Abstract
Kv2.1, the primary delayed rectifying potassium channel in neurons, is extensively regulated by phosphorylation. Previous reports have described Kv2.1 phosphorylation events affecting channel gating and the impact of this process on cellular excitability. Kv2.1, however, also provides the critical exit route for potassium ions during neuronal apoptosis via p38 MAPK-dependent membrane insertion, resulting in a pronounced enhancement of K+ currents. Here, electrophysiological and viability studies using Kv2.1 channel mutants identify a p38 phosphorylation site at Ser-800 (S800) that is required for Kv2.1 membrane insertion, K+ current surge, and cell death. In addition, a phospho-specific antibody for S800 detects a p38-dependent increase in Kv2.1 phosphorylation in apoptotic neurons and reveals phosphorylation of S800 in immunopurified channels incubated with active p38. Consequently, phosphorylation of Kv2.1 residue S800 by p38 leads to trafficking and membrane insertion during apoptosis, and remarkably, the absence of S800 phosphorylation is sufficient to prevent completion of the cell death program.
Keywords: apoptosis, ion channel, MAPK
Enhancement of voltage-gated K+ channel activity, resulting in K+ efflux, is an essential step in neuronal apoptosis (1). Blocking K+ channels, or increasing the extracellular K+ concentration, effectively attenuate cell death in many apoptotic models (2–6), including oxidant exposure in cortical and midbrain dopaminergic neurons (7–10). With the use of dominant negative mutant subunits, Kv2.1, the major component of the delayed rectifier K+ current in neurons (11, 12), was identified as the channel responsible for mediating the apoptotic K+ current surge in cortical neurons (13). During oxidant-induced neuronal apoptosis, the liberation of intracellular Zn2+ from metal binding proteins (7) leads to the activation of p38 MAPK, which precedes and is necessary for the characteristic K+ current surge (8, 9). This enhancement of K+ currents is caused by a soluble N-ethylmaleimide-sensitive factor attachment protein receptor-dependent insertion of new Kv2.1-encoded channels, rather than an alteration in the properties of existing surface channels, such as a change in activation kinetics (3, 14). Despite this information, the molecular process connecting p38 activation and the apoptotic membrane insertion of Kv2.1 K+ channels had heretofore remained undefined.
Here, we combine several experimental approaches to establish a direct link between active p38 and Kv2.1 during apoptosis after a sequence-based prediction model (15) was used to identify a putative phosphorylation site for the MAPK on the C terminal of the channel. First, alanine substitution of Ser-800 (S800) in Kv2.1 completely abolished the apoptotic enhancement of K+ currents. Second, a cysteine-containing mutant of Kv2.1 and a thiol-reactive covalent inhibitor were used to demonstrate that S800 is critical for membrane insertion of the channel during apoptosis. Third, expression of phospho-mimetic mutant channels resulted in significant increases in basal K+ current densities. Fourth, a phospho-specific antibody directed at S800 detected a p38-dependent increase in phospho-Kv2.1 levels in apoptotic neurons and revealed phosphorylation of immunopurified Kv2.1, but not of Kv2.1(S800A), incubated with active p38. Most significantly, Kv2.1(S800A) did not support apoptosis mediated by the WT channel in a recombinant expression system. This study establishes that p38-mediated phosphorylation of Kv2.1 is necessary and sufficient for its apoptotic trafficking and completion of the cell death program.
Results
S800 in Kv2.1 Is Necessary for the Apoptotic Surge in K+ Currents.
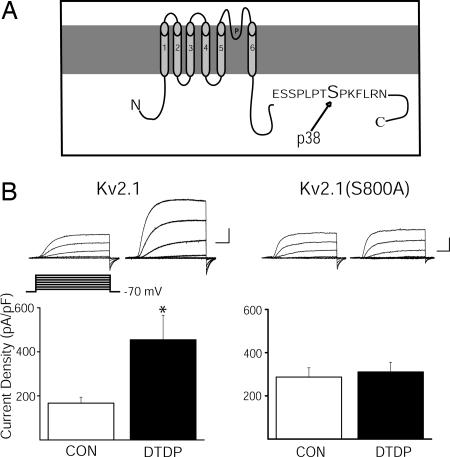
Scansite (http://scansite.mit.edu), a program that predicts protein phosphorylation sites based on proteomic and biochemical data (15), was used to search for potential MAPK targets in Kv2.1 (GenBank accession no. NM_013186). A medium-stringency scan revealed a single serine residue at position 800 (Fig. 1A) as a potential phosphorylation target for both p38 and ERK. However, the Scansite score for this sequence suggested a substantially better match for p38 than for ERK. S800 resides in the serine- and threonine-rich intracellular C-terminal tail of the protein (Fig. 1A), where other phosphorylation sites exist (16–18). We thus examined the role of S800 in the up-regulation of K+ currents during oxidant-induced apoptosis in a recombinant expression system.
Fig. 1.
Ser-800 of Kv2.1 is critical for apoptosis-associated K+ current enhancement. (A) Schematic of Kv2.1 showing the putative p38 phosphorylation site on the cytoplasmic C terminus. (B) (Upper Left) Representative whole-cell K+ currents from Kv2.1-expressing CHO cells recorded under control and DTDP treatment conditions. Currents were obtained 48 h posttransfection and evoked by sequential 15-mV voltage steps to +35 mV from a holding potential of −70 mV. Calibration was 5 nA, 15 ms. (Lower Left) Mean ± SEM. K+ current densities from Kv2.1-expressing CHO cells under control (n = 25) and DTDP (n = 12) treatment conditions (∗, P < 0.05; t test). Currents were induced with a voltage step to +5 mV from a holding potential of −70 mV and normalized to cell capacitance. (Upper Right) Representative whole-cell K+ currents from Kv2.1(S800A)-expressing CHO cells recorded under control and DTDP treatment conditions. (Lower Right) Mean ± SEM. K+ current densities from Kv2.1(S800A)-expressing CHO cells recorded under control (n = 24) and DTDP (n = 24) treatment conditions.
Whole-cell electrophysiological recordings were performed on CHO cells transiently expressing either WT Kv2.1 channels or a nonphosphorylatable mutant, Kv2.1(S800A). CHO cells have no endogenous voltage-gated K+ channels (19), but can be induced to readily undergo oxidant-induced apoptosis after expressing Kv2.1 (13), and, like neurons, show a pronounced K+ current enhancement during this process (14). Recordings were obtained under control conditions and after treatment with the oxidant apoptogen 2,2′-dithiodipyridine (DTDP; refs. 7 and 14). Electrophysiological measurements were routinely performed 3 h after oxidant exposure, a time when a robust K+ current surge is well established (8, 13, 14). As shown (14), currents mediated by WT Kv2.1-encoded channels were substantially enhanced after the apoptotic stimulus (Fig. 1B Left). In contrast, no current surge was observed in CHO cells expressing Kv2.1(S800A) after the oxidant treatment (Fig. 1B Right), indicating that S800, a putative target for p38-mediated phosphorylation, is required for the increase in K+ currents during apoptosis.
S800 Is Required for Apoptogen-Induced Membrane Insertion of Kv2.1.
The apoptotic surge in K+ current observed in both cortical neurons and CHO cells is the result of trafficking and soluble N-ethylmaleimide-sensitive factor attachment protein receptor-dependent exocytotic insertion of new Kv2.1 channels into the plasma membrane (14). New channel membrane insertion was detected by using the cysteine-containing K+ channel mutant Kv2.1(I379C) (20). The presence of this mutation allows the channel to be functionally blocked by the membrane impermeant thiol reagent (2-trimethylammoniumethyl) methanethiosulfate (MTSET) (21, 22). Virtually all previously existing surface Kv2.1(I379C) channels can therefore be silenced after a 5- to 10-min exposure to MTSET. With existing plasma membrane Kv2.1 channels permanently blocked, the insertion of new channels is easily detected electrophysiologically by the appearance of new currents under MTSET-free conditions (14). This assay, which has the distinct advantage of detecting the insertion of new functional channels, has been validated by parallel biotinylation studies (14).
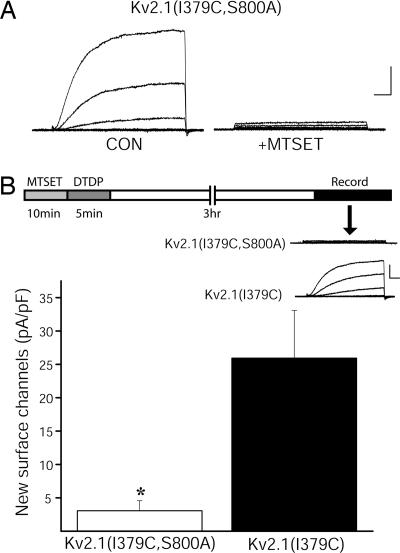
To investigate the role of S800 in the apoptotic insertion of new channels, the double mutant Kv2.1(I379C, S800A) was generated. Whole-cell recordings first confirmed the preservation of functional silencing by MTSET in this double mutant channel (Fig. 2A). Next, CHO cells expressing either Kv2.1(I379C) or Kv2.1(I379C, S800A) were exposed to MTSET for 10 min to block all existing surface channels. The cells were then exposed to oxidant to trigger the apoptotic cascade. Electrophysiological recordings 3 h later revealed the presence of K+ currents in Kv2.1(I379C)-expressing cells (Fig. 2B), similar to what was reported earlier (14). However, little or no current could be measured in CHO cells expressing Kv2.1(I379C, S800A), indicating a lack of apoptotic membrane insertion of these mutant channels (Fig. 2B). This result not only accounts for the lack of Kv2.1(S800A)-mediated apoptotic current surge observed earlier, but demonstrates that phosphorylation of S800 is necessary for Kv2.1 membrane insertion during the cell death program.
Fig. 2.
S800A mutation blocks apoptogen-induced membrane insertion of Kv2.1. (A) MTSET blocks Kv2.1(I379C) channel containing the Kv2.1(S800A) mutation. Calibration was 2 nA, 10 ms. (B) (Upper) Diagram representing the experimental protocol designed to measure the appearance of new K+ channels during the apoptotic process. Shown are representative whole-cell K+ currents obtained from Kv2.1(I379C)- and Kv2.1(I379C, S800A)- expressing CHO cells 3 h after sequential treatment with MTSET and DTDP. Currents were evoked by sequential 15-mV voltage steps to +35 mV from a holding potential of −70 mV. Calibration was 1 nA, 10 ms. (Lower) Mean ± SEM. K+ current densities recorded from Kv2.1(I379C)-expressing CHO cells (n = 17) and Kv2.1(I379C, S800A)-expressing CHO cells (n = 20) 3 h after the sequential MTSET/DTDP treatments (∗, P < 0.01; t test). These currents represent the newly inserted channels during apoptosis.
Negatively Charged Amino Acids at Position 800 Mimic the Apoptotic Surge.
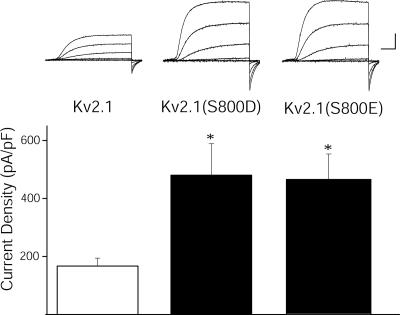
Recordings were performed from CHO cells transiently expressing two additional variants of Kv2.1 to test whether substitution of S800 with negatively charged residues would mimic the apoptotic increase in currents observed in WT channels. Indeed, basal current densities in untreated Kv2.1(S800D) and Kv2.1(S800E) mutant-expressing cells displayed significantly enhanced amplitudes, comparable to those observed in DTDP-treated Kv2.1-expressing cells (Fig. 3). Oxidant treatment of cells expressing these mutations did not produce any additional current enhancement (data not shown). Rather, a slight decrease in the currents was observed under these conditions, likely the result of cellular damage by the injurious stimulus. These results further support the role of S800 phosphorylation as a key mediator of the K+ current surge during apoptosis.
Fig. 3.
Substitution of S800 with negatively charged amino acids mimics apoptotic K+ current densities. (Upper) Representative whole-cell K+ currents from Kv2.1-expressing CHO cells (Left), Kv2.1(S800D)-expressing CHO cells (Center), and Kv2.1(S800E)expressing CHO cells (Right). Currents were obtained 48 h posttransfection and evoked by sequential 15-mV voltage steps to +35 mV from a holding potential of −70 mV. Calibration was 5 nA, 15 ms. (Lower) Mean ± SEM. Current densities from Kv2.1-expressing CHO cells (n = 13) (Left), Kv2.1(S800D)-expressing CHO cells (n = 17) (Center), and Kv2.1(S800E)-expressing CHO cells (n = 14) (Right) (∗, P < 0.05; t test).
S800 Phosphorylation Is p38-Dependent in Neurons Undergoing Apoptosis.
Biochemical studies were performed to detect phospho-S800 levels in primary cortical neurons after oxidative injury. Cell lysates were subjected to gel electrophoresis, and the resulting blots were probed with a commercially available antibody directed against amino acids 841–857 of the C terminus of Kv2.1 (Kv2.1; Alomone Labs, Jerusalem, Israel) or with an antibody that we generated against the phosphorylated form of S800 [pKv2.1; supporting information (SI) Fig. 6]. Immunoblots probed with Kv2.1-directed antibodies revealed the presence of two major bands in cortical extracts: a diffuse band near 100 kDa and another, more compact band, at ≈80 kDa (SI Fig. 7; see also refs. 23–25). The diffuse band near 100 kDa likely represents multiple, non-p38 dependent phosphorylation states of the predicted full-length channel (18, 26), whereas the 80-kDa band may reflect the presence of a protein generated by an alternative mRNA isoform (23, 27) or a proteolytic fragment. Importantly, preincubation of the two antibodies with their respective immunizing peptides completely blocked both immunoreactive signals (SI Fig. 7).
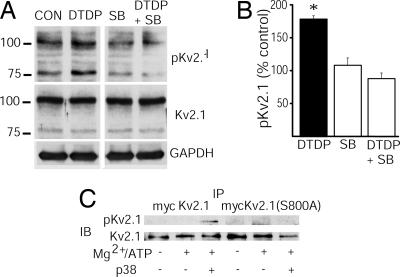
Phospho-S800 levels in cortical neuronal lysates were assessed by using the pKv2.1 antibody under control conditions and after oxidative injury. We observed a significant increase in pKv2.1 immunoreactivity in cortical neurons undergoing apoptosis, compared with vehicle-treated controls (Fig. 4 A and B). This increase was particularly apparent in the compact, 80-kDa band. Importantly, SB-239063, a selective p38 inhibitor (8, 28), prevented oxidant-mediated increases in pKv2.1 immunoreactivity (Fig. 4 A and B). These data confirm a p38-dependent increase in phospho-S800 levels in endogenous Kv2.1 channels from cortical neurons undergoing apoptosis.
Fig. 4.
Phosphorylation of Ku2.1 by p38. (A) DTDP-induced phosphorylation of Kv2.1-S800 is regulated by p38 MAPK in cultured cortical neurons. Cortical cultures were pretreated with either vehicle or the specific p38 MAPK inhibitor SB293063 (SB; 20 μM for 10 min) and then incubated with either vehicle or DTDP (100 μM for 10 min). Immunoblots were probed with anti-pKv2.1 antibody (1:5,000; Top), a commercially available anti-Kv2.1 antibody (Alomone Labs; 1:1,000; Middle), and an anti-GAPDH antibody (Novus Biologicals, Littleton, CO; 1:5,000; Bottom) to confirm equal protein loading between lanes. These data are representative of results from three independent experiments. (B) Oxidative injury significantly increases S800 phosphorylation in cortical neurons in vitro. Optical density measurements of the phosphorylation level and its protein loading control at both the 100-kDa and the 80-kDa band were quantified by using Scion Image software. Values represent the mean ± SEM normalized pKv2.1 signal (to Kv2.1 immunoreactivity) of both bands as a percentage of vehicle-treated control from three separate, independent experiments (∗, P < 0.01; ANOVA/Dunnett). Note that the observed increase in S800 phosphorylation was effectively blocked with a p38 MAPK inhibitor. (C) S800 of Kv2.1 is phosphorylated by p38α in a cell-free kinase assay. Myc-Kv2.1 and Myc-Kv2.1(S800A) proteins were immunoprecipitated (IP) from CHO cell lysates and reacted at 30°C for 1 h with 15 μl of kinase buffer (25 mM Hepes, pH 8.0/2 mM DTT/0.1 mM vanadate), 15 μl of Mg/ATP (50 mM MgCl and 50 μM ATP), and 50 μg of activated p38α kinase. Reactions containing kinase buffer and Mg/ATP and kinase buffer alone were used as controls. Immunoblots (IB) were probed with the pKv2.1 antibody (1:12,000) and the Kv2.1 antibody (1:3,000; Alomone Labs).
Active p38 Phosphorylates S800 in Cell-Free Assays.
To determine whether p38 could phosphorylate Kv2.1, we performed a cell-free assay in which recombinantly expressed, immunoprecipitated poly myc-tagged WT Kv2.1 (myc-Kv2.1; refs. 14 and 29) was used as a substrate for purified, active p38. We observed that myc-Kv2.1 protein exposed to active p38 produced a phospho-specific immunoreactive band (Fig. 4C). Similar experiments performed with an S800A mutant of the myc-tagged Kv2.1 yielded no phospho-specific signal (Fig. 4C). These results indicate that active p38 can directly induce selective phosphorylation of residue S800 of Kv2.1.
S800A Mutation Disrupts Kv2.1-Mediated Apoptosis.
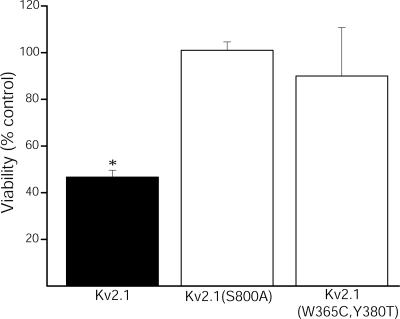
Unlike truncated or pore mutants of the channel (13), Kv2.1(S800A) does not function as a dominant interfering form in neurons undergoing apoptosis, but simply enhances the overall basal current amplitude without eliminating the contribution of endogenous channels (data not shown). As such, the requirement for S800 phosphorylation in apoptosis was evaluated in Kv2.1-expressing CHO cells under oxidant exposure conditions that are normally sublethal to vector-expressing control cells (13). A 15-min exposure to 30 μM DTDP was sufficient to induce apoptosis in ≈50% of WT Kv2.1-expressing cells (Fig. 5). However, the same oxidative insult was completely innocuous to CHO cells expressing Kv2.1(S800A) mutant channels. As a negative control, we assayed the viability of CHO cells expressing a nonconducting, double-pore mutant Kv2.1(W365C, Y380T) channel (12). As in cells expressing Kv2.1(S800A), the oxidant was not toxic to cells expressing the double-pore mutant channels. These data demonstrate the requirement for functional Kv2.1 channels containing the phosphorylatable amino acid residue S800 to complete the apoptotic program.
Fig. 5.
S800A mutation blocks Kv2.1-mediated apoptosis. CHO cells were cotransfected with EGFP plus pRBG4 vector and either Kv2.1, Kv2.1(S800A), or nonconducting Kv2.1(W365C, Y380T) and 24 h later exposed to 30 μM DTDP (15 min). Viability was assayed 24 h posttreatment by counting GFP+ cells and expressed as a percent of vehicle-treated control. Note that DTDP induced ≈50% cell death in Kv2.1-expressing CHO cells but was not lethal to either Kv2.1(S800A)- or Kv2.1(W365C, Y380T)-expressing CHO cells. Values represent the mean ± SEM viability from three separate, independent experiments (∗, P < 0.05; ANOVA/Dunnett).
Discussion
The results presented in this study reveal an important and previously unrecognized mechanism of Kv2.1 regulation that has a critical impact on apoptosis. Kv2.1 is subject to extensive phosphorylation and dephosphorylation reactions, which alter the functional properties of this channel by influencing gating, and thereby, cell firing properties (18, 30). We have found, however, a phosphorylation target for p38 on the C-terminal of Kv2.1 that is vital for the apoptotic surge of K+ currents observed in apoptosis and the completion of the cell death program. Interestingly, a recent report by Park et al. (18) reported that S800 is a phosphorylation target, albeit by a then-unidentified kinase. They further showed that S800 was not one of several C-terminal sites subject to calcineurin-dependent dephosphorylation and did not participate in the graded regulation of Kv2.1 gating. We have found here that S800 is a unique p38 phosphorylation target on Kv2.1 that is required for channel trafficking and cell death.
Phosphorylation of S800 is not necessary, in and of itself, for normal Kv2.1 trafficking and plasma membrane insertion, as Kv2.1(S800A) mutant channels can be functionally expressed. Indeed, normal neurons overexpressing this construct simply have larger K+ currents, which explains why this mutated form of Kv2.1 cannot function as a dominant interfering channel during neuronal apoptosis. Nonetheless, our studies in CHO cells reveal that under injurious situations this site is critical for de novo channel insertion, resulting in the current surge necessary for cytoplasmic K+ loss and cell death.
Earlier studies revealed that the Kv2.1-mediated current surge during apoptosis is a result of the insertion of new channels into the plasma membrane (14), and the p38 MAPK signaling pathway is instrumental in this process (8, 9, 31). Interestingly, p38 activation by TNFα has also been implicated in the potentiation of tetrodotoxin-resistant Na+ currents in sensory neurons, although a direct phosphorylation of the channel by p38 has not been documented (32). The MAPK can phosphorylate Nav1.6-encoded Na+ channels, but in this case the resulting interaction produces a decrease, rather than an increase, in Na+ currents (33). In addition, another member of the MAPK family, ERK1/2, phosphorylates Kv4.2, a potassium channel responsible for A-type K+ currents in neurons (34, 35), resulting in a down-regulation of dendritic K+ currents in CA1 hippocampal pyramidal neurons (36). Collectively, this information indicates that MAPKs participate in cell signaling events that alter the contribution of voltage-gated ion channels to overall neuronal excitability. Our results demonstrate that p38 phosphorylation of Kv2.1 is required for an entirely different, but critical process: completion of the apoptotic program.
Several issues remain to be addressed. Primarily among them, the molecular mechanism linking p38-induced phosphorylation of Kv2.1 to the membrane insertion of this channel remains to be determined. We previously observed that cleavage and inactivation of the exocytotic soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins SNAP-25 and syntaxin was sufficient to prevent the apoptotic K+ current surge (14). Importantly, both of these proteins are known to directly interact with Kv2.1 (37): SNAP-25 with the cytoplasmic N-terminal (38), and syntaxin with two domains of the cytoplasmic C-terminal tail (39). Overexpression of syntaxin and Kv2.1 inhibits channel surfacing and leads to a decrease in Kv2.1-mediated current density (39). It is thus tempting to speculate that p38 phosphorylation of Kv2.1 leads to changes in its association with syntaxin or other proteins, promoting, in turn, exocytosis of channel containing vesicles. In addition, Kv2.1 subunits consistently colocalize with cortical neuron subsurface cisterns, compressed stacks of smooth endoplasmic reticulum situated ≈5–8 nm beneath the plasma membrane (40). Thus, the possibility exists for cisternae to serve as a nearby holding area for channels destined for the plasma membrane (41), with p38 phosphorylation serving as the molecular switch necessary to free Kv2.1 from cisternal retention during apoptosis.
In summary, we have identified a putative p38 phosphorylation target residue on the C-terminal of Kv2.1. Importantly, mutation of S800 to a nonphosphorylatable residue is sufficient to disrupt the apoptotic cascade initiated by oxidative injury and block cell death. The work described here illustrates a critical mechanistic link between oxidant-induced Zn2+ release (7), p38 MAPK activation (8, 9), the ensuing Kv2.1-mediated apoptotic K+ current surge (13, 14), and cell death. Thus, prevention of Kv2.1 phosphorylation by p38 is a potential novel therapeutic target for promoting neuronal survival during injurious oxidative stress conditions.
Materials and Methods
Plasmids and Site-Directed Mutagenesis.
The mammalian expression vector encoding WT Kv2.1 was the gift of J. Trimmer (University of California, Davis, CA). The polymyc-tagged Kv2.1 plasmid was from K. Takimoto (University of Pittsburgh, Pittsburgh, PA). The FLAG-tagged dominant negative Kv2.1(W365, Y380T) plasmid was provided by J. Nerbonne (Washington University, St. Louis, MO). S. Korn (National Institutes of Health, Bethesda, MD) provided the Kv2.1(I379C) vector. Mutagenesis of these cDNAs was performed with a QuikChange XL kit (Stratagene, La Jolla, CA) according to the manufacturer's directions. Primers containing the desired mutations (S800A, S800D, S800E) were obtained from Integrated DNA Technologies (Coralville, IA). Mutations were confirmed by sequencing. A plasmid encoding EGFP (pCMVIE-eGFP; Clontech, Palo Alto, CA) was used for the identification of positively transfected cells.
Tissue Culture.
CHO cells were plated at a density of 5.6 × 104 cells per well on coverslips in 24-well plates 24 h before transfection. Cells were treated for 4 h in serum-free medium (F12 nutrient medium with 10 mM Hepes) with a total of 1.2 μl of Lipofectamine (Invitrogen, Carlsbad, CA) and 0.28 μg of DNA per well (0.14 μg of both EGFP and potassium channel cDNA). Cells were briefly washed in MEM with Earle's salts containing 25 mM Hepes and 0.01% BSA. After transfection, cells were maintained in F12 medium containing FBS at 37°C, 5% CO2 for 48 h before recordings.
Cortical neurons were prepared from embryonic day 16 rat embryos and grown in 6-well plates according to McLaughlin et al. (8). Cultures were exposed to drug treatment procedures at 25–29 days in vitro. Cells were briefly washed in MEM with Earle's salts containing 25 mM Hepes and 0.01% BSA and maintained in D2C growth medium until harvesting. Cells were harvested 3 h posttreatment in lysis buffer [1% Triton X-100/0.1% SDS/0.25% Na deoxycholate/50 mM Hepes/150 mM NaCl/protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN), pH 7.5] after two washes with PBS. Cell lysate samples were combined in a 1:1 ratio with reducing sample prep buffer and incubated for 5 min at 100°C to denature proteins before gel electrophoresis.
Drug Treatment.
The apoptotic stimulus for the electrophysiological experiments in CHO cells consisted of a 5-min treatment with 25 μM DTDP at 37°C, 5% CO2. The DTDP-containing solution was then removed and replaced with fresh F12 medium containing 10 μM 1–3-boc-aspartyl (Ome)-fluoromethyl-ketone (BAF), a broad-spectrum cysteine protease inhibitor. BAF was necessary to maintain cells viable for electrophysiological recordings because Kv2.1-expressing cells are highly susceptible to DTDP-induced apoptosis (13). Cells were subsequently maintained in BAF-containing medium, and electrophysiological recordings were performed ≈3 h after oxidative injury. For channel insertion experiments, cells were first treated with 4 μM MTSET for 10 min to covalently block all Kv2.1(I379C) or Kv2.1(I379C, S800A) channels present on the plasma membrane surface before the usual DTDP exposure (14). The apoptotic stimulus for the biochemical experiments on rat primary cortical neurons consisted of a 10-min treatment with 100 μM DTDP at 37°C, 5% CO2 (7, 8). Cortical neurons were pretreated with either vehicle or the specific p38 MAPK inhibitor SB-293063 (20 μM for 10 min).
Electrophysiological Measurements.
Current recordings were performed on EGFP-positive cells by using the whole-cell patch clamp configuration technique as described (8). We observed that 97% of GFP-positive CHO cells cotransfected with Kv2.1 had measurable K+ currents. The intracellular electrode solution contained 100 mM K-gluconate, 10 mM KCl, 1 mM MgCl2, 1 mM CaCl2 × 2H2O, 10 mM Hepes; pH adjusted to 7.2 with concentrated KOH; 0.22 mM ATP was added and the osmolarity was adjusted to 280 mOsm with sucrose. The extracellular solution contained 115 mM NaCl, 2.5 mM KCl, 2.0 mM MgCl2, 10 mM Hepes, 0.1 mM 1,2-bis(2-aminophenoxy)ethane-N, N, N′, N′-tetraacetate acid, 10 mM d-glucose, and 0.1 mM tetrodotoxin; pH was adjusted to 7.2. Measurements were obtained under voltage clamp conditions with an Axopatch 1-D amplifier (Axon Instruments, Foster City, CA) and pClamp software (Axon Instruments) using 2- to 3-MΩ electrodes. Recording electrodes were pulled from 1.5-mm borosilicate glass (Warner Instruments, Hamden, CT) with a model P-97 mechanical pipette puller (Sutter Instruments, Novato, CA). Series resistance was partially compensated (80%) in all cases. Currents were filtered at 2 kHz and digitized at 10 kHz with Digidata software (Axon Instruments). Potassium currents were evoked with incremental 15-mV voltage steps to +35 from a holding potential of −70 mV. To determine current density values, steady-state current amplitudes were measured at 80 ms after the initiation of the +5-mV step and normalized to cell capacitance. All data are expressed as mean ± SEM, and statistical analysis was performed with InStat software (GraphPad, San Diego, CA).
Generation of Phospho-Specific Antibody.
To generate an antibody specific for the phosphorylated p38 MAPK consensus sequence of Kv2.1 (pKv2.1; SI Fig. 6), we synthesized the peptide C-KNHFESSPLPTS(p)PKFLR (Tufts University Core Facility, Boston, MA). The HPLC-purified peptide was conjugated to keyhole limpet hemocyanin (Pierce, Rockford, IL) with Sulfo-Link (44895; Pierce) following the manufacturer's protocol. Antiserum was generated in New Zealand White rabbits at Covance (Princeton, NJ). After prebleed screening, animals were initially injected with 1.5 mg of immunogen, followed by three monthly boosts of 0.75 mg and three monthly boosts of 0.5 mg. The crude serum was affinity-purified by using an Immunopure (A) IgG Purification Kit (Pierce) according to the methods described by the manufacturer, and the resulting product was used in the experiments reported here. A commercially available Kv2.1 polyclonal antibody (Alomone Labs), which was not targeted to the p38 site, was used as a control in immunoblots.
Cell-Free Kinase Assay.
Polymyc-Kv2.1- and polymyc-Kv2.1(S800A)-expressed protein from transfected CHO cells were immunoprecipitated by incubating cell lysates with an anti-myc-tag rabbit polyclonal antibody (40 μg/μl) (MBL International Corp., Woburn, MA) followed by a protein A/G PLUS-Agarose immunoprecipitation reagent (Santa Cruz Biotechnology, Santa Cruz, CA). The immunopurified substrate was incubated in 15 μl of kinase buffer (25 mM Hepes, pH 8.0/2 mM DTT/0.1 mM vanadate), 15 μl of Mg/ATP (50 mM MgCl and 50 μM ATP), and 50 μg activated p38α (Roche Protein Expression Group, Indianapolis, IN) for 1 h at 30°C. Reactions containing kinase buffer alone and kinase buffer plus Mg/ATP were used as controls. The reaction was stopped with sample preparation buffer (625 mM Tris/25% glycerol/2% SDS/0.01% bromophenol blue/5% β-mercaptoethanol) and incubated at 100°C for 5 min before SDS/PAGE and immunoblotting. Immunoblotting was performed with the pKv2.1 antibody (1:12,000) and a commercially available polyclonal Kv2.1 antibody (1:3,000) (Alomone Labs).
Electrophoresis and Immunoblotting.
SDS/PAGE was carried out by standard procedures using the Mini Protean 3 System (Bio-Rad, Hercules, CA). Equal amounts of cell lysate were separated by reducing 6% or 10% SDS/PAGE gels. Separated protein bands were transferred onto a 0.2-μm nitrocellulose membrane (Bio-Rad). The membranes were then blocked with 1% BSA in PBS with 0.05% Tween 20 (PBST) at room temperature for 1 h and probed with the appropriate primary antibodies diluted in PBST. Blots were then incubated with goat secondary antibody conjugated to HRP at room temperature for 1 h. Blots were visualized with a SuperSignal CL-HRP Substrate System (Pierce) and exposed to BioMax films (Kodak, New Haven, CT). In the cortical culture experiments, optical density measurements (Scion Image software, National Institutes of Health) were taken of both pKv2.1 immunoreactive bands, normalized to their respective Kv2.1 immunoreactive bands, and the resulting values were pooled.
Viability Assays.
CHO cells were plated and transfected with EGFP and pRBG4 vector plus Kv2.1, Kv2.1(S800A) or Kv2.1(W365C/Y380T) according to the protocol described above, with 0.28 μg of DNA added per well (0.14:0.13:0.0028 μg; EGFP/empty vector/potassium channel cDNA). Twenty-four hours after transfection, cells were exposed to either 30 μM DTDP or vehicle for 15 min at 37°C, 5% CO2. Twenty-four hours after treatment, counts of GFP-positive cells were obtained by a person blinded to the experimental treatment groups from 15 fields with a ×20 objective per coverslip; three coverslips were counted per condition in three independent experiments.
Supplementary Material
Acknowledgments
We thank D. Jimenez for preliminary electrophysiological measurements, M. Aras for helpful suggestions, and Drs. J. Trimmer, K. Takimoto, J. Nerbonne, and S. Korn for the gifts of plasmids. This work was supported by National Institutes of Health Grants NS043277 (to E.A.) and HL080632 (to E.S.L.) and Mental Retardation Research Center Grant HD018655 (to P.A.R. and L.H.).
Abbreviations
- DTDP
2,2′-dithiodipyridine
- MTSET
(2-trimethylammoniumethyl) methanethiosulfate.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610159104/DC1.
References
- 1.Yu SP. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 2.Bortner CD, Hughes FM, Jr, Cidlowski JA. J Biol Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- 3.Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- 4.Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. J Biol Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- 5.Zaks-Makhina E, Kim Y, Aizenman E, Levitan ES. Mol Pharmacol. 2004;65:214–219. doi: 10.1124/mol.65.1.214. [DOI] [PubMed] [Google Scholar]
- 6.Grishin A, Ford H, Wang J, Li H, Salvador-Recatala V, Levitan ES, Zaks-Makhina E. Am J Physiol. 2005;289:G815–G821. doi: 10.1152/ajpgi.00001.2005. [DOI] [PubMed] [Google Scholar]
- 7.Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin BA, Reynolds IJ. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin BA, Pal S, Tran MP, Parsons AA, Barone FC, Erhardt JA, Aizenman E. J Neurosci. 2001;21:3303–3311. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossy-Wetzel E, Talantova MV, Lee WD, Schlozke MN, Harrop A, Matthews E, Gotz T, Han J, Ellisman MH, Perkins GA, et al. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 10.Redman PT, Jefferson BS, Ziegler CB, Mortensen OV, Torres GE, Levitan ES, Aizenman E. Neuroscience. 2006;143:1–6. doi: 10.1016/j.neuroscience.2006.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakoshi H, Trimmer JS. J Neurosci. 1999;19:1728–1735. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malin SA, Nerbonne JM. J Neurosci. 2002;22:10094–10105. doi: 10.1523/JNEUROSCI.22-23-10094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal S, Takimoto K, Aizenman E, Levitan ES. Cell Death Differ. 2006;13:661–667. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obernauer JC, Cantley LC, Yaffe MB. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Mol Pharmacol. 1997;52:821–828. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- 17.Mohapatra DP, Trimmer JS. J Neurosci. 2006;26:685–695. doi: 10.1523/JNEUROSCI.4620-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 19.Yu SP, Kerchner GA. J Neurosci Res. 1998;52:612–617. doi: 10.1002/(SICI)1097-4547(19980601)52:5<612::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Kurz LL, Zuhlke RD, Zhang HJ, Joho RH. Biophys J. 1995;68:900–905. doi: 10.1016/S0006-3495(95)80266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HJ, Liu Y, Zuhlke RD, Joho RH. Biophys J. 1996;71:3083–3090. doi: 10.1016/S0006-3495(96)79502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Shikano S, Xiong Q, Li M. Proc Natl Acad Sci USA. 2004;101:16964–16969. doi: 10.1073/pnas.0404178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trimmer JS. FEBS. 1993;324:205–210. doi: 10.1016/0014-5793(93)81394-f. [DOI] [PubMed] [Google Scholar]
- 24.Scannevin RH, Murakoshi H, Rhodes KJ, Trimmer JS. J Cell Biol. 1996;135:1619–1632. doi: 10.1083/jcb.135.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung JJ, Li M. FEBS. 2005;272:3743–3755. doi: 10.1111/j.1742-4658.2005.04802.x. [DOI] [PubMed] [Google Scholar]
- 26.Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 27.Roder K, Koren G. Biol Chem. 2006;387:1237–1246. doi: 10.1515/BC.2006.153. [DOI] [PubMed] [Google Scholar]
- 28.Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF, McVey MJ, Legos JJ, et al. J Pharmacol Exp Ther. 2001;296:312–321. [PubMed] [Google Scholar]
- 29.Ren X, Shand SH, Takimoto K. J Biol Chem. 2003;278:43564–43570. doi: 10.1074/jbc.M302337200. [DOI] [PubMed] [Google Scholar]
- 30.Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. J Physiol (London) 2000;522:19–31. doi: 10.1111/j.1469-7793.2000.t01-2-00019.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal S, He K, Aizenman E. Pflügers Arch. 2004;448:296–303. doi: 10.1007/s00424-004-1256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin X, Gereau RW, IV J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD. J Neurosci. 2005;25:6621–6630. doi: 10.1523/JNEUROSCI.0541-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- 35.Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. Am J Physiol. 2006;290:C852–C861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- 36.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaelevski I, Chikvashvili D, Tsuk S, Singer-Lahat D, Kang Y, Linial M, Gaisano HY, Fili O, Lotan I. J Biol Chem. 2003;278:34320–34330. doi: 10.1074/jbc.M304943200. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald PE, Wang G, Tsuk S, Dodo C, Kang Y, Tang L, Wheeler MB, Cattral MS, Lakey JR, Salapatek AM, et al. Mol Endocrinol. 2002;16:2452–2461. doi: 10.1210/me.2002-0058. [DOI] [PubMed] [Google Scholar]
- 39.Leung YM, Kang Y, Gao X, Xia F, Xie H, Sheu L, Tsuk S, Lotan I, Tshushima RG, Gaisano HY. J Biol Chem. 2003;278:17532–17538. doi: 10.1074/jbc.M213088200. [DOI] [PubMed] [Google Scholar]
- 40.Du J, Tao-Cheng JH, Zerfas P, McBain CJ. Neuroscience. 1998;84:37–48. doi: 10.1016/s0306-4522(97)00519-8. [DOI] [PubMed] [Google Scholar]
- 41.O'Connell KM, Tamkun MM. J Cell Sci. 2005;118:2155–2166. doi: 10.1242/jcs.02348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.