Abstract
Frugivores are highly variable in their contribution to fruit removal in plant populations. However, data are lacking on species-specific variation in two central aspects of seed dispersal, distance of dispersal and probability of dispersal among populations through long-distance transport. We used DNA-based genotyping techniques on Prunus mahaleb seeds dispersed by birds (small- and medium-sized passerines) and carnivorous mammals to infer each seed's source tree, dispersal distance, and the probability of having originated from outside the study population. Small passerines dispersed most seeds short distances (50% dispersed <51 m from source trees) and into covered microhabitats. Mammals and medium-sized birds dispersed seeds long distances (50% of mammals dispersed seeds >495 m, and 50% of medium-sized birds dispersed seeds to >110 m) and mostly into open microhabitats. Thus, dispersal distance and microhabitat of seed deposition were linked through the contrasting behaviors of different frugivores. When the quantitative contribution to fruit removal was accounted for, mammals were responsible for introducing two-thirds of the immigrant seeds into the population, whereas birds accounted for one-third. Our results demonstrate that frugivores differ widely in their effects on seed-mediated gene flow. Despite highly diverse coteries of mutualistic frugivores dispersing seeds, critical long-distance dispersal events might rely on a small subset of large species. Population declines of these key frugivore species may seriously impair seed-mediated gene flow in fragmented landscapes by truncating the long-distance events and collapsing seed arrival to a restricted subset of available microsites.
Keywords: dispersal vectors, fragmented landscapes, frugivorous vertebrates, long-distance dispersal, seed dispersal kernel
Seed dispersal establishes the initial template for regeneration in natural plant populations, influencing demography, genetic structure, and spatial distribution of future generations (1, 2). Successful seed dispersal consists of removal from a source tree and deposition into sites (the seed shadow) where seeds can germinate and seedlings can establish themselves (3). A wide diversity of dispersal agents (both biotic and abiotic) can contribute to successful dispersal. Because these agents are differentially effective, dispersal of a given species is poorly described if only one or a few dispersal vectors are considered (4–8). For instance, seeds already dispersed by animal frugivores can be significantly reshuffled by water (9).
Functional roles of different types of frugivores are well documented for the fruit removal stage of dispersal but not for the seed deposition stage. Frugivores may differ not only quantitatively in terms of how many fruits they consume and seeds they disperse but also qualitatively in terms of where and how far they deposit seeds. Previous analyses of frugivore assemblages have focused on species-specific differences in quantitative aspects of visitation, fruit removal, postfeeding behavior, and effects on germination (see refs. 10 and 11). These studies often show that patterns of seed rain reflect the distinct spatial signatures of different dispersal agents (8, 12, 13).
For example, large-sized mammals and birds can transport large quantities of seeds over long distances, thereby connecting distant populations (6, 14–18). In contrast, small- to medium-sized birds tend to deposit seeds near the source tree, although they are capable of dispersing seeds much further (19–21). The combined seed dispersal curve (i.e., the dispersal kernel) thus results from the interaction between the feeding behavior of a diverse suite of small and large frugivores and the landscape structure, mediated by habitat preferences and the dynamics of digestion processes. The likely result is that a few key frugivore species may contribute disproportionately to seed-mediated gene flow, population connectivity, and genetic structure. Despite the importance of these processes to plant populations and communities, they are still underinvestigated and remain poorly understood (22).
How can one determine which frugivore species dispersed a given seed and which tree was the source for that seed? This is a central question in seed dispersal studies that only recently has been successfully tackled. Analytical models used to estimate dispersal kernels (both inverse modeling and mechanistic/phenomenological models) cannot fully reflect the complexities of dispersal, especially long-distance dispersal (2). Direct tracking of individual seeds, using molecular markers, remains the most accurate method to obtain reliable estimates of seed dispersal distances reached by different frugivores (22–25), yet determining which frugivore species was responsible for dispersing a given seed is highly complex.
Here, we dissect the relative contribution of medium-sized birds (Corvus corone and Turdus viscivorus), small-sized birds (e.g., warblers), and large carnivorous mammals to the seed rain of a common tree, Prunus mahaleb (Rosaceae). Specifically, we (i) assessed the contribution of each frugivore type to the seed dispersal kernel and quantified their contribution to seed dispersal immigration into the study population; (ii) disentangled the relationship between dispersal of seeds by particular types of frugivores and the microhabitats into which those seeds were placed; and (iii) determined the contribution of such frugivores to long-distance dispersal events.
Results
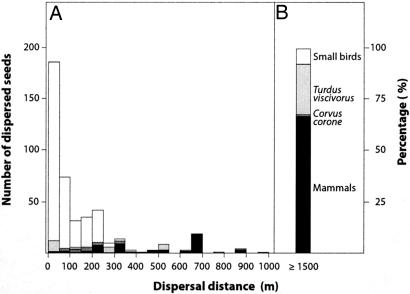
By combining direct observations of fruit consumption, sampling of seed rain by setting seed traps and collecting feces, and linking dispersed seeds to source trees by genotyping seed endocarps and fruiting trees, we were able to assess how different types of frugivores move seeds different distances to different microhabitats and how they contribute to seed immigration into the study population. Our data demonstrate that different types of frugivores indeed accounted for different proportions of seeds dispersed to each distance class (Fig. 1A). Although small-sized birds (<110 g) were by far the major seed dispersers of seeds that were moved <250 m, larger frugivores (110–500 g) were the major dispersers of seeds that were moved 250–990 m. Medium-sized birds (T. viscivorus and C. corone) contributed to short-distance dispersal (<100 m), but they dispersed most seeds beyond 100 m. In contrast, small birds rarely dispersed seeds >100 m. Seed dispersal distances by carnivorous mammals ranged from 0 m (i.e., under the canopy of the source tree) to 990 m, with a peak at 650–700 m. These distance intervals correspond to within-population dispersal events (seeds from trees growing in the study population). However, seeds were clearly moved longer distances, as evidenced by immigration of seeds into our study population from outside our 26-ha study site (Fig. 1B).
Fig. 1.
Frequency distribution of seeds dispersed over given distance classes (seed dispersal kernel). (A) Shown are the relative contributions of major frugivore groups to different distance classes within the study population. Open bars, small- to medium-size frugivorous birds, including, e.g., E. rubecula, P. ochruros, T. merula, and Sylvia spp.; light grey, T. viscivorus; dark grey, C. corone; black, carnivorous mammals, including V. vulpes, M. foina, and M. meles. (B) Shown is the weighted contribution of each dispersal vector to seed immigration to the study population (dispersal distances ≥ 1,500 m); i.e., fruits consumed in fruiting trees growing in other populations with the seeds being regurgitated or defecated in the study population. For each disperser group, the proportion of immigrant seeds in the genotyped sample was weighted by the overall contribution to fruit removal.
Most immigrant seeds were dispersed by mammals (Fig. 1B); their weighted contribution to the immigrant seed pool (considering both the proportion of immigrant seeds in the total sample and the total number of seeds removed) was 66.9%, whereas frugivorous birds accounted for the remaining 33.1%. Among these, 0.07% was contributed by C. corone, 21.5% was contributed by T. viscivorus, and 7.8% was contributed by the small bird species (Fig. 1). Considering each dispersal vector separately, 74.2% of the seeds dispersed by mammals came from outside the population, whereas 21.9% of the seeds dispersed by birds came from other populations (Table 1). Among birds, 20.6% of seeds dispersed by T. viscivorus and 56.5% of the seeds dispersed by C. corone were inferred to be immigrants.
Table 1.
Description of the Prunus mahaleb seed rain generated by four different frugivore types in the study population
| Vector | Sampling |
Source tree location |
Deposition per microhabitat, % (Piout, %) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Sampling points, no. | Niin | Niout | P. mahaleb | High shrub | Low shrub | Pinus | Acer-Quercus | Open | |
| Small-birds | 292 | 143 | 234 | 58 | 23 (17.0) | 42 (20.0) | 5 (7.2) | 14 (18.6) | 8 (10) | 8 (7.2) |
| T. viscivorus | 173 | 38 | 137 | 36 | 18 (19.4) | 2 (33.0) | — | 66 (21.5) | 7 (16.7) | 7 (16.7) |
| C. corone | 23 | 4 | 10 | 13 | — | — | — | — | — | 100 (56.5) |
| Mammals | 167 | 20 | 43 | 124 | 24 (79.4) | — | — | — | — | 76 (67.2) |
The frugivore types studied are as follows: small- to medium-sized birds, including warblers (Sylvia spp.), redstarts (Phoenicurus spp.), and robin Erithacus rubecula, mistle thrushes (T. viscivorus), carrion crows (C. corone), and mammals. For each frugivore type, we report the total number of genotyped endocarps (Ni), the number of sampling points, the number of seeds coming from source trees growing within (Niin) and outside (Niout) the population, and the percentage of seeds deposited per microhabitat type, with the percentage of them coming from outside the population (Piout) in parentheses. Dashes indicate areas wherein no seeds were dispersed.
The frequency of seed deposition in different microhabitats differed significantly among dispersal vectors (χ2 = 596.93, df = 15, P < 0.001). Whereas small-sized birds dispersed seeds mainly beneath the canopies of P. mahaleb and other fleshy-fruited trees or shrubs, mammals deposited seeds preferentially in open sites (rocky soils and open ground with little woody vegetation or grass cover) (Table 1). Medium-sized birds dispersed seeds mainly to open areas (C. corone) and beneath pine trees (T. viscivorus). The differential use of the microhabitats by different frugivores translates into a variable percentage of immigrant seeds received by each microhabitat (Table 1). Thus, almost 80% of the seeds dispersed by mammals under P. mahaleb canopies were immigrant seeds, whereas this proportion dropped to 17% when small- to medium-sized birds were the dispersal vectors. Similarly, >50% of the seeds deposited in open sites by C. corone (56.5%) and mammals (67.2%) came from other populations, whereas immigrant seeds only represented 7.2% and 16.7% of the seeds dispersed by small-birds and T. viscivorus, respectively.
Discussion
By combining DNA-based genotyping methods and field observations, we found that seed dispersal by different types of frugivores resulted in distinct contributions to different distance classes and microhabitats, with only a few species responsible for long-distance dispersal events. Small-sized birds accounted for most short-distance dispersal, and larger frugivores (both birds and mammals) accounted for most long-distance dispersal. Our data thus allow direct estimates of two relevant components of dispersal, namely dispersal distances and the frequency of seed immigration into the population (seeds dispersed from other populations).
Our results are largely congruent with field observations of postfeeding behavior of frugivores in our study system (19) but add evidence for nonrandom dispersal of immigrant seeds to specific microhabitats. Variable shapes of seed dispersal kernels have been reported by a series of field studies based on direct field observation and/or tracking of frugivores, often in heterogeneous landscapes (14, 16, 20, 21, 25–28). None of these previous studies, however, documents the frequency of long-distance dispersal, especially for immigrant seeds. Mechanistic models involving direct tracking of frugivores and modeling gut-passage dynamics have been used to infer dispersal distances. Although these techniques are quite precise in identifying the dispersal agent, they are imprecise with respect to identifying the seed source and hence the dispersal distance (25). Molecular-based techniques are powerful for source tree and distance estimation but rely on indirect methods (e.g., identification of the feces or regurgitations) for determining which dispersal agent deposited the seed(s). Ideally, a combination of techniques (essentially, observational and genetic identification, as used in this study) will help to address the three basic questions for a sampled dispersed seed: which tree was the source for it, what the dispersal distance is, and which frugivore species contributed the dispersal event.
Our data show that the P. mahaleb dispersal kernel is comprised of two distinct components: short-distance seed-dispersal events primarily due to a diverse array of small-sized frugivores and long-distance dispersal events primarily due to medium-sized birds and carnivorous mammals. It follows that larger birds and carnivorous mammals are mostly responsible for the observed seed exchange among populations, which in our study system involves dispersal events between 1.5 and 17 km (P.J., personal observation). Even though large-bodied dispersers are uncommon (19), neglecting their role would lead one to underestimate seriously the frequency of long-distance dispersal events. On the other hand, ignoring small-sized vectors would seriously underestimate the frequency of short-distance dispersal events and overestimate dispersal distance. Thus, analysis of the seed dispersal kernel should be approached as a composite function that results from the differential contribution of different frugivore types (the total dispersal kernel, in the sense of ref. 5).
Interestingly, although small-sized birds dominated the left section of the dispersal kernel (short-distance dispersal events), almost 20% of the seeds they dispersed originated from another population. This type of frugivore likely accounts for a nonnegligible number of immigrant seeds, because they are responsible for most fruit removal (19), with consumption rates well above those by mammals. Our data indicate that they dispersed seeds to all microhabitats, whereas larger birds and mammals selectively dispersed seeds to open microhabitats (C. corone and carnivorous mammals) and pine forests (T. viscivorus). More importantly, small birds are the main contributors to the seed rain beneath covered microhabitats (under P. mahaleb and high and low shrubs), where seedlings have higher chances of establishing (ref. 30; E. W. Schupp and P.J., unpublished data). This finding demonstrates how dispersal kernels can be combined with ecological information on microhabitat type to assess dispersal effectiveness, i.e., the contribution each disperser makes to the future reproduction of a plant (in the sense of ref. 30). Quantifying disperser effectiveness remains an elusive but important goal. Once it is known, one can predict contributions of seed dispersal to gene flow and better forecast the consequences of local extinctions of key frugivores with respect to gene flow patterns via seed and loss-of-connectivity among fragments. For instance, estimates of gene flow mediated by pollen (31), effective dispersal distances obtained for seedlings (32), and thorough demographic data can be combined to better understand the genetic consequences of population fragmentation.
Finally, our data highlight that seed dispersal kernels emerge from the interaction of three main components (8): frugivore abundance (determining which species remove most fruits and disperse most seeds), frugivore feeding and postfeeding behavior (determining feeding preferences and dispersal movement), and the structure of the landscape (describing the relative position of fruiting trees and the deposition sites). As the frugivore community or the landscape changes, the dispersal kernel and the two-dimensional pattern of the seed shadows will also be modified. Thus, if carnivorous mammals go extinct in our study population, the movement of seeds among populations would strongly decrease, resulting in a significant truncation of dispersal distances and an increase in population isolation (33). In addition, the reduction of some habitats (e.g., the pine forest in our study) might shift the dispersal kernel to the left, resulting in a strong restriction of the seed dispersal distance. We may also envision sizeable changes in seed shadows and the dispersal kernel accompanying year-to-year variation in the frugivore assemblage (34). These types of effects have broad implications for the conservation of frugivore species, especially when some dispersers disproportionately deposit seeds in microhabitats where the probability of successful recruitment is high (e.g., refs. 28 and 35). For instance, the fast-paced extinction of large tropical frugivores will not only reduce removal rates, a quantitative effect repeatedly documented (36, 37), but will also truncate dispersal kernels and severely limit seed-mediated gene flow. Overall, a comprehensive approach to seed dispersal in plant populations requires the incorporation of a new paradigm in seed dispersal studies, moving from simple to complex seed dispersal systems (5, 22) and envisioning seed dispersal kernels as an emergent property of plant–animal interactions with context-dependent outcomes.
Materials and Methods
Species and Study Site Characteristics.
The study species, P. mahaleb (L.) (Rosaceae), is a shrub or small tree that produces fleshy fruits that are ingested by frugivores, who disperse seeds after regurgitating or defecating them. This species is frequently visited during July to mid-August by small- and medium-sized birds (19) and carnivorous mammals that include fruits in their diets during late summer to winter (38). P. mahaleb occurs in a patchy distribution at the regional scale, with isolated populations consisting of dozens to hundreds of trees. Our study population consisted of 196 adult trees distributed over an area of ≈26 ha in patches of variable density. Other populations within 20 km exist as scattered patches of 10–150 trees, with some containing >1,000 trees. The nearest population is 1.5 km away. Additional information on the study population is reported in refs. 19 and 38.
Relative Contribution of Each Dispersal Vector to the Seed Rain.
Here, we differentiate three major frugivore types: large carnivorous mammals (such as foxes, badgers, and stone martens); two species of medium-sized frugivorous birds, mistle thrushes (T. viscivorus), and carrion crows (C. corone); and a pool of small-sized frugivorous birds, including warblers, redstarts, and robins (19). These frugivores vary widely in their relative contributions to seed removal in the study population. Although small birds and T. viscivorus account for a large fraction of the seed rain (up to 71.0% and 13.8%, respectively) (refs. 19, 34, and 38; P.J., personal observation), removal by C. corone is marginal (0.2%, estimated from visit frequencies and consumption rates) (ref. 19; P.J. personal observation). The overall contribution of mammals, estimated by seed trap data and direct sampling of scats in the area, is ≈15% (refs. 19, 34, 38, and 39 report data supporting this estimate). We combined these estimates with the direct assignment of dispersed seeds to their source tree based on molecular markers (see Seed Dispersal Kernel) to dissect the relative contribution of each frugivore group in terms of distance, frequency of seed immigration from other populations, and distribution of immigrant seeds among microhabitat types. For each distance class, the contribution of each disperser group was the sum of seeds originating from its sample and identified having reached that distance. The contribution of a disperser group to seed immigration is the percentage of immigrant seeds in the genotyped endocarp sample weighted by the overall quantitative contribution to fruit removal, as described in detail in refs. 19 and 34. Briefly, during direct watches at the trees, for each species, we quantified visitation frequency and multiplied it by their feeding rates (number fruits taken per visit) and by the fraction of seeds estimated to leave the tree (fruits ingested, not dropped beneath the tree). We assumed that the contribution of each frugivore group to the sample of immigrant seeds would be proportional to their overall contribution to fruit removal.
Sampling of Dispersed Seeds.
To estimate the relative contribution of each dispersal vector to the seed dispersal kernel and the seed deposition in different microhabitat types, we first collected dispersed seeds, following different sampling schemes according to the dispersal vector. Seeds were collected in 1997–1999 and 2003–2005. These sampling schemes were as follows.
Seeds dispersed by mammals.
We haphazardly collected 130 samples of feces during the dispersal period and recorded their microhabitat and location relative to potential source trees. Feces contained 106.2 ± 66.2 seeds (mean ± SD). From the frequency-distribution curve of number of seeds per fecal sample, we randomly chose five samples from each quartile and genotyped 10% of the seeds in each of these samples. Overall, we genotyped 167 seeds from 20 fecal samples. Most samples were from red fox (Vulpes vulpes) and stone marten (Martes foina); some (<10 samples) were from badger (Meles meles) (ref. 38; C.G. and J.L.G.-C., personal observation).
Seeds dispersed by C. corone.
Regurgitation pellets of this species can be visually identified. The species is a scarce visitor of fruiting P. mahaleb trees, but it is one of the few large-bodied frugivorous birds in the disperser assemblage. We used all of the samples we had available for it. We collected a total of four pellets, which contained 54.5 ± 16.9 seeds per pellet, and randomly chose 10% of the seeds for genetic analysis. A total of 23 seeds were genotyped. The spatial location and the microhabitat type for each pellet were recorded.
Seeds dispersed by small birds.
We collected seeds deposited in seed traps, using a random sampling protocol that was stratified by microhabitats (see refs. 38 and 40). We characterized six different microhabitats (MH) according to the type of plant cover at the sampling point. The three microhabitats that contained fleshy-fruited species are as follows: (i) MH-Prunus, dominated by adult P. mahaleb trees; (ii) MH-high shrub, dominated by high-shrub, fleshy-fruited, woody cover with a height of >1.0 m (Crataegus monogyna, Juniperus phoenicea, Lonicera arborea, Rosa spp., Taxus baccata); and (iii) MH-low shrub, dominated by low-shrub species with a height of <1.5 m (e.g., Berberis vulgaris, Juniperus communis, and Rhamnus saxatilis).
The three microhabitats that were dominated by non-fleshy-fruited species are as follows: (iv) MH-Pinus, dominated by pine trees (Pinus nigra, ssp. salzmannii); (v) MH-Acer-Quercus dominated by Acer granatense and Quercus faginea or Quercus ilex, which presented a scattered distribution; and (vi) MH-open, including rocky soil, very low (height of <0.20 m) woody vegetation, and/or grassland. We genotyped the endocarp of all dispersed seeds collected in each microhabitat except for MH-Prunus and MH-high shrub (40), where seed density was frequently too high. For these microhabitats, we genotyped a subsample. This seed sample included seeds dispersed by small and medium-sized passerine species, such as Phoenicurus ochruros, Turdus merula, Erithacus rubecula, Sylvia communis, Sylvia atricapilla, etc. (19). Seeds in these samples also include a fraction of those dispersed by T. viscivorus (see below).
Seeds dispersed by T. viscivorus.
Seeds collected in the Pinus microhabitat were all assigned to T. viscivorus, based on our previous and extensive feeding observations and foraging data (19, 38). In addition, most seeds in the seed traps in this microhabitat appeared in scats with 5–10 seeds, which matched the number expected for a single scat of T. viscivorus. This species also contributed small numbers of seeds to the samples from seed traps in other microhabitats described in Seeds dispersed by small birds, but visual identification was not possible. Therefore, from the seed sample genotyped for each microhabitat type, we estimated the percentage dispersed by T. viscivorus by extrapolation from the relative contribution of this species to the seed rain in each specific microhabitat, as described in ref. 19. Given N seeds in microhabitat i and a relative contribution p by T. viscivorus, we estimated Ni × p seeds to be contributed by this species. The contribution of T. viscivorus to immigrant seeds in microhabitat i would be Ni × p × (NoutTvis/NTvis), where NoutTvis/NTvis is the proportion of immigrant seeds (NoutTvis) in the seed sample of T. viscivorus (NTvis).
Seed Dispersal Kernel.
The seed dispersal distance kernel was obtained directly by measuring the distance between the seed deposition site (scats, pellet, or seed trap) and the location of the source tree, as determined by maternal assignment based on molecular markers (41). We genotyped all adult trees in the population (n = 196) along with the endocarp of the seeds collected from the seed traps (n = 465), mammal scats (n = 167), and C. corone pellets (n = 23). Because the endocarp is a tissue of maternal origin, the multilocus genotype of a given endocarp and the genotype of its source tree are identical (41). We considered each dispersed seed as an independent replicate, because each represented a dispersal event from the perspective of plant population genetics, i.e., an independent “arrival” event resulting from the dispersal process mediated by the frugivore (24, 25).
We used a set of 10 polymorphic microsatellite markers (simple DNA sequence repeats) to obtain the multilocus genotypes of both of the adult trees (candidate source trees) and the sample of seed endocarps (for details, see ref. 41). All adult trees in the population had a distinct multilocus genotype. Thus, an unambiguous assignment of each seed to its source tree could be made. When a full match between the endocarp genotype and any of the adult-tree genotypes in the population was not possible, we assumed that the seed came from another population. To assess the effect of genotyping errors, we reexamined the exclusion of genotypes due to a single locus mismatch, two loci mismatches, etc. At the analysis level, any exclusion of identity between a seed and a potential mother tree based on mismatches of only one or two loci was rechecked. We used GIMLET software (42) to find the matching adult multilocus genotype for each endocarp with eight or more loci successfully typed. Because each seed belonged to one of the four groups of dispersers, we could thus derive the relative contribution of each frugivore group to different distance classes and microhabitats and to seed immigration.
Contribution of Each Dispersal Vector to Seed Immigration.
Based on the total number of genotyped endocarps, we estimated for each frugivore type i the number of seeds coming from source trees growing within (Nini) and outside (Nouti) the population. In addition, based on the percentage of seeds deposited per microhabitat type by each frugivore group, we estimated the fraction of seeds coming from outside the population (Pouti) for each microhabitat. The weighted contribution to overall seed immigration for each frugivore type (seeds coming from outside the population) was obtained as the product of the fraction of immigrant seeds in each frugivore type sample (Nouti/Ni) and their proportional contribution to overall fruit removal. All these values are provided in Table 1.
Acknowledgments
We thank J. M. Arroyo for his invaluable help in the laboratory; J. G. P. Rodríguez Sánchez and M. Carrión for their extended help with field work; and Jordi Bascompte, Arndt Hampe, Enrico Rezende, Alfredo Valido, Abhay Krishna, Jofre Carnicer, Pete Buston, Eugene W. Schupp, and Eva Albert for their constructive comments and discussions of the manuscript. Douglas Levey and an anonymous reviewer helped to improve the manuscript with insightful, detailed comments. The Consejería de Medio Ambiente (Junta de Andalucía) greatly facilitated our work in Parque Natural de las Sierras de Cazorla, Segura y Las Villas. This work was supported by Spanish Ministerio de Ciencia y Tecnología Grants FP2000-5627 (to C.G.), AP96-27318040 (to J.L.G.-C.) PB96-0857, BOS2000-1366-C02-01, REN2003-00273, CGL2006-00373, and Junta de Andalucía Grant RNM-305.
Abbreviation
- MH
microhabitats.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Levey DJ, Silva WR, Galetti M. Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. Wallingford, UK: CAB International; 2002. [Google Scholar]
- 2.Nathan R, Muller-Landau HC. Trends Ecol Evol. 2000;15:278–285. doi: 10.1016/s0169-5347(00)01874-7. [DOI] [PubMed] [Google Scholar]
- 3.Janzen DH. Amer Nat. 1970;104:501–528. [Google Scholar]
- 4.Chambers JC, MacMahon JA. Ann Rev Ecol Syst. 1994;25:263–292. [Google Scholar]
- 5.Nathan R. In: Frugivory and Seed Dispersal: Theory and Applications in a Changing World. Dennis AJ, Schupp EW, Green R, Westcott D, editors. Wallingford, UK: CAB International; 2007. in press. [Google Scholar]
- 6.Poulsen JR, Clark CJ, Connor EF, Smith TB. Ecology. 2002;83:228–240. [Google Scholar]
- 7.Vander Wall SB, Longland WS. Trends Ecol Evol. 2004;19:155–161. doi: 10.1016/j.tree.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Westcott DA, Bentrupperbaumer J, Bradford MG, McKeown A. Oecologia. 2005;146:57–67. doi: 10.1007/s00442-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 9.Hampe A. J Ecol. 2004;92:797–807. [Google Scholar]
- 10.Fleming T, Estrada A. Frugivory and Seed Dispersal: Ecological and Evolutionary Aspects. Dordrecht, The Netherlands: Kluwer Academic; 1993. [Google Scholar]
- 11.Jordano P. In: Seeds: the Ecology of Regeneration in Plant Communities. Fenner M, editor. Wallingford, UK: CAB International; 2000. pp. 125–166. [Google Scholar]
- 12.Howe HF, Smallwood J. Ann Rev Ecol Syst. 1982;13:201–228. [Google Scholar]
- 13.Schupp EW, Milleron T, Russo S. In: Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. Levey DJ, Silva WR, Galetti M, editors. Wallingford, UK: CAB International; 2002. pp. 19–33. [Google Scholar]
- 14.Fragoso JMV. J Ecol. 1997;85:519–529. [Google Scholar]
- 15.Willson MF. Oikos. 1993;67:159–176. [Google Scholar]
- 16.Holbrook KM, Smith TB, Hardesty BD. Ecography. 2002;25:745–749. [Google Scholar]
- 17.Balcomb SR, Chapman CA. Ecol Monogr. 2003;73:625–642. [Google Scholar]
- 18.Clark CJ, Poulsen JR, Bolker BM, Connor EF, Parker VT. Ecology. 2005;86:2684–2694. [Google Scholar]
- 19.Jordano P, Schupp EW. Ecol Monogr. 2000;70:591–615. [Google Scholar]
- 20.Westcott DA, Graham DL. Oecologia. 2000;122:249–257. doi: 10.1007/PL00008853. [DOI] [PubMed] [Google Scholar]
- 21.Levey DJ, Bolker BM, Tewksbury JJ, Sargent S, Haddad NM. Science. 2005;309:146–148. doi: 10.1126/science.1111479. [DOI] [PubMed] [Google Scholar]
- 22.Nathan R. Science. 2006;313:786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- 23.Jordano P, Godoy JA. In: Frugivores and Seed Dispersal: Ecological, Evolutionary, and Conservation Issues. Levey DJ, Silva W, Galetti M, editors. Wallingford, UK: CAB International; 2002. pp. 305–321. [Google Scholar]
- 24.Ouborg NJ, Piquot Y, vanGroenendael JM. J Ecol. 1999;87:551–568. [Google Scholar]
- 25.Jordano P. In: Frugivory and Seed Dispersal: Theory and Applications in a Changing World. Dennis AJ, Schupp EW, Green R, Westcott D, editors. Wallingford, UK: CAB International; 2007. in press. [Google Scholar]
- 26.Mack AL. Ecography. 1995;18:286–295. [Google Scholar]
- 27.Gómez JM. Ecography. 2003;26:573–584. [Google Scholar]
- 28.Wenny DG, Levey DJ. Proc Natl Acad Sci USA. 1998;95:6204–6207. doi: 10.1073/pnas.95.11.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holbrook KM, Loiselle BA. In: Seed Dispersal: Theory and Its Application in a Changing World. Dennis AJ, Schupp EW, Green R, Westcott D, editors. Wallingford, UK: CAB International Publishing; 2007. in press. [Google Scholar]
- 30.Schupp EW. In: Frugivory and Seed Dispersal: Ecological and Evolutionary Aspects. Fleming TH, Estrada A, editors. Dordrecht, The Netherlands: Kluwer Academic; 1993. pp. 15–29. [Google Scholar]
- 31.García C, Arroyo JM, Godoy JA, Jordano P. Mol Ecol. 2005;14:1821–1830. doi: 10.1111/j.1365-294X.2005.02542.x. [DOI] [PubMed] [Google Scholar]
- 32.Hardesty BD, Hubbell SP, Bermingham E. Ecol Lett. 2006;9:516–525. doi: 10.1111/j.1461-0248.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 33.Loiselle BA, Blake JG. In: Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. Levey DJ, Silva WR, Galetti M, editors. Wallingford, UK: CAB International; 2002. pp. 397–406. [Google Scholar]
- 34.Jordano P. Ecology. 1995;76:2627–2639. [Google Scholar]
- 35.Schupp EW. In: Frugivory and Seed Dispersal: Theory and Applications in a Changing World. Dennis AJ, Schupp EW, Green R, Westcott D, editors. Wallingford, UK: CAB International; 2007. in press. [Google Scholar]
- 36.Wright SJ, Zeballos H, Domínguez I, Gallardo MM, Moreno MC, Ibáñez R. Cons Biol. 2000;14:227–239. [Google Scholar]
- 37.Cordeiro NJ, Howe HF. Proc Natl Acad Sci USA. 2003;100:14052–14056. doi: 10.1073/pnas.2331023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Castaño JL. Spain: Universidad de Sevilla; 2001. PhD thesis. [Google Scholar]
- 39.Herrera CM, Jordano P. Ecol Monogr. 1981;51:203–221. [Google Scholar]
- 40.García C, Jordano P, Godoy JA. Mol Ecol. 2007;62 doi: 10.1111/j.1365-294X.2006.03126.x. in press. [DOI] [PubMed] [Google Scholar]
- 41.Godoy JA, Jordano P. Mol Ecol. 2001;10:2275–2283. doi: 10.1046/j.0962-1083.2001.01342.x. [DOI] [PubMed] [Google Scholar]
- 42.Valière N. Mol Ecol Notes. 2002;2:377–379. [Google Scholar]