Abstract
Severe hearing loss during early development is associated with deficits in speech and language acquisition. Although functional studies have shown a deafness-induced alteration of synaptic strength, it is not known whether long-term synaptic plasticity depends on auditory experience. In this study, sensorineural hearing loss (SNHL) was induced surgically in developing gerbils at postnatal day 10, and excitatory synaptic plasticity was examined subsequently in a brain slice preparation that preserves the thalamorecipient auditory cortex. Extracellular stimuli were applied at layer 6 (L6), whereas evoked excitatory synaptic potentials (EPSPs) were recorded from L5 neurons by using a whole-cell current clamp configuration. In control neurons, the conditioning stimulation of L6 significantly altered EPSP amplitude for at least 1 h. Approximately half of neurons displayed long-term potentiation (LTP), whereas the other half displayed long-term depression (LTD). In contrast, SNHL neurons displayed only LTD after the conditioning stimulation of L6. Finally, the vast majority of neurons recorded from control prehearing animals (postnatal days 9–11) displayed LTD after L6 stimulation. Thus, normal auditory experience may be essential for the maturation of synaptic plasticity mechanisms.
Keywords: deafness, long-term depression
Severe hearing loss is known to affect auditory processing in humans. This loss includes impairments of signal detection in noise or in a multiple source environment, and disruption of intensity discrimination, frequency discrimination, and temporal resolution (1–4). Even transient bouts of conductive hearing loss can disrupt auditory processing, and months or years may be required for normal perception to resolve (5, 6). Because severe hearing loss during development is implicated in the deficient acquisition of auditory perceptual skills and language, which presumably require learning, we have examined whether synaptic plasticity is also affected.
At a structural level, hearing impairments can lead to neuron death or atrophy and alter process outgrowth (7). Furthermore, direct examination of neuron function in brain slice preparations from deaf animals has revealed significant physiological changes to intrinsic and synaptic properties. In both the inferior colliculus and auditory cortex (ACx), early sensorineural hearing loss (SNHL) alters the balance of excitatory and inhibitory synaptic strength (8–10). A similar disruption in synaptic physiology and certain intrinsic properties also occurs in the auditory brainstem of the congenitally deaf mutant mouse (11, 12).
Both long-term potentiation (LTP) and depression (LTD) have been characterized in a series of studies on the rat ACx (13–18). For example, an NMDA receptor-mediated LTP in the ACx can be induced by stimulation of afferents innervating the ACx via the white matter (13), whereas thalamo- and corticocortical synapse activation can induce LTD (16). These cellular mechanisms may serve as a substrate for use-dependent alteration of coding properties in the auditory cortex of adult and developing animals, including humans (19). For example, repetitive presentation of sound stimuli can lead to a long-lasting increase in the auditory-evoked potential in human and mouse cortex as assessed, respectively, with surface electrodes and transcranial fluorescence imaging (20, 21). These findings suggest that LTP and LTD serve as important bases for the modification of excitatory synapses within the ACx.
Despite a growing literature on central auditory synaptic plasticity in developing and adult animals, it remains unknown whether acoustic experience is required for its expression. There is some reason to expect that sensory experience may regulate synaptic plasticity. During development, the magnitude of inhibitory long-term depression in the auditory brainstem declines after hearing onset (22). Visual deprivation has been shown to occlude LTP of inhibitory synaptic connections in the visual cortex (23), and chronic blockade of the NR2B subunit of the NMDA receptor eliminates excitatory synaptic LTP in adult mouse auditory cortex (24). Given the profound descending projection from auditory cortex to the amygdala, thalamus, and brainstem (25), any modification in synaptic plasticity within L5 of the ACx could influence both processing and learned responses (26, 27). Here, we asked whether excitatory synaptic plasticity could be induced in ACx layer 5 (L5) neurons and, if so, whether it was affected by the loss of normal hearing. Our primary findings are that excitatory LTP emerges at the time of hearing onset in ACx L5 neurons, and this particular form of plasticity is eliminated by the loss of hearing.
Results
The data in this paper were collected from a total of 62 neurons recorded from an equal number of brain slices obtained from control postnatal day 8–11 (P8–11) (prehearing), control P14–P21 (posthearing), and SNHL (deafened) P14–21 gerbils. To assess whether the synaptic strength of L5 neurons could be modified, L6-evoked EPSPs were recorded in a whole-cell current-clamp configuration. We chose to record from L5 because these neurons exert a profound descending influence on the inferior colliculus (in gerbil: 28). We stimulated L6 because, unlike L2–4 neurons, L5 neurons do not receive a strong projection from the medial geniculate nucleus, MGv (in mice: 29).
Because resting membrane potential of ACx neurons changes significantly during the developmental period in which we recorded (30), we held each neuron at −70 mV. At the end of each recording, this holding potential was canceled, and we confirmed that the cell returned to its original resting membrane potential. After withdrawal of the recording electrode from the neuron, the recoded potential was 0 ± 2 mV. Thus, the VREST did not change during the course of these experiments.
Stimuli were applied to L6 at incremental intensity steps (0.1 Hz) until an EPSP-evoked spike was generated. The stimulus intensity was then adjusted to 50% of the value that elicited the maximum subthreshold EPSP (Fig. 1B), and the evoked EPSPs had an average amplitude of 6.5 ± 2.1 mV. This strategy permitted us to observe a bidirectional modification in EPSP amplitude. The initial mean EPSP amplitude of neurons that underwent LTD or LTP showed a small but significant difference (Wilcoxon's test; X2 = 4.3, n = 22, P = 0.04).
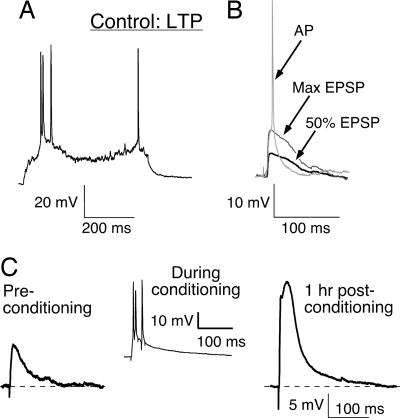
Fig. 1.
LTP was induced in a control posthearing L5 auditory cortex pyramidal neuron. (A) A 50-pA suprathreshold current pulse was injected to characterize the firing pattern. (B) An example of L6-evoked EPSPs at increasing stimulus intensities. For induction of synaptic plasticity, the stimulus intensity was chosen to produce a 50% amplitude EPSP. This strategy allowed normalization of EPSP amplitude to create a window for synaptic potentiation or depression by the subsequently conditioning stimuli applied at the same site (L6). (C) An EPSP trace recorded at the beginning of the experiment (Preconditioning) (Left) and an enhanced EPSP trace (Right) 1 h after the conditioning protocol. (Center) The neuron's response to one of several bursts within a conditioning stimulus is shown.
In slices from P14–21 control animals, the conditioning protocol induced either LTP (79% average EPSP amplitude increase; Figs. 1, 3A, and 4A) or LTD (47% average EPSP amplitude decrease, Figs. 2, 3C, and 4B). For those neurons displaying LTP, the mean EPSP amplitude increased significantly from 6.0 ± 0.4 mV before conditioning (average of first three EPSPs for each neuron), to 10.5 ± 0.7 mV (mean of last three EPSPs) 1 h after conditioning (t test, P = 0.0002, n = 10; Fig. 4A). For those neurons exhibiting LTD, the preconditioning mean EPSP amplitude was 7.3 ± 0.3 mV and decreased significantly to 3.8 ± 0.3 mV at 1 h after the conditioning stimuli (t test, P = 0.0001, n = 12; Fig. 4B).
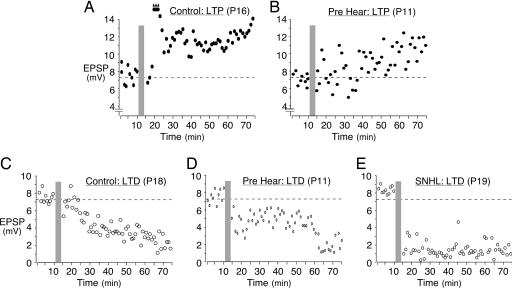
Fig. 3.
Examples of bidirectional plasticity in L5 neurons. Baseline EPSPs were acquired for 10 min before the conditioning stimulus (gray bars). EPSPs were then acquired for an additional hour. (A) Expression of LTP in a control posthearing neuron. (B) Relatively weak LTP in a prehearing neuron. (C) Expression of LTD in a control posthearing neuron. (D) LTD in a prehearing neuron. (E) LTD in a SNHL neuron. The plasticity induction protocol was similar for all cases. Postnatal age is in parentheses.
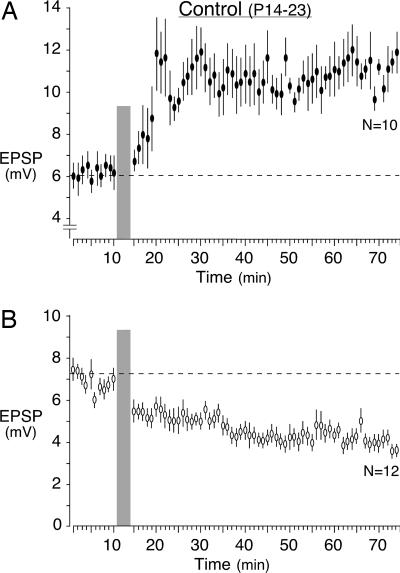
Fig. 4.
Expression of either LTP or LTD for all control posthearing neurons. Baseline EPSPs were acquired for 10 min before the conditioning stimulus (gray bar). EPSPs were then acquired for an additional hour. (A) Approximately half of the neurons displayed LTP (filled symbols). (B) Approximately half of the neurons displayed LTD (open symbols) (mean EPSP amplitude ± SEM).
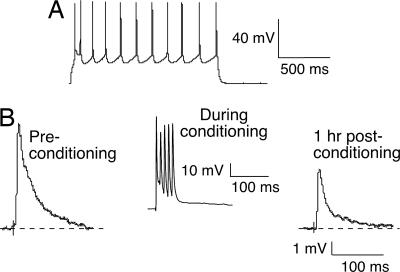
Fig. 2.
LTD was induced in a control posthearing L5 ACx pyramidal neuron. (A) A 90-pA suprathreshold depolarizing current pulse was injected to characterize the firing pattern (regular spiking). (B) An EPSP trace recorded at the beginning of the experiment (Preconditioning) (Left) and a significantly depressed EPSP trace (Right) 1 h after the conditioning protocol. (Center) The neuron's response to one of several bursts within a conditioning stimulus is shown.
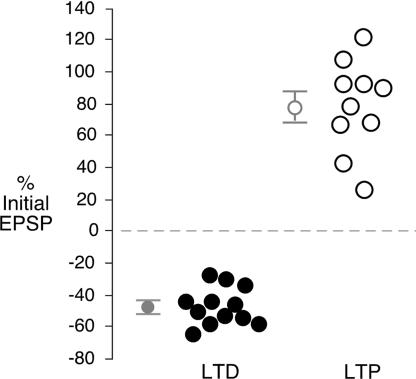
To determine whether the magnitude of synaptic modification was actually bimodally distributed, we plotted the percent change in EPSP amplitude for each recorded neuron (Fig. 5). The minimum change in synaptic strength that was observed for any individual neuron (LTP or LTD) was 26% (mean = 79%) for LTP and −27% for LTD (mean = −47%). Thus, the control population did not display a single-peaked distribution.
Fig. 5.
Difference in the magnitude of control LTP versus LTD. Percent change in the amplitude of the last three EPSPs after the conditioning stimulus were compared with the mean of first three EPSPs for all control posthearing neurons. This analysis showed the minimum change in synaptic strength in an individual neuron (LTP or LTD) was 26% for LTP (mean = 79%) and 27% for LTD (mean = 47%). Thus, the control population did not display a single-peaked distribution.
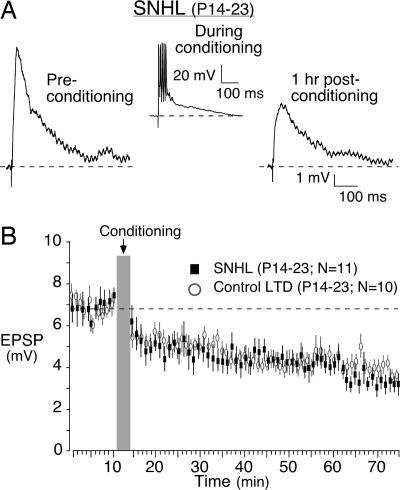
To determine whether auditory experience affected plasticity, recordings were obtained in thalamocortical slices obtained from age-matched animals in which SNHL had been induced surgically. In contrast to controls, all SNHL neurons displayed LTD in response to the conditioning stimulus (Figs. 3E and 6). The mean EPSP amplitude was 7.2 ± 1.6 mV (mean of first three EPSPs) before conditioning and declined significantly to 4.4 ± 1.9 mV (mean of last three EPSPs) 1 h after the conditioning protocol (t test, P = 0.004, n = 11). Thus, the plasticity induction protocol was not effective at producing LTP after hearing loss.
Fig. 6.
Hearing loss eliminates LTP. Baseline EPSPs were acquired for 10 min before the conditioning stimulus (gray bar). EPSPs were then acquired for an additional hour. (A) An EPSP trace recorded from an SNHL neuron at the beginning of the experiment (Preconditioning) (Left) and a significantly depressed EPSP trace (Right) obtained 1 h after the conditioning protocol. (Center) The neuron's response to one of several bursts within a conditioning stimulus is shown. (B) Control LTD (open circles) data are plotted for comparison (mean EPSP amplitude ± SEM). See Results for statistics.
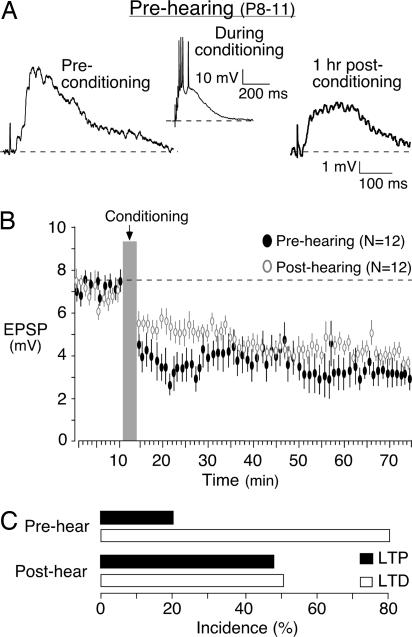
It is possible that SNHL either prevented LTP from developing or led to its loss. Therefore, recordings were obtained in slices generated from P8–11 animals (prehearing) to determine whether LTP had emerged at the time of the surgery to induce hearing loss. EPSPs from these slices displayed an induction of LTD in 12 of 15 neurons tested and a modest potentiation in the remaining 3 neurons (Figs. 3 B and D and 7). The mean EPSP amplitude for LTD was 7.1 ± 0.3 mV before the conditioning stimulus, and this declined significantly to 3.5 ± 0.4 mV 1 h after the conditioning protocol (Wilcoxon's test; X2 = 15.4, n = 12, P = 0.001). Of the three neurons that did display an increase in EPSP amplitude, there was not a significant difference before and after conditioning (X2 = 1.2, n = 3, P = 0.26). Thus, the prehearing neurons primarily displayed LTD, suggesting that SNHL prevented the emergence of LTP.
Fig. 7.
LTD is prominent in prehearing animals. (A) An EPSP trace recorded from a prehearing animal at the beginning of the experiment (Preconditioning) (Left) and a significantly depressed EPSP trace (Right) obtained 1 h after the conditioning protocol. (Center) The neuron's response to one of several bursts within a train is shown. (B) Baseline EPSPs were acquired for 10 min before the conditioning protocol (gray bar). EPSPs were then acquired for an additional hour. Control LTD (open circles) data are plotted for comparison (mean EPSP amplitude ± SEM). (C) The bar graph displays the incidence of LTP and LTD in pre- versus posthearing neurons. Note the bias toward LTD in prehearing neurons.
Consistent with previous reports (30, 31), the recorded L5 neurons displayed three different firing patterns in response to suprathreshold depolarizing current pulses: intrinsic bursting (IB; Fig. 1A), regular spiking (RS; Fig. 2A), or sustained. To explore whether the pattern of discharge was associated with the direction of synaptic plasticity, the data were subcategorized in those cells where firing pattern was determined. This analysis revealed no discernible trend; the three cell types displayed both LTP and LTD. LTP was generated in 2/5 IB cells, 4/8 RS cells, and 2/5 sustained cells. LTD was displayed in 3/5 IB cells, 4/8 RS cells, and 3/5 sustained cells.
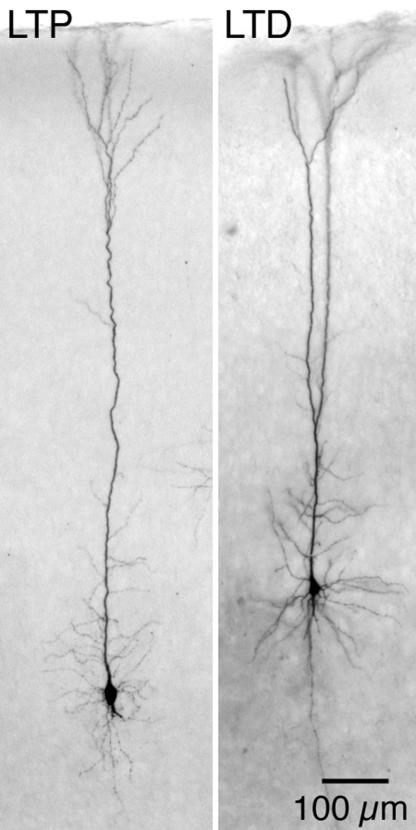
In addition to the physiological characterization of cells, we asked whether there was a correlation between the morphological identity of the two cell types and the direction of plasticity (Fig. 8). The cell types were categorized by the spread of dendritic arbor across the auditory cortical laminae, as described previously (31). By this anatomical criterion, three control and three SNHL neurons with IB dendritic morphology displayed LTP, whereas two control and three SNHL neurons with RS dendritic morphology displayed LTD. Four prehearing neurons with RS dendritic morphology displayed LTD. Thus, the dendritic morphology of L5 neurons was not associated with a particular form of plasticity.
Fig. 8.
Dendritic morphology does not correspond with the direction of plasticity. The photomicrographs show two biocytin-labeled IB neurons, each recorded in L5 ACx of a control posthearing animal. The neuron on the left displayed LTD, whereas the neuron on the right displayed LTD.
Discussion
The developmental appearance of synaptic plasticity mechanisms is thought to support activity-dependent alterations in neural connectivity, driven either by patterned spontaneous or sensory-driven activity (32). However, the maintenance and maturation of these mechanisms are poorly understood, particularly their dependence on experience. There is no study testing whether synaptic plasticity in the central auditory system depends on normal hearing experience during development. The major findings of this study are that: (1) L5 pyramidal neurons in ACx display both LTP and LTD after hearing onset (Figs. 1–4), and (2) bilateral sensorineural hearing loss selectively eliminates use-dependent LTP, leaving excitatory synaptic LTD unchanged (Figs. 3 and 6).
In prehearing animals, LTP could not be induced reliably (Figs. 3 and 7). This is consistent with several developmental studies showing the emergence of LTP with postnatal age (33, 34). In many CNS regions, LTD expression diminishes during postnatal aging (22, 35–37).
Many studies have described an association between deprivation and synaptic strength. Wiesel and Hubel (38) found that binocular light deprivation resulted in a large fraction of cortical neurons being unresponsive to light. In the barrel cortex, selective excision of whiskers leads to enhanced responses in barrel cortex corresponding to intact whiskers and depressed responses in columns corresponding to trimmed whisker regions (39). However, these studies have not examined the effect of disuse on the ability of synapses to undergo LTP or LTD.
There are a few recent indications that experience does influence the maturation of synaptic plasticity mechanisms. In the perirhinal cortex, for example, excitatory synaptic LTD changes from being metabotropic glutamate receptor-dependent to muscarinic acetylcholine receptor-dependent, and this shift is prevented by dark rearing, demonstrating a dependence on visual experience (40). Likewise, in vestibular medial nucleus neurons, dark rearing can prevent a developmental transformation from excitatory LTD to LTP (41). Interestingly, visual cortex neurons of dark-reared rats display LTP that persists later into development (42). Therefore, deprivation results in the persistence of the immature state (presence of LTP in V1, absence of LTP in A1) in both studies. There are also several methodological differences between the two studies that make comparison difficult. Specifically, the Kirkwood study (42) examined much older animals (4- to 6-week-old juvenile rats versus 2- to 3-week old gerbils in the present study), used different conditioning stimuli, and examined neurons in L4 and stimulated in L5 (versus L6 stimulation and L5 recording in the present study). These differences may also explain why developing ACx is more susceptible to bidirectional plasticity by the same conditioning protocol (Figs. 1–4).
The plasticity displayed in control gerbil ACx (Figs. 1–5) confirms previous reports on the induction of cortico- and thalamocortical LTP and LTD (13, 15, 43). The behavioral relevance of NMDA receptor-mediated plasticity in ACx has also been demonstrated in adult rats (44). A change in the balance between LTP and LTD may explain the decreased responsiveness of ACx in sound-deprived mice (21) and the decline of electrically evoked responses observed in congenitally deaf cats (45). L5 synaptic plasticity may have a significant impact on subthalamic processing because of the massive descending projection from ACx (28, 46–50). Furthermore, the specific complement of cortical plasticity mechanisms may support the functional takeover of ACx by other sensory modalities. For example, in congenitally deaf mice and humans, auditory cortical neurons respond to visual and somatosensory stimuli (51, 52). It now appears that a fundamental cellular mechanism that supports auditory-based learning, long-term potentiation in ACx, may not develop properly when developing animals are deprived of normal hearing. This opens the possibility that we might understand associated cognitive deficits in terms of the residual level of synaptic plasticity after a period of auditory deprivation.
Materials and Methods
Animals.
Gerbil (Meriones unguiculatus) pups at P8–21 were used for these studies. The age range represents the time during which auditory functional properties are known to mature (53–58). All protocols were reviewed and approved by the New York University Institutional Animal Care and Use Committee.
Surgically Induced Hearing Loss.
Cochlear ablations were performed using procedures described previously (8, 10). Gerbil pups at P10 were anesthetized (methoxyflurane), and each cochlea was rapidly removed with a forceps. Animals were reared for 4–11 days with their parents under conditions identical to those for control pups. The age of surgery was chosen on the basis of evidence that anteroventral cochlear nucleus cell number is unaffected by cochlear ablation after P9 (59).
Brain Slice Preparation.
Animals were anesthetized (chloral hydrate, 400 mg/kg), and the brain was dissected in chilled oxygenated artificial cerebrospinal fluid (ACSF: 123 mM NaCl, 4 mM KCl, 1.2 mM KH2PO4, 1.3 mM MgSO4, 24 mM NaHCO3, 15 mM glucose, 2.4 mM CaCl2, 0.2 mM ascorbic acid; pH = 7.35 after bubbling with 95% 02/5% CO2). The brain was vibratome-sectioned at 500 μm, which preserves the connectivity from the medial geniculate (MG) to the ACx (8, 29). ACSF superfusion rate was 3 ml/min, and the bath temperature was maintained at 32 ± 1°C.
Whole-Cell Recordings.
The recording electrodes (10–15 MΩ) were pulled from borosilicate glass (1.5-mm OD) and backfilled with a solution containing: 130 mM K+-gluconate, 5 mM KCl, 2 mM MgCl2, 2 mM ATP, 0.3 mM GTP, 0.6 mM EGTA, 5 mM phosphocreatine, 10 mM Hepes, and 0.5% biocytin (pH 7.2 with KOH). ACx neurons with a resting potential of −50 mV or better and responding to MGv stimulation (500-μs pulse) were included. Series resistance was compensated up to 50 MΩ.
Before each experiment, extracellular recordings were obtained while stimulating MGv to confirm that the recording site was thalamorecipient ACx. We recorded only within an L5 region that had previously responded extracellularly to the direct electrical stimulation of the MGv (100 μA/500 μS), validating that the recording site was thalamorecipient ACx. In addition, the criteria for including cells were consistent to those we applied for L2/3 neurons (8). These criteria included a resting membrane potential of −50 mV or less, overshooting action potentials in response to suprathreshold depolarizing current injection (1,500 ms), compensation of access resistance up to 40 MΩ, and a verification of a stable VREST after the completion of each recording (the potential returned to 0 mV ± 2 mV after withdrawing the recording electrode from the neuron). Cells that did not meet these criteria were excluded from the analyses.
Computer-Automated Stimulation, Acquisition, and Analysis.
Data were acquired by using a Macintosh G4 running an Igor-based macro (WaveMetrics, Lake Oswego, OR; v5.03), called SLICE, and analyzed off-line by using a second macro, SLICE ANALYSIS (22). Both the data acquisition and analysis macros are available with documentation at www.cns.nyu.edu/∼sanes/slice_software/. The SLICE macro controls stimulus delivery (Dagan BSI-950) and data acquisition (Warner PC-501A) via an ITC-18 interface (Instrutech, Mineola, NY). Data were sampled at 10 kHz while current pulses were delivered under computer control.
EPSP amplitudes were analyzed offline by using SLICE ANALYSIS. The algorithm for this measurement defines the poststimulus artifact wave for each trace consisting of all points from the original wave after the start of the stimulus pulse and subtracts the mean of the trace during the prestimulus period from this wave. Thus, the amplitudes of EPSPs were calculated. Statistical comparisons (ANOVA, Student's t test) or Wilcoxon tests were performed with JMP 5.0.1 (SAS Institute, Cary, NC).
Induction of Plasticity.
Submaximal stimuli were applied at L6 just above its juncture with the white matter to evoke an EPSP at 50% of maximum amplitude in L5 neurons (Fig. 1B). To minimize damage to afferents by the current buildup by bipolar stimulating electrode, especially during the condition protocol, the stimulus duration of each pulse was kept at a minimum (100 μS). In addition, EPSP acquisition at long intervals (1 per min) makes unlikely that use-dependent plasticity would be elicited during the control intervals. The EPSPs were recorded every minute for 10 min to establish a preconditioning baseline of synaptic amplitude before treatment with a plasticity induction protocol (conditioning stimuli). This conditioning protocol was comprised of five stimulus repetitions: each repetition consisted of five trains (five pulses, 100 Hz) delivered at 1-sec intervals. The stimulus repetitions were delivered at intervals of 30 sec. The conditioning stimulus intensity was three times larger than that used to acquire preconditioning EPSPs. In general, neurons responded with a robust depolarization and discharge during the conditioning stimulus, and further postsynaptic depolarization by direct current injection was not necessary. Immediately after the conditioning protocol, EPSPs were acquired once per minute for an additional hour with the same stimulus intensity used during the preconditioning period.
Anatomy.
Biocytin-filled neurons were stained with a standard ABC-HRP-diaminobenzidine protocol. The soma location was confirmed to be in L5, the dendritic arborization was confirmed to be intact, and the characteristic cell morphologies were determined (31).
Acknowledgments
This work was supported by the National Institute on Deafness and Other Communication Disorders Grant DC006864 (to D.H.S. and V.C.K.).
Abbreviations
- SNHL
sensorineural hearing loss
- ACx
auditory cortex
- EPSP
excitatory postsynaptic potential
- LTD
long-term depression
- LTP
long-term potentiation
- Pn
postnatal day n
- Ln
layer n
- RS
regular spiking.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Halliday LF, Bishop DV. J Speech Lang Hear Res. 2005;48:1187–1203. doi: 10.1044/1092-4388(2005/083). [DOI] [PubMed] [Google Scholar]
- 2.Iverson P. J Acoust Soc Am. 2003;113:1056–1064. doi: 10.1121/1.1531985. [DOI] [PubMed] [Google Scholar]
- 3.Kidd G, Jr, Arbogast TL, Mason CR, Walsh M. J Assoc Res Otolaryngol. 2002;3:107–119. doi: 10.1007/s101620010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojtczak M, Donaldson GS, Viemeister NF. J Acoust Soc Am. 2003;114:396–407. doi: 10.1121/1.1579007. [DOI] [PubMed] [Google Scholar]
- 5.Hogan SC, Meyer SE, Moore DR. Audiol Neurootol. 1996;1:104–111. doi: 10.1159/000259189. [DOI] [PubMed] [Google Scholar]
- 6.Wilmington D, Gray L, Jahrsdoerfer R. Hear Res. 1994;74:99–114. doi: 10.1016/0378-5955(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 7.Parks TN. In: The Biology of Early Influences. Hyson RL, Johnson F, editors. New York: Academic; 1999. pp. 15–34. [Google Scholar]
- 8.Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vale C, Sanes DH. J Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vale C, Sanes DH. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- 11.Leao RN, Oleskevich S, Sun HH, Bautista M, Fyffe REW, Walmsley B. J Neurophysiol. 2004a;91:1006–1012. doi: 10.1152/jn.00771.2003. [DOI] [PubMed] [Google Scholar]
- 12.Leao RN, Berntson A, Forsythe ID, Walmsley B. J Physiol. 2004b;559:25–33. doi: 10.1113/jphysiol.2004.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudoh M, Shibuki K. Exp Brain Res. 1996;110:21–27. doi: 10.1007/BF00241370. [DOI] [PubMed] [Google Scholar]
- 14.Kudoh M, Shibuki K. J Neurosci. 1997;17:9458–9465. doi: 10.1523/JNEUROSCI.17-24-09458.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seki K, Kudoh M, Shibuki K. Neurosci Res. 1999;34:187–197. doi: 10.1016/s0168-0102(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 16.Seki K, Kudoh M, Shibuki K. J Physiol. 2001;533:503–518. doi: 10.1111/j.1469-7793.2001.0503a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seki K, Kudoh M, Shibuki K. Brain Res. 2003;988:114–120. doi: 10.1016/s0006-8993(03)03351-1. [DOI] [PubMed] [Google Scholar]
- 18.Wakatsuki H, Gomi H, Kudoh M, Rimura S, Takahashi K, Takeda M, Shibuki K. J Physiol. 1998;513:71–81. doi: 10.1111/j.1469-7793.1998.071by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberger NM. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clapp WC, Kirk IJ, Hamm JP, Shepherd D, Teyler TJ. Eur J Neurosci. 2005;22:1135–1140. doi: 10.1111/j.1460-9568.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Hishida R, Kubota Y, Kudoh M, Takahashi S, Shibuki K. Eur J Neurosci. 2006;23:1365–1376. doi: 10.1111/j.1460-9568.2006.04662.x. [DOI] [PubMed] [Google Scholar]
- 22.Kotak VC, Sanes DH. J Neurosci. 2000;20:5820–5826. doi: 10.1523/JNEUROSCI.20-15-05820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 24.Mao Y, Zang S, Zhang J, Sun X. Brain Res. 2006;1073–1074:131–138. doi: 10.1016/j.brainres.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 25.Syka J, Popelár J, Druga R, Vlková A. In: Auditory Pathway—Structure and Function. Syka J, Masterton RB, editors. New York: Plenum; 1988. pp. 279–292. [Google Scholar]
- 26.Suga N, Ma X. Nat Rev. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- 27.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 28.Bajo VM, Moore DR. J Comp Neurol. 2005;486:101–116. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- 29.Cruikshank SI, Rose HJ, Metherate R. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- 30.Metherate R, Aramakis VB. Dev Brain Res. 1999;115:131–144. doi: 10.1016/s0165-3806(99)00058-9. [DOI] [PubMed] [Google Scholar]
- 31.Hefti BJ, Smith PH. J Neurophysiol. 2000;83:2626–2638. doi: 10.1152/jn.2000.83.5.2626. [DOI] [PubMed] [Google Scholar]
- 32.Constantine-Paton M, Cline HT. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, Constantine-Paton M. Neuron. 2004;43:237–249. doi: 10.1016/j.neuron.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 34.Quinlan EM, Olstein DH, Bear MF. Proc Natl Acad Sci USA. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battistin T, Cherubini E. Eur J Neurosci. 1994;6:1750–1755. doi: 10.1111/j.1460-9568.1994.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 36.Dudek SM, Friedlander MJ. Neuron. 1996;16:1097–1106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 37.Feldman DE, Nicoll RA, Malenka RC, Issac JT. Neuron. 1998;21:347–357. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- 38.Wiesel TN, Hubel DH. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong-James M, Diamond ME, Ebner FF. J Neurosci. 1994;14:6978–6991. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jo J, Ball SM, Seok H, Oh SB, Massey PV, Molnar E, Bashir ZI, Cho K. Nat Neurosci. 2006;9:170–172. doi: 10.1038/nn1637. [DOI] [PubMed] [Google Scholar]
- 41.Grassi S, Dieni C, Frondaroli A, Pettorossi VE. J Physiol. 2004;560:767–777. doi: 10.1113/jphysiol.2004.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkwood A, Rioult MC, Bear MF. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 43.Kudoh M, Sakai M, Shibuki K. J Neurophysiol. 2002;88:3167–3174. doi: 10.1152/jn.00928.2001. [DOI] [PubMed] [Google Scholar]
- 44.Sakai M, Kudoh M. Behav Neurosci. 2005;119:961–973. doi: 10.1037/0735-7044.119.4.961. [DOI] [PubMed] [Google Scholar]
- 45.Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Cereb Cortex. 2005;15:552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- 46.Coomes DL, Schofield BR. Eur J Neurosci. 2004;19:2188–2200. doi: 10.1111/j.0953-816X.2004.03317.x. [DOI] [PubMed] [Google Scholar]
- 47.Coomes DL, Schofield RM, Schofield BR. Brain Res. 2005;1042:62–72. doi: 10.1016/j.brainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Schofield BR, Coomes DL, Schofield RM. J Assoc Res Otolaryngol. 2006;7:95–109. doi: 10.1007/s10162-005-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schofield BR, Coomes DL. Hear Res. 2005a;206:3–11. doi: 10.1016/j.heares.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Schofield BR, Coomes DL. Hear Res. 2005b;199:89–102. doi: 10.1016/j.heares.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Finney EM, Fine I, Dobkins KR. Nat Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- 52.Hunt DL, Yamoah EN, Krubitzer L. Neurosci. 2006;139:1507–1524. doi: 10.1016/j.neuroscience.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 53.Harris DM, Dallos P. Science. 1984;225:741–743. doi: 10.1126/science.6463651. [DOI] [PubMed] [Google Scholar]
- 54.McFadden SL, Walsh EJ, McGee J. Hear Res. 1996;100:68–79. doi: 10.1016/0378-5955(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 55.Ryan AF, Woolf NK. Dev Brain Res. 1988;41:61–70. doi: 10.1016/0165-3806(88)90169-1. [DOI] [PubMed] [Google Scholar]
- 56.Sanes DH, Rubel EW. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woolf NK, Ryan AF. Hear Res. 1984;13:277–283. doi: 10.1016/0378-5955(84)90081-9. [DOI] [PubMed] [Google Scholar]
- 58.Woolf NK, Ryan AF. Dev Brain Res. 1985;17:131–147. doi: 10.1016/0165-3806(85)90138-5. [DOI] [PubMed] [Google Scholar]
- 59.Tierney TS, Russell FA, Moore DR. J Comp Neurol. 1997;378:295–306. doi: 10.1002/(sici)1096-9861(19970210)378:2<295::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]