi: 10.1046/j.1365-2605.1999.00191.x. [DOI] [PubMed] [Google Scholar]
- 44.Papaconstantinou AD, Umbreit TH, Goering PL, Brown KM. J Steroid Biochem Mol Biol. 2002;82:305–314. doi: 10.1016/s0960-0760(02)00221-2. [DOI] [PubMed] [Google Scholar]
- 45.Turner KJ, Morley M, MacPherson S, Millar MR, Wilson JA, Sharpe RM, Saunders PT. Mol Cell Endocrinol. 2001;178:73–87. doi: 10.1016/s0303-7207(01)00413-0. [DOI] [PubMed] [Google Scholar]
- 46.Roselli CE, Salisbury RL, Resko JA. Endocrinology. 1987;121:2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- 47.Sneddon SF, Walther N, Saunders PT. Endocrinology. 2005;146:5304–5312. doi: 10.1210/en.2005-0914. [DOI] [PubMed] [Google Scholar]
- 48.Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Biol Reprod. 2000;62:310–317. doi: 10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- 49.Delbes G, Levacher C, Pairault C, Racine C, Duquenne C, Krust A, Habert R. Endocrinology. 2004;145:3395–3403. doi: 10.1210/en.2003-1479. [DOI] [PubMed] [Google Scholar]
- 50.Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- 51.Print CG, Loveland KL. BioEssays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 52.Lambrot R, Coffigny H, Pairault C, Donnadieu AC, Frydman R, Habert R, Rouiller-Fabre V. J Clin Endocrinol Metab. 2006;91:2696–2703. doi: 10.1210/jc.2005-2113. [DOI] [PubMed] [Google Scholar]
- 53.Buzzard JJ, Wreford NG, Morrison JR. Reproduction. 2002;124:633–641. doi: 10.1530/rep.0.1240633. [DOI] [PubMed] [Google Scholar]
- 54.Moilanen AM, Poukka H, Karvonen U, Hakli M, Janne OA, Palvimo JJ. Mol Cell Biol. 1998;18:5128–5139. doi: 10.1128/mcb.18.9.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seidenfaden R, Desoeuvre A, Bosio A, Virard I, Cremer H. Mol Cell Neurosci. 2006;32:187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Bouma GJ, Hart GT, Washburn LL, Recknagel AK, Eicher EM. Gene Expr Patterns. 2004;5:141–149. doi: 10.1016/j.modgep.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 57.O'Shaughnessy PJ, Willerton L, Baker PJ. Biol Reprod. 2002;66:966–975. doi: 10.1095/biolreprod66.4.966. [DOI] [PubMed] [Google Scholar]
- 58.Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]