Abstract
The atypical antipsychotic drugs (AAPDs) have markedly enhanced the treatment of schizophrenias but their use has been hindered by the major weight gain elicited by some AAPDs. We report that orexigenic AAPDs potently and selectively activate hypothalamic AMP-kinase, an action abolished in mice with deletion of histamine H1 receptors. These findings may afford a means of developing more effective therapeutic agents and provide insight into the hypothalamic regulation of food intake.
Keywords: atypical antipsychotic drugs, obesity, hypothalamus
The antipsychotic actions of classic neuroleptics revolutionized the therapy of schizophrenia, but their use has been impeded by side effects such as extrapyramidal symptoms, tardive dyskinesia, a high incidence of nonresponders, and the failure of negative symptoms such as apathy to respond. The atypical antipsychotic drugs (AAPDs), pioneered by clozapine, represent an important advance in improving negative symptoms, benefiting patients who do not respond to the typical drugs, and displaying fewer side effects (1–5). A major limitation of AAPDs is pronounced weight gain, predominantly mediated by increased food intake (6–10). Weight gain elicited by AAPDs is primarily related to increased food intake, although there may also be metabolic alterations (11–13).
To directly address central systems that mediate appetite and weight gain, we have explored hypothalamic AMPK phosphorylation, which activates the enzyme (14, 15). In the periphery, AMPK activation is associated with decreased lipid formation, because AMPK phosphorylates acetyl-CoA carboxylase (ACC) inhibiting the generation of malonyl-CoA. Malonyl-CoA is a substrate for fatty acid synthase so that inhibition of ACC diminishes formation of fatty acids and lipid (14–16). In the hypothalamus, AMPK acts in a seemingly reciprocal fashion to regulate food intake (15, 17–19). Kahn and collaborators (20) showed that AMPK activity in the arcuate and paraventricular hypothalamic nuclei is inhibited by anorexigenic agents such as leptin and augmented by the orexigenic agouti-related protein (AGRP) (20).
We now show that orexigenic AAPDs selectively and potently stimulate hypothalamic AMPK, which has been linked to the regulation of food intake (20), and reverse the actions of the anorexigenic hormone leptin. This action involves the histamine H1 receptor (H1R), because clozapine augmentation of AMPK is abolished in B6.129P-Hrh1tm1Wat (H1RKO) mice, and orexigenic potencies of neuroleptics correlate with their affinities for H1R.
Results
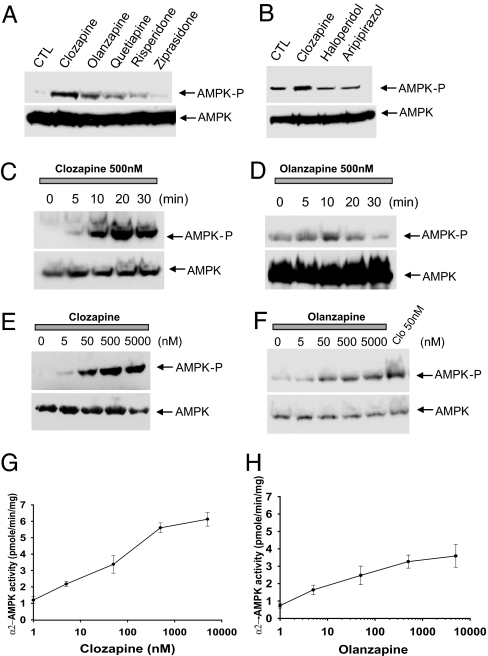
In hypothalamic slices, clozapine and olanzapine markedly enhance levels of phospho-AMPK, and quetiapine, which is also orexigenic, produces similar effects (Fig. 1A). However, risperidone, ziprasidone, haloperidol, and aripiprazole, which are much less orexigenic (Table 1), fail to stimulate AMPK (Fig. 1B). Increased AMPK phosphorylation is observed as early as 5 min after treatment with clozapine or olanzapine (Fig. 1 C and D). The drug actions are potent and substantial with EC50 values for both of ≈10 nM and with 6- and 3.5-fold maximal increases, respectively, with clozapine and olanzapine (Fig. 1 E–H).
Fig. 1.
AAPDs activate AMPK in hypothalamic slices. (A and B) Hypothalamic slices were incubated in oxygenated artificial cerebrospinal fluid buffer with 500 nM drugs for 30 min. Phospho-AMPK (AMPK-P) and total AMPK were detected by Western blotting. (C and D) Hypothalamic slices were incubated with 500 nM clozapine or olanzapine for various times as indicated. Phospho-AMPK and total AMPK were detected by Western blotting. (E and F) Hypothalamic slices were incubated with various concentrations of clozapine or olanzapine for 30 min. (G and H) α2-APMK enzymatic activity was measured with SAMS peptides as a substrate.
Table 1.
Neuroleptic affinities for the H1R correlate with orexigenic actions
| Drugs | IC50, nM | Orexigenic effects |
|---|---|---|
| Clozapine | 9 | ++++ |
| Olanzapine | 13 | +++ |
| Quetiapine | 40 | ++ |
| Risperidone | 80 | +/− |
| Ziprasidone | 150 | − |
| Haloperidol | 2,000+ | − |
| Aripipirazole | 3,000+ | − |
Receptor binding was assayed by using rat brain membranes incubated with [3H]mepyramine and 12 concentrations of drugs ranging from 30 pM to 10 μ M, in triplicate. Data are means of three independent determinations that varied <10%. Orexigenic action of drugs was obtained from published literature, indicating reproducible differences among AAPDs in eliciting weight gain when administered at comparable therapeutic doses (5, 8, 14).
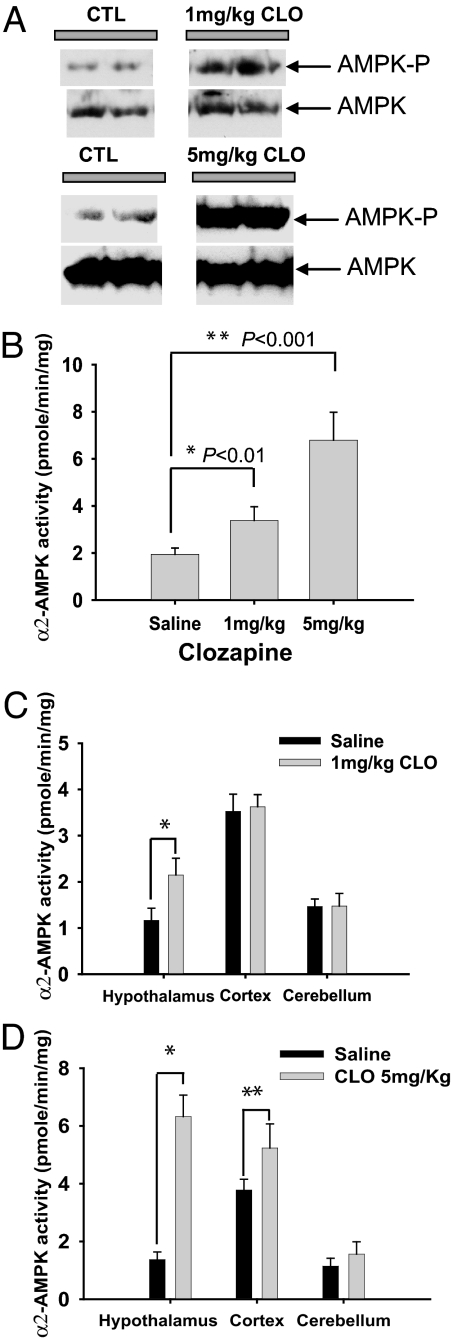
Clozapine also potently and selectively augments hypothalamic AMPK in intact animals. As little as 1 mg/kg of clozapine markedly stimulates levels of phospho-AMPK (Fig. 2A) as well as AMPK catalytic activity, with 5 mg/kg producing a 3.5-fold augmentation of activity (Fig. 2B). The increase of phospho-AMPK and AMPK catalytic activity is relatively selective for the hypothalamus, because clozapine (1 mg/kg) fails to increase phospho-AMPK levels in the cerebellum and liver [see supporting information (SI) Fig. 5 A and B], and AMPK catalytic activity is not affected in the cerebral cortex or cerebellum by clozapine (1 mg/kg) (Fig. 2C). At 5 mg/kg, clozapine elicits a 20% increase in cortical AMPK activity, much less than the quadrupling of hypothalamic AMPK activity, whereas no increase is apparent in the cerebellum (Fig. 2D). The effect of clozapine is maximal 3 h after drug administration and gradually decreases to basal levels in 24 h (SI Fig. 5F).
Fig. 2.
AAPDs activate AMPK in intact animals. (A and B) Mice received clozapine (1 or 5 mg/kg) and were killed at 3 h. Hypothalami were removed and tissue lysates analyzed for phospho-AMPK or α2-AMPK activity. (C and D) Mice received clozapine (1 or 5 mg/kg) and were killed at 3 h. Various parts of brain were isolated, and α2-AMPK activity was assayed. Bars represent the mean ± SE of three independent lysates performed in triplicate. ∗, Student's t test (n = 5).
Kahn and colleagues (20) reported that the anorexigenic peptide leptin reduces hypothalamic AMPK activity, which we confirm. Clozapine reverses reductions in hypothalamic phospho-AMPK elicited by leptin (Fig. 3A) and insulin (20) (SI Fig. 6). In intact mice, leptin (3 mg/kg) reduces hypothalamic phospho-AMPK (Fig. 3B) and catalytic activity (Fig. 3C), and clozapine reverses these actions.
Fig. 3.
Clozapine reverses effects of leptin on phospho-AMPK in hypothalamic slices and in intact animals. (A) Hypothalamic slices were incubated with various concentrations of leptin in the absence or presence of 50 nM clozapine for 30 min. Phospho-AMPK and total AMPK were detected by Western blotting. (B and C) Mice received leptin (3 mg/kg), followed at 1 h by clozapine (5 mg/kg) and were killed at 3 h. Phospho-AMPK and total AMPK were detected by Western blotting, and α2-AMPK activity was assayed. Bars represent the mean ± SE of three independent lysates performed in triplicate. ∗, P < 0.001, Student's t test (n = 5).
The arcuate and paraventricular hypothalamic nuclei display the greatest alterations of AMPK activity in response to feeding stimuli (20). In immunohistochemical experiments phospho-AMPK is selectively augmented in these two nuclei with clozapine (1 and 5 mg/kg), whereas much lesser effects are evident in the cerebral cortex (SI Fig. 7 A and B). By contrast, ziprasidone fails to alter phospho-AMPK in the paraventricular nucleus (SI Fig. 8).
We wondered whether the influence of AAPDs on hypothalamic AMPK is secondary to actions of the drugs on specific neuropeptide receptors that have been implicated in appetite regulation. Clozapine and olanzapine (10 and 100 nM) fail to influence ligand binding to receptors for leptin, α-MSH, and neuropeptide Y (data not shown).
Relative potencies of AAPDs in blocking H1R have been reported to correlate with their orexigenic potencies (21, 22), which we confirm (Table 1). Moreover, in hypothalamic slices, the H1R antagonist triprolidine stimulates phospho-AMPK to the same extent as clozapine both in hypothalamic slices (Fig. 4A) and in intact animals (SI Fig. 9). Conversely, histamine decreases phospho-AMPK with reversal of this effect by clozapine (Fig. 4B). To explore whether augmentation of phospho-AMPK by drugs stems from H1R blockade, we administered clozapine to H1R knockout mice. Whereas the drug elicits a quadrupling of phospho-AMPK in wild-type mice, no effect is evident in H1R knockout animals (Fig. 4 C and D).
Fig. 4.
Clozapine activates AMPK through histamine H1 receptors. (A) Hypothalamic slices were incubated with triprolidine (50 or 500 nM) or clozapine (200 nM) for 30 min. Phospho-AMPK and total AMPK were detected by Western blotting. (B) Hypothalamic slices were incubated with various concentrations of histamine in the absence or presence of 200 nM clozapine for 30 min. Phospho-AMPK and total AMPK were detected by Western blotting. (C) Mice were administered saline or 3 mg/kg clozapine and perfused with 4% paraformaldehyde. Immunohistochemistry was performed with an antibody specific for phospho-AMPK. (D) Quantification of immunohistochemistry. Bars represent the mean ± SE of five independent slides. ∗, P < 0.005; ∗∗, P < 0.001; Student's t test (n = 5).
Discussion
Our findings indicate that the appetite stimulation–weight gain associated with AAPDs is mediated by activation of hypothalamic AMPK linked to blockade of the histamine H1R. AMPK stimulation parallels the orexigenic actions of the drugs, with clozapine and olanzapine producing the most marked effects. The drug actions are very potent, with substantial effects evident at 5 nM concentration. They are selective, with effects restricted largely to the arcuate and paraventricular nuclei of the hypothalamus. Orexigenic potencies of AAPDs parallel their affinities for histamine H1Rs, and stimulation by AAPDs of AMPK is lost in H1R-deleted mice. These findings are in accord with studies implicating central histamine (23, 24) and AMPK (20) in weight control as well as the orexigenic role of the paraventricular and arcuate nuclei (25, 26). Moreover, mice, like humans, manifest weight gain in response to AAPDs, although inhibition of locomotor activity sometimes impairs characterization of orexigenic actions (27).
Numerous mechanisms have been advanced to explain the orexigenic influences of AAPDs. Because therapeutic actions of the drugs have been linked to serotonin receptors, these have also been hypothesized to mediate orexigenic effects. Thus, agonists at 5HT2C receptors, such as fenfluramine and m-chlorophenylpiperazine, are anorexigenic (28), whereas mice with targeted deletion of 5HT2C receptors are obese (29). Orexigenic potencies of neuroleptics correlate significantly with affinity for 5HT2C receptors, although there are notable exceptions, such as ziprasidone, which is not orexigenic yet has high receptor affinity (30). Neuroleptics elicit antipsychotic actions by blocking dopamine D2 receptors. Although blockade in vitro of these receptors does not correlate with orexigenic potencies, positron emission tomographic studies reveal a relationship between obesity and D2 receptor occupancy in humans (31). Moreover, blocking D2 sites in the lateral hypothalamus increases feeding behavior in rodents (32). Some anorectic drugs act by augmenting synaptic levels of norepinephrine in the hypothalamus (33), and affinity of drugs for noradrenergic α-2 receptors correlates with orexigenic potency (34). Although these correlations suggest some role for serotonin, norepinephrine, and dopamine in obesity, the present study establishes definitively that orexigenic AAPDs act via histamine H1 receptors and AMPK. Our findings predict that H1R-deleted mice should be resistant to the orexigenic actions of AAPDs and that H1 antihistamines should be orexigenic. In rats (35) and humans (36), H1 antihistamines have been reported to be orexigenic. Because these drugs typically are used sporadically and in substantially lower doses than AAPDs, effects on weight are less prominent for H1 antihistamines.
Weight gain elicited by AAPDs can be massive and associated with the “metabolic syndrome” leading to diabetes (5, 7, 37, 38). Thus, the orexigenic actions of AAPDs, especially olanzapine and clozapine, have precluded their use in large numbers of patients. Ignorance of the mechanism of these orexigenic actions has hindered efforts to develop alternative therapeutic agents. Evaluation of candidate drugs for influences on H1R and hypothalamic AMPK provides a straightforward approach to developing better drugs and may advance our understanding of the hypothalamic regulation of food intake.
Methods
Drug Preparation.
All drugs were purchased from Toronto Research Chemicals (Toronto, ON, Canada). Clozapine was dissolved in 0.1 M HCl (0.8 ml) and neutralized by 0.1 M NaOH (0.7 ml). The drug was diluted with 8.5 ml of saline solution, and the appropriate doses were administrated to mice. For control mice, the same solution was injected without a drug. For in vitro assays, drugs were dissolved in DMSO.
Hypothalamic Slices.
Hypothalami from 8- to 10-week-old mice were cut at 0.4-mm intervals in sagittal and coronal planes by using a Mcllwain tissue chopper. The slices were dispersed in artificial cerebrospinal fluid buffer.
Immunohistochemistry.
Phospho-AMPK immunohistochemistry was performed as described (39, 40), and all solutions before and including the primary antibody incubation contained 2 mM sodium fluoride. C57BL/6 mice or B6.129P-Hrh1tm1Wat (H1R knockout) mice (8–10 weeks of age) were perfused with 4% paraformaldehyde maintained at 37°C. Organs were postfixed for 2 h at room temperature and cryoprotected overnight at 4°C (30% sucrose in PBS). Free-floating sections (45 μm) were quenched with 3% H2O2 in water for 10 min at room temperature, washed in TBS-T (16 mM Tris, pH 7.4/140 mM sodium chloride/0.1% Tween 20), and antigen retrieved for 30 min in a 70°C water bath (10 mM sodium citrate in TBS-T). Sections were blocked (5% NGS in TBS-T) for 1 h at room temperature and incubated with a mouse anti-phospho-AMPKα antibody (Cell Signaling Technologies, Danvers, MA) diluted 1:200 into the blocking solution overnight at 4°C. Subsequent washes were conducted in TBST, and labeling was visualized with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Images were quantified with the AlphaEaseFC program.
Effect of Neuroleptics on H1R Binding.
The IC50 values of neuroleptics on H1R were determined as described (41). Briefly, rats were killed by decapitation and the forebrains removed. Brains were homogenized in 30 vol of Na-K phosphate buffer, pH 7.5, and centrifuged at 48,000 × g for 10 min. The tissue was resuspended in buffer and recentrifuged an additional three times. Tissue was resuspended in buffer at 15 mg/ml.
Tissue (0.2 ml) was added to tubes containing 25 μl of drug and 25 μl of [3H]mepyramine (30 nM). Nonspecific binding was determined in the presence of 1 μM triprolidine. Tubes were incubated for 1 h at 25°C, and the samples were filtered over 0.5% poly(ethyleneimine)-coated filters washed with 2 × 5 ml of cold 50 mM NaCl.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service Grants DA000266 and MH18501 and Research Scientist Award DA00074 (to S.H.S.); Canadian Institute of Health Research Fellowship (to S.F.K.); and National Institutes of Health Grants NS36526, AI4515, AI41747, and AI45666 and National Multiple Sclerosis Society Grant RG-3129 (to C.T.).
Abbreviations
- AAPD
atypical antipsychotic drug
- H1R
H1 receptor.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 3019.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611417104/DC1.
References
- 1.Kane J, Honigfeld G, Singer J, Meltzer H. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer HY. Hosp Community Psychiatry. 1990;41:1356–1357. doi: 10.1176/ps.41.12.1356. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT., Jr Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- 4.Tuunainen A, Wahlbeck K, Gilbody S. Schizophr Res. 2002;56:1–10. doi: 10.1016/s0920-9964(01)00212-2. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, et al. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 6.Gothelf D, Falk B, Singer P, Kairi M, Phillip M, Zigel L, Poraz I, Frishman S, Constantini N, Zalsman G, et al. Am J Psychiatry. 2002;159:1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- 7.Newcomer JW. CNS Drugs. 2005;19(Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 8.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 9.Blin O, Micallef J. J Clin Psychiatry. 2001;62(Suppl 7):11–21. [PubMed] [Google Scholar]
- 10.Isaac MB, Isaac MT. Am J Psychiatry. 2005;162:1764–1765. doi: 10.1176/appi.ajp.162.9.1764. [DOI] [PubMed] [Google Scholar]
- 11.Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Am J Psychiatry. 2003;160:290–296. doi: 10.1176/appi.ajp.160.2.290. [DOI] [PubMed] [Google Scholar]
- 12.Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, Lynch CJ. Obesity (Silver Spring) 2006;14:36–51. doi: 10.1038/oby.2006.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haupt DW. Eur Neuropsychopharmacol. 2006;16(Suppl 3):S149–S155. doi: 10.1016/j.euroneuro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Hardie DG. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 15.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG, Pan DA. Biochem Soc Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- 17.Hardie DG, Hawley SA, Scott JW. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramamurthy S, Ronnett GV. J Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfgang MJ, Lane MD. Annu Rev Nutr. 2006;26:23–44. doi: 10.1146/annurev.nutr.25.050304.092532. [DOI] [PubMed] [Google Scholar]
- 20.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 21.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 22.Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR. J Clin Psychiatry. 1999;60:358–363. [PubMed] [Google Scholar]
- 23.Sakata T, Fukagawa K, Ookuma K, Fujimoto K, Yoshimatsu H, Yamatodani A, Wada H. Physiol Behav. 1988;44:539–543. doi: 10.1016/0031-9384(88)90316-2. [DOI] [PubMed] [Google Scholar]
- 24.Fukagawa K, Sakata T, Shiraishi T, Yoshimatsu H, Fujimoto K, Ookuma K, Wada H. Am J Physiol. 1989;256:R605–R611. doi: 10.1152/ajpregu.1989.256.3.R605. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 26.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 27.Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Int J Obes (London) 2005;29:607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- 28.Goodall E, Oxtoby C, Richards R, Watkinson G, Brown D, Silverstone T. Br J Psychiatry. 1988;153:208–213. doi: 10.1192/bjp.153.2.208. [DOI] [PubMed] [Google Scholar]
- 29.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 30.Stahl SM, Shayegan DK. J Clin Psychiatry. 2003;64(Suppl 19):6–12. [PubMed] [Google Scholar]
- 31.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 32.Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 33.Jackson HC, Needham AM, Hutchins LJ, Mazurkiewicz SE, Heal DJ. Br J Pharmacol. 1997;121:1758–1762. doi: 10.1038/sj.bjp.0701312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 35.Orthen-Gambill N. Pharmacol Biochem Behav. 1988;31:81–86. doi: 10.1016/0091-3057(88)90315-2. [DOI] [PubMed] [Google Scholar]
- 36.Navarro-Badenes J, Martinez-Mir I, Palop V, Rubio E, Morales-Olivas FJ. Ann Pharmacother. 1992;26:928–930. doi: 10.1177/106002809202600715. [DOI] [PubMed] [Google Scholar]
- 37.Bergman RN, Ader M. J Clin Psychiatry. 2005;66:504–514. doi: 10.4088/jcp.v66n0414. [DOI] [PubMed] [Google Scholar]
- 38.Casey DE. J Clin Psychiatry. 2004;65(Suppl 18):27–35. [PubMed] [Google Scholar]
- 39.Huang AS, Beigneux A, Weil ZM, Kim PM, Molliver ME, Blackshaw S, Nelson RJ, Young SG, Snyder SH. J Neurosci. 2006;26:2814–2819. doi: 10.1523/JNEUROSCI.5060-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- 41.Chang RS, Tran VT, Snyder SH. Eur J Pharmacol. 1978;48:463–464. doi: 10.1016/0014-2999(78)90177-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.