Abstract
Most instances of colorectal cancer are due to abnormalities in the Wnt signaling pathway, resulting in nuclear accumulation of β-catenin. β-Catenin activates transcription of target genes primarily by associating with the T cell factor/lymphoid enhancer-binding factor (TCF/Lef) family of transcription factors. In this report, we use serial analysis of chromatin occupancy (SACO) to identify 412 high-confidence β-catenin targets in HCT116 colorectal carcinoma cells. Of these targets, 84% contained a consensus TCF motif and were occupied by TCF4 in vivo. Examination of the flanking 5-bp residues in each consensus revealed motif-specific enrichment at neighboring sites. β-Catenin binding was localized to the 5′ promoters, internal regions, and 3′ UTRs of protein-coding genes. Furthermore, 15 components of the canonical Wnt pathway were identified as β-catenin target genes, suggesting that feed-forward and feedback mechanisms exist to modulate the Wnt signal in colon cancer cells.
Keywords: chromatin immunoprecipitation, human genome
The β-catenin transcription coactivator is a key transducer of the Wnt signal in the canonical pathway. In the absence of Wnt, a multiprotein destruction complex containing glycogen synthase kinase β (GSK3β), axin, disheveled, casein kinase 1 (CK1), and adenomatous polyposis coli (APC) facilitates β-catenin degradation by the proteosome (for a review, see ref. 1). Upon Wnt binding to Frizzled/low-density lipoprotein receptor complexes, GSK3β is inactivated and β-catenin accumulates. β-Catenin is subsequently translocated into the nucleus where it activates transcription via association with T cell factor/lymphoid enhancer-binding factor (TCF/Lef; hereafter TCF) family of DNA binding proteins. Mutations in components of the canonical Wnt pathway, most notably in APC, are found in 90% of colorectal cancers (2–4). Because these mutations contribute to stabilization of β-catenin, the identification of nuclear β-catenin targets would facilitate an understanding of the pathophysiology of colorectal cancer. However, since the discovery of Wnt 25 years ago (5), only 30 direct β-catenin target genes have been identified in mammalian species (listed at http://www.stanford.edu/∼rnusse/wntwindow.html).
Some of the earliest β-catenin targets, including c-myc, cyclin D1, ultrabithorax, and siamois were identified through biochemical and genetic methods (6–10). Recently, three different approaches have been used to identify additional putative β-catenin targets: RNA profiling, serial analysis of gene expression (SAGE), and bioinformatic analysis of TCF4 enhancer elements (11–14). Although RNA profiling and SAGE have identified hundreds of transcripts that are affected by Wnt signaling (12, 13) or are differentially expressed in transformed versus normal colon (14, 15), neither of these technologies identify direct β-catenin targets. Hallikas et al. (11) recently developed a computational enhancer element locator to predict Wnt target genes and identified hundreds of potential TCF4 enhancer elements. Only a few of these sites have been confirmed, however, and only a fraction is likely to bind TCF4 in vivo.
ChIP has emerged as a method of interrogating transcription factor-binding regions in vivo on a gene-by-gene basis (16). Two experimental methodologies were developed to provide genome-wide localization of transcription factor targets isolated by ChIP. The first involves using high-density tiled microarrays that can be probed with fluorescently labeled ChIP DNA (ChIP-on-chip). However, difficulties in interpreting microarray data may result in a failure to identify low-affinity or low-abundance targets (17, 18). Furthermore, repetitive elements, which constitute a significant fraction of the genome, are largely excluded in the design of current arrays. As an alternative to ChIP-on-chip, our laboratory and others have developed sequence-based techniques that identify and localize DNA fragments isolated by ChIP in an unbiased manner (19–24). Serial analysis of chromatin occupancy (SACO) is a method that combines ChIP with long SAGE (19, 25). In this approach, the immunoprecipitated DNA is ultimately represented by 20- to 22-bp genomic tags termed genomic signature tags (GSTs), that are mapped to a genomic database. Because sequence-based approaches do not require a microarray for interrogating genomic binding sites, every binding event in the genome can potentially be sampled, cataloged, and quantified.
In this report, we used SACO to identify β-catenin genomic targets in human HCT116 colorectal carcinoma cells. HCT116 cells contain a mutated β-catenin allele that stabilizes the β-catenin protein. This provides an ideal cellular context to identify the set of β-catenin genomic targets that become activated upon stimulation of the canonical Wnt pathway. Here we report the identification of >400 previously unrecognized β-catenin target genes. This analysis greatly expands the list of direct β-catenin targets in mammalian cells.
Results
Generation of a β-Catenin SACO Library.
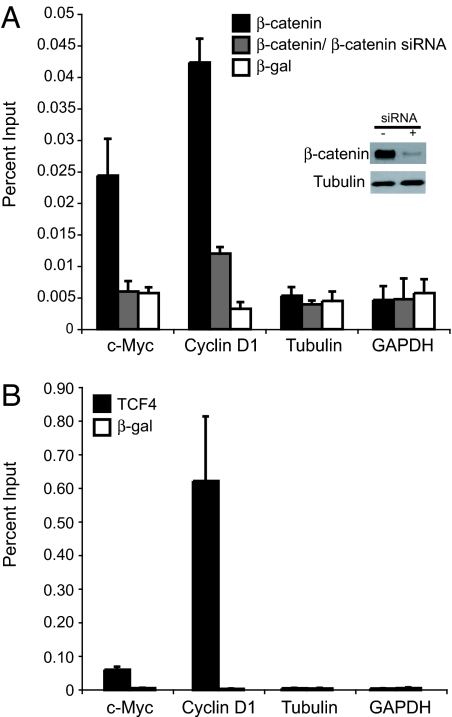
A key parameter for each SACO library is the quality of the antibody used for the ChIP. To test the efficacy of the β-catenin antibody, we assayed its binding to two known β-catenin target genes, c-myc and cyclin D1, in HCT116 cells. Enhanced β-catenin binding was seen at both promoters, relative to the control GAPDH and tubulin genes (Fig. 1A). Additionally, binding to c-myc and cyclin D1 was reduced significantly in cells incubated with an siRNA against β-catenin. TCF4, the protein that recruits β-catenin to c-myc and cyclin D1, was also present at these promoters, but not at the tubulin or GAPDH genes (Fig. 1B).
Fig. 1.
β-Catenin and TCF4 bind c-myc and cyclin D1 promoters in HCT116 colorectal carcinoma cells. (A) Real-time PCR analysis of DNA fragments precipitated in a ChIP assay by using a β-catenin antibody (black bars) or a β-galactosidase antibody (white bars) as a control. Primers designed to the 5′ promoters of c-myc and cyclin D1 were used to detect specific β-catenin binding, and primers designed to the 5′ promoters of tubulin and GAPDH were used to detect nonspecific interactions. As an additional control, cells were incubated with siRNAs to β-catenin for 48 h before ChIP. (Inset) A Western blot demonstrates that β-catenin protein is diminished in siRNA treated cells, whereas tubulin levels remain unchanged. β-Catenin binding to c-myc and cyclin D1 is lowered in siRNA-treated cells (gray bars). (B) The same as in A except that a TCF4 antibody was used in ChIP. In A and B, standard curves were derived from PCRs with serially diluted input chromatin DNA as the template. Data are represented as percent input. Error bars represent SEM.
We used this β-catenin antibody to generate a SACO library containing over two million GSTs. Of the 56,822 GSTs sequenced, 31,699 (56%) could be assigned to a unique position in the genome. GSTs that cannot be assigned uniquely most often map to repeat elements, and these were not considered further. GSTs that fell within 2 kb of one another were defined as comprising a cluster. This span was chosen because it represents the largest β-catenin chromatin fragments that were included in generation of the library (data not shown). Two thousand eight hundred and nine clusters contained two or more GSTs, and 412 clusters contained three or more GSTs.
ChIP Validation of β-Catenin Targets.
On the basis of the observation from our previous study that loci with a greater number of GSTs have a higher probability of confirming in repeat ChIP assays (19), we initially tested 36 randomly chosen loci containing three or more GSTs. A locus was considered positive for β-catenin binding if the signal using a β-catenin antibody was two-fold or greater than that using an antibody directed against β-galactosidase. This threshold was established because nontarget genes (tubulin and GAPDH) have equal background binding with the two antibodies (Fig. 1A). Thirty-five of these targets (97%) were positive in repeat screening (Fig. 2A). An additional 37 targets were then analyzed, all of which were confirmed to bind β-catenin (Fig. 2A). Overall, therefore, 72 of 73 (98.6%) targets containing three or more clustered GSTs were confirmed to bind β-catenin by a repeat ChIP assay. By extrapolation, we predict that β-catenin binds 407 of the 412 targets in our library characterized by three or more GSTs. Therefore, the false discovery rate in this population is ≈1%. Most of the targets in the SACO library are represented by either one or two GSTs. Within these populations, 50% (10 of 20) containing one GST confirmed in a repeat ChIP assay, and this percentage increased to 85% (17 of 20) in targets containing two GSTs (data not shown). Thus, even targets represented by only one or two GSTs can readily be identified by repeat ChIP assays.
Fig. 2.
Validation of β-catenin-binding sites identified in the SACO library. (A) Real-time PCR quantitation of DNA fragments precipitated in a ChIP assay with a β-catenin antibody (filled bars) or a β-galactosidase antibody as a control (open bars). Genomic targets were chosen at random from 412 loci containing three or more clustered β-catenin GSTs. Primers were designed to a 500-bp region surrounding the mean GST by using primer3 software (Massachusetts Institute of Technology). The mean GST is the midpoint of GST anchors that define the cluster. A locus on the x axis labeled with a known gene symbol indicates that the position of mean GST is within or near 2.5 kb of that gene. All other loci are indicated by the chromosomal position of the mean GST cluster. Data are represented as percent input. Regions interrogated as negative controls included tubulin, GAPDH, the cyclin D1 3′ UTR, and six 5-kb regions of the genome chosen at random, which lacked a consensus TCF motif (CTTTG A/T A/T). (B) The same as in A except that a TCF4 antibody was used to precipitate the putative targets in a ChIP assay. Two independent clusters in the villin 2 gene (VIL2) were confirmed. Error bars represent SEM.
β-Catenin is believed to bind DNA primarily through recruitment via TCF (26). However, other transcription factors, including FOXO, Pit1, and Prop1, can also recruit β-catenin (27–29). In colon cells, the predominant TCF is TCF4 (30). To identify SACO targets that were potentially capable of recruiting β-catenin via TCF4, we searched for a consensus TCF site, CTTTG A/T A/T, in 5-kb regions of DNA defined by clusters of three or more GSTs. Three hundred and forty-five of 412 (84%) high-confidence β-catenin targets also had the consensus TCF4 motif. We tested 34 of the 345 and found that 33 bound both β-catenin and TCF4 (Fig. 2B; labeled consensus TCF). The one target that did not bind TCF4, CACNA2D2, also failed to bind β-catenin and is a false positive. Primers designed to six 5-kb genomic regions lacking the TCF consensus motif were included as negative controls; these regions failed to bind TCF4. This unbiased analysis suggests that most β-catenin recruitment occurs through TCF in colon cancer cells; presumably, β-catenin binding to the remaining small fraction of targets occurs through an alternative mechanism. Sixty-seven (16%) of the high-confidence targets did not have a consensus TCF motif. Of 37 tested, 28 lacked significant TCF4 binding (Fig. 2B, labeled no consensus TCF). However, 9 of the 37 targets that lacked a consensus TCF4 motif nevertheless showed significant levels of TCF4 binding. In fact, one target (chr5:156824232) had the same level of binding as cyclin D1. TCF4 binding to this group of targets likely occurs through a nonconsensus motif or indirectly through an unrelated factor(s).
Characterization of TCF Motifs.
To establish the significance of the enrichment of the consensus TCF motif in our high-confidence targets, we first generated a background model of five sets of 412 5-kb DNA sequences centered around random NlaIII sites, The distribution of the consensus TCF motifs. CTTTGAA, CTTTGTT, CTTTGAT, or CTTTGTA, was significantly different in the high-confidence target sequences compared with each of the in silico mapped sets (all P values <0.05, average P value, 0.0092). A total of 1,016 TCF consensus motifs localized to regions defined by the β-catenin clusters. These motifs were found to occur at the following frequencies: CTTTGAA, 30.8%; CTTTGTT, 28.8%; CTTTGAT, 24.2%; and CTTTGTA, 16.2%. The flanking 5-bp windows of each consensus motif were examined by categorical data analysis to determine whether there was motif-specific enrichment for particular nucleotides. We indentified three flanking sites with consensus motif-specific nucleotide usage (positions +1, +2, and +4) (Table 1). As previously reported in a TCF4 binding site selection study (11), we found that a G was enriched at the +1 position in the CTTTGAT consensus (Table 1). There was also a perference for particular nucleotides at all three flanking sites for CTTTGAA and at positions +1 and +2 for CTTTGTT (Table 1). Thus, nucleotides outside the TCF consensus may provide additional information for each motif.
Table 1.
Enrichment of flanking nucleotides for TCF consensus motifs
Identification of flanking sites to TCF consensus motifs for which there was a significant motif-specific enrichment. Position +1 refers to the nucleotide immediately 3′ to the seventh base in the motif. For each site for which there was a significant association between motif and nucleotide usage, the most enriched site(s) is reported on the basis of contribution from the adjusted residuals.
*, P < 0.05;
**, P < 0.0001.
Genomic Location of Putative β-Catenin Binding Sites.
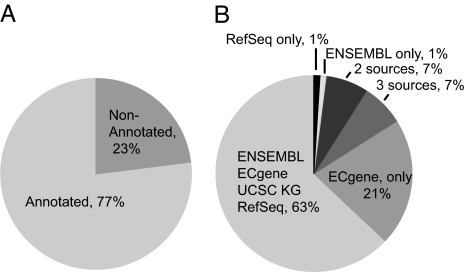
Several databases were interrogated to determine whether β-catenin binding occurs near known regions of transcription. Of the 412 high-confidence β-catenin GST clusters, 77% were within 2.5 kb of annotation supported by ECgene, RefSeq, Ensembl, or University of California, Santa Cruz known gene databases (Fig. 3A). Within the group of 316 targets mapping to annotated regions, most were supported by evidence from all four databases, however a significant portion (21%) were supported only by ECgene transcripts (Fig. 3B). This is likely due to the large number of ESTs that are unique to ECgene. Of the 192 protein-coding (RefSeq) genes in the high-confidence β-catenin target list, the majority (73%) had binding sites at internal positions, defined as a location at least 2.5 kb inside the transcriptional start and stop sites. Of the predicted β-catenin binding sites, 15% fell within the 5′ UTR, defined as an inclusive region 2.5 kb upstream and downstream of the transcription start; 11% were within the 3′ UTR, defined as an inclusive region 2.5 kb upstream and downstream of the transcription stop. In addition, 13% of β-catenin binding sites were within 5 kb of a CpG island. β-Catenin, therefore, is similar to other mammalian transcription factors, including CREB, myc, SP1, and p53, in binding to regions of genes outside the 5′ promoter region (19, 31). Particularly striking, however, was the large percentage of genes that had internal β-catenin binding sites. Whether these sites regulate alternative promoters or function cooperatively with the 5′ promoter has not been determined.
Fig. 3.
Distribution of high-confidence β-catenin targets relative to annotation features. (A) Mapping of β-catenin targets containing three or more GSTs within 2.5 kb of annotation features defined in ECgene, Ensembl, RefSeq, and University of California, Santa Cruz known gene databases. Of the targets, 77% align to features in these databases. (B) Assignment of clusters within annotation features listed in A.
We organized the 192 high-confidence protein-coding β-catenin target genes by function [supporting information (SI) Table 2]. We then used SAGE data from the Cancer Genome Anatomy Project (CGAP) to determine whether these genes were associated with transcripts (http://cgap.nci.nih.gov). Because our library was constructed from HCT116 cells, which were also the source of a SAGE library, we were able to align the SAGE tags with our SACO loci. Of 192 protein-coding genes, 105 had at least one tag identified by SAGE, suggesting that more than half of the genes bound by β-catenin are expressed in HCT116 cells (SI Table 2).
Two hundred one high-confidence targets did not map to protein-coding genes in the RefSeq database. We used the Functional Annotation of Mouse 3 (FANTOM3) database to determine whether these β-catenin binding sites were associated with transcripts (http://fantom.gsc.riken.go.jp/). The FANTOM3 data clusters groups of closely associated capped analysis of gene expression (CAGE) tags to define transcriptional start sites (TSSs) (32). Of the noncoding β-catenin targets, 44% had at least one TSS within a 5-kb region of a GST cluster, suggesting that these noncoding targets were also transcribed.
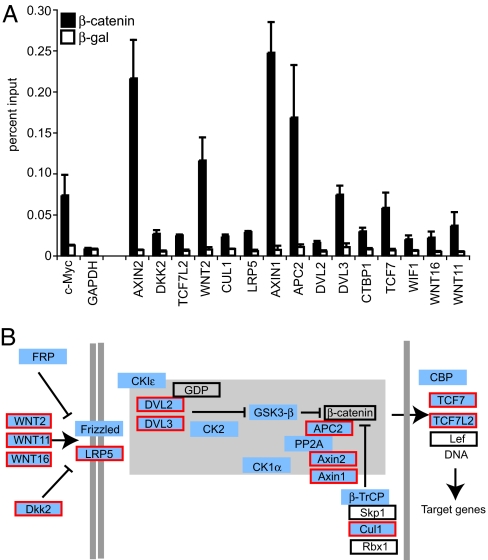
We then determined whether components of particular signaling pathways were over-represented in the β-catenin SACO library. By using pathway data from the Kyoto Encyclopedia of Genes and Genomes (KEGG), BioCarta, and the Cancer Pathway Database (cPath), we found that 113 potential β-catenin genomic targets, represented by one, two, or three or more GSTs, belong to the Wnt signaling pathway. We tested 24 of these targets for β-catenin binding by ChIP and found that 15 displayed significant interaction (Fig. 4A). Each layer of the Wnt signaling pathway is represented in this target list, from cell surface receptors to nuclear transcription factors (Fig. 4B). This is consistent with reports demonstrating that components of the Wnt pathway are themselves β-catenin targets (33, 34) and suggests that autoregulation within the Wnt pathway is more extensive than previously appreciated. Components of the hedgehog and TGFβ pathways were also significantly enriched in our library.
Fig. 4.
β-Catenin binds targets in the canonical Wnt signaling pathway. (A) Real-time PCR quantitation of fragments precipitated in a ChIP assay using a β-catenin antibody (filled bars) or a β-galactosidase antibody (open bars) as a control. Targets that have significant binding are indicated on the x axis. The amount of DNA precipitated is indicated on the y axis and is relative to levels obtained with input chromatin serially diluted to generate standard curves. The c-myc promoter is a positive control, and GAPDH is a negative control for the ChIP. Error bars represent SEM. (B) Overlay of putative β-catenin SACO targets on the Wnt pathway. The Wnt signaling diagram is a modified version from the Kyoto Encyclopedia of Genes and Genomes (KEGG) available at the Cancer Genome Anatomy Project (CGAP) pathways web page. Targets identified in the SACO library are blue. Targets in blue with a red border were confirmed by ChIP in A. Proteins in an open box were not identified as targets in the SACO library. Proteins in the β-catenin destruction complex are represented within the gray-shaded region.
Discussion
Although bioinformatic characterization of transcription factor binding motifs can have important predictive value, experimental approaches are still required to localize transcription factor binding sites on a genome-wide level. Hybridization-based methods (i.e., ChIP-on-chip) have been used most extensively to accomplish this goal. Sequence-based methodologies (which couple ChIP and long SAGE), are somewhat more laborious than ChIP-on-chip approaches but are also potentially less biased and avoid limitations involving hybridization specificity, genome coverage, and reproducibility (17). Additionally, the decreasing price of DNA sequencing may soon equalize the costs of the two approaches (35). Here, we use a sequence-based approach, SACO, to identify β-catenin-binding sites in HCT116 colorectal carcinoma cells. We provide a list of 412 new high-confidence targets bound by β-catenin in vivo, which expands the number of direct β-catenin binding targets in mammalian cells by >15-fold.
The primary mechanism of β-catenin-mediated transcriptional activation involves association with members of the TCF family of transcription factors bound at specific chromosomal locations. Presumably, only a fraction of the potential TCF binding sites is actually occupied, however, and only a fraction of these recruit β-catenin. Moreover, β-catenin can associate with DNA-binding proteins other than TCF. Our finding that 84% of the 412 β-catenin targets colocalize with a consensus TCF motif and bind TCF4 in vivo supports the view that TCFs are responsible for most β-catenin-dependent transcriptional signaling. Furthermore, this association supports the observation that dominant negative TCF4 disrupts the proliferative capacity of both colon cancer cells and the colonic crypt (13). Nine β-catenin targets lacked a TCF motif but still bound TCF4 by ChIP, suggesting that TCF4 either recognizes a novel binding element that deviates significantly from the consensus sequence in these instances or that it can bind DNA indirectly through some other factor. Other targets lack a TCF motif and fail to bind TCF4 in a ChIP assay. Efforts to identify factors responsible for β-catenin recruitment at these TCF4-independent sites are underway.
The first TCF (TCF1) was identified as a factor that bound an enhancer downstream of exon IX in the CD3-ε gene (36). TCF1 is found only in T cells, and its binding to the CD3-ε enhancer was conferred by a AACAAAG motif (this motif is the inverse of the CTTTGTT motifs described above). What has been elusive, due to the paucity of known β-catenin/TCF direct targets, is whether flanking residues also contribute to β-catenin/TCF binding. Our analysis allowed us to examine a large collection of target genes identified empirically. Surprisingly, different TCF consensus motifs were associated with different preferred flanking residues. We propose that this enrichment offers an additional layer of specificity for β-catenin/TCF targets. Whether these motifs bind TCF with different affinities has not been determined. It is possible that variant motifs may specify classes of genes that are under distinct developmental regulation.
β-Catenin binding was detected within annotated regions of the genome and in regions that lack known transcripts. For protein-coding genes, putative β-catenin-binding elements localized within 5′ promoters, 3′ untranslated regions, and internal positions. A regulatory role for β-catenin binding at the 5′ ends of protein-coding genes has been established for many genes that were identified in our library, including c-met, c-myc, cyclin D1, PPARδ, c-jun, axin2, and others (10, 33, 37, 38). In a previous report, we characterized a β-catenin binding site that localized to the 3′ UTR of the E2F4 gene (39). We found that β-catenin recruitment to this site by TCF4 resulted in production of an E2F4 antisense transcript. Importantly, β-catenin-dependent induction of the E2F4 antisense transcript reduced levels of E2F4 protein and decreased the association of E2F4 with several target promoters. Interestingly, a confirmed β-catenin binding site also localized to the 3′ end of the c-myc gene (data not shown). Binding to this site raises the possibility that c-myc may also be regulated by an antisense transcript, as has been shown for N-myc (40). We used CAGE data from the FANTOM3 consortium to determine whether other high-confidence target genes with 3′ β-catenin-binding sites were associated with antisense transcripts. Of the 3′ targets, 76% have CAGE evidence for antisense transcription, suggesting that β-catenin regulation of these transcripts may be a common phenomenon. The propensity for β-catenin to occupy internal positions within protein-coding genes is also of interest. In a separate SACO screen using TFIIB to identify core promoter elements, we noticed a similar abundance of internal sites (G.S.Y. and S.M., unpublished work). In that study, we found that internal TFIIB sites demarcated promoters that drove expression of novel transcripts. The′ FANTOM3 consortium similarly provided evidence for internally derived RNAs. Although β-catenin has been found to regulate 5′ promoters through binding sites in the first intron, the function of the internal β-catenin binding sites remains an open question.
It is recognized that this study does not provide a comprehensive list of β-catenin target genes. Absent from our high-confidence list, for example, were the well characterized targets c-myc and cyclin D1. However, these targets were, in fact, represented by single GSTs. Because these two genes bind β-catenin in ChIP assays (Fig. 1A), it is likely that deeper sequencing would have increased their representation, and also identified additional targets. We therefore searched all 33,699 loci in our library for targets listed on the Nusse website, the most comprehensive compilation of β-catenin regulated genes. Thirty-six putative targets from the Nusse list were represented in the β-catenin SACO library by one or multiple GSTs, and 15 of these were tested for β-catenin-binding by ChIP. Nine had significant levels of β-catenin binding, demonstrating that SACO library contains many previously identified β-catenin target genes.
An extensive analysis of gene targets in the β-catenin SACO library revealed a striking enrichment for components of the canonical Wnt signaling pathway. This finding suggests that the pathway itself acts as a rheostat to regulate the Wnt signal. For example, the colonic crypt is divided into discrete zones of proliferating and differentiating cells (41, 42). Transit-amplifying cells, produced by multipotent stem cells occupying the base of the crypt, proliferate rapidly in the presence of Wnt. We predict that expression of positive regulators of the pathway, such as DVLs, Wnts, and LRPs, would potentiate the action of Wnt signaling. It is somewhat surprising that negative regulators of the Wnt pathway, including Axins, Ctbp1, and Cul1, were also found as direct β-catenin target genes. Expression of these factors may be envisioned to aid in ceasing the Wnt signal. This is necessary for proliferative progenitors to differentiate into the enterocytes, goblet cells, and enteroendocrine cells. For colorectal carcinomas that develop by prolonged Wnt/β-catenin signaling, we propose that positive regulators of the pathway would be preferentially expressed over negative regulators. Analyzing the expression of these genes in normal and Min mice (which develop colon cancer) may allow us to test this hypothesis (43, 44).
In summary, we provide evidence that the number of genomic β-catenin targets is much greater than previously envisioned. Because the targets in this screen were identified in a colon cancer cell line with hyperactive Wnt signaling, a deeper analysis of these targets should provide further insight into the mechanisms underlying colorectal carcinogenesis. In addition, it will be of interest to determine whether these targets are functional in other Wnt signaling paradigms (i.e., stem cell self-renewal, hematopoesis, and neurogenesis).
Materials and Methods
Cell Culture.
HCT116 cells (ATCC no. CCL-247) were grown in McCoy's 5A modified medium [American Type Culture Collection (ATCC)]. Media was supplemented with 10% FBS (HyClone, Logan, UT), 100 units/ ml penicillin, 100 units/ ml streptomycin, and 5 mM l-glutamine. Cells were maintained at 37°C and 5% CO2.
ChIP.
Antibodies used for ChIP included: 3 μg of anti-β-catenin (no. 610154; BD Transduction, Lexington, KY), 3 μg of anti-TCF4 (no. 05-511; Upstate Biotechnology, Lake Placid, NY), 3 μg of anti-β-galactosidase (Z378B; Promega, Madison, WI), and 6 μg of rabbit anti-mouse IgG (no. 315-005-003; Jackson ImmunoResearch, West Grove, PA). ChIP assays contained 5 × 106 cells and were conducted as described in ref. 19 with the following modifications: Chromatin in formaldehyde-fixed cell lysates was sonicated to an average size of 600 bp by using a Misonex cup horn sonicator (5 × 20 sec, 140- to 150-W pulses with 60-sec rest intervals on ice). Lysates were clarified by centrifugation at 20,800 × g for 10 min at 4°C and incubated with primary antibody overnight at 4°C. Secondary rabbit anti-mouse IgG was then added for 6 h. Immunocomplexes were captured with BSA/glycogen-blocked protein A Sepharose (Repligen, Cambridge, MA), washed, and the bead pellet was resuspended in 100 μl of TE, pH 8.0. RNA was digested for 30 min at 37°C with 50 μg of RNase A (Roche, Indianapolis, IN). SDS was added to 0.25% and proteins were digested with 250 μg of proteinase K (Roche) for 12 h at 37°C. Formaldehyde cross-links were reversed at 65°C for 6 h. Samples were phenol/chloroform-extracted, and the DNA was precipitated in 100% ethanol. DNA fragments were quantified by RT-PCR, as described in ref. 19. Reactions were run on an Opticon 346 thermocycler (MJ Research, Cambridge, MA) for one cycle at 95°C for 35 sec and for 50 cycles at 94°C for 15 sec and at 70°C for 40 sec. ChIP data are presented as percent input by dividing values obtained from total input multiplied by 100. Primers were designed with Massachusetts Institute of Technology's Primer3 software. Primers were synthesized at Integrated DNA Technologies (Coralville, IA) and sequences are available upon request. A modified version of the fast-ChIP (45) has also been successfully used to precipitate β-catenin target genes (39).
SACO Library.
For a complete description of the protocol used to construct the SACO library, see ref. 19. For the β-catenin SACO library, pZERO-2 (Invitrogen, Carlsbad, CA) was used as the sequencing vector. The SphI site in the kanamycin resistance gene of pZER0–2 was mutated with the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). The raw data for the 31, 699 mapped β-catenin GSTs is available upon request.
Sequencing.
Sequencing was performed at High-Throughput Sequencing Solutions at the University of Washington, Seattle.
Western Blot.
Western blots were conducted as described in ref. 46. Dilutions of antibodies used were as follows: 1:1,000 β-catenin (no. 610154; BD Tansduction) and 1:10,000 α-tubulin (T3526; Sigma, St. Louis, MO).
siRNAs and Transfection of HCT116 Cells.
SMARTpool siRNAs to β-catenin were from Dharmacon (Lafayette, CO). siRNA (100 pmols) were transfected into 5 × 106 HCT116 cells with the Nucleofector Kit V (Amaxa, Gaithersburg, MD). siRNAs were incubated with cells for 48 h before either ChIP or Western blotting. Transfections with GFP indicated that >90% of cells were transfected at the time of analysis.
Bioinformatic Analysis.
Initial processing and placement of the GSTs followed the pipeline created by Impey et al. (19). Chromosome locations, start, end, and orientation of human RefSeq features aligned to the hg17 build of the human genome were obtained from the UCSC Annotation database. Known Genes were obtained from the University of California, Santa Cruz. ECGene data were downloaded from http://genome.ewha.ac.kr/ECgene/download/v1.2_ECgene in November of 2004. Ensembl gene annotations were downloaded from http://www.ensembl.org/multi/martview in November 2004. Gene ontology (GO) associations were made with RefSeq genes by using GO data from NCBI (ftp://ftp.ncbi.nih.gov/gene/DATA) downloaded on July 17, 2006. The Fantom3 CAGE data from http://gerg01.gsc.riken.jp/cage_analysis/export/hg17prmtr/ctss_summary.tsv.bz2 (March 11, 2005) was used for transcriptional start site analysis.
Supplementary Material
Acknowledgments
We thank Gail Mandel, Soren Impey, and members of the R.H.G. laboratory for useful discussions on SACO. This research was supported by grants from the National Institutes of Health.
Abbreviations
- GST
genomic signature tag
- SACO
serial analysis of chromatin occupancy
- SAGE
serial analysis of gene expression
- TCF/Lef
T cell factor/lymphoid enhancer binding factor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611576104/DC1.
References
- 1.Clevers H. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Nagase H. Hum Mutat. 1993;2:425–434. doi: 10.1002/humu.1380020602. [DOI] [PubMed] [Google Scholar]
- 3.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 4.Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. Proc Natl Acad Sci USA. 1997;94:10330–10334. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nusse R, Varmus HE. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 6.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu SC, Grosschedl R, Bienz M. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 8.Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 10.Tetsu O, McCormick F. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 11.Hallikas O, Palin K, Sinjushina N, Rautiainen R, Partanen J, Ukkonen E, Taipale J. Cell. 2006;124:47–59. doi: 10.1016/j.cell.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, et al. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 14.Buckhaults P, Rago C, St Croix B, Romans KE, Saha S, Zhang L, Vogelstein B, Kinzler KW. Cancer Res. 2001;61:6996–7001. [PubMed] [Google Scholar]
- 15.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 16.Das PM, Ramachandran K, vanWert J, Singal R. Biotechniques. 2004;37:961–969. doi: 10.2144/04376RV01. [DOI] [PubMed] [Google Scholar]
- 17.Buck MJ, Lieb JD. Genomics. 2004;83:349–360. doi: 10.1016/j.ygeno.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Draghici S, Khatr P, Eklund AC, Szallasi Z. Trends Genet. 2006;22:101–109. doi: 10.1016/j.tig.2005.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Bhinge AA, Morgan XC, Iyer VR. Nat Methods. 2005;2:47–53. doi: 10.1038/nmeth726. [DOI] [PubMed] [Google Scholar]
- 23.Roh TY, Cuddapah S, Zhao K. Genes Dev. 2005;19:542–552. doi: 10.1101/gad.1272505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roh TY, Ngau WC, Cui K, Landsman D, Zhao K. Nat Biotechnol. 2004;2:1013–1016. doi: 10.1038/nbt990. [DOI] [PubMed] [Google Scholar]
- 25.Saha S, Sparks AB, Rago C, Akmaev V, Wang CJ, Vogelstein B, Kinzler KW, Velculescu VE. Nat Biotechnol. 2002;20:508–512. doi: 10.1038/nbt0502-508. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MD, Nusse R. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 27.Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, et al. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 28.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 29.Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, et al. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- 30.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 31.Cawley S, Bekiranov S, Ng HH, Kapranov P, Sekinger EA, Kampa D, Piccolboni A, Sementchenko V, Cheng J, Williams AJ, et al. Cell. 2004;116:499–509. doi: 10.1016/s0092-8674(04)00127-8. [DOI] [PubMed] [Google Scholar]
- 32.Carninci P, Sandelin A, Lenhard B, Katayama S, Shimokawa K, Ponjavic J, Semple CA, Taylor MS, Engstrom PG, Frith MC, et al. Nat Genet. 2006;38:626–635. doi: 10.1038/ng1789. [DOI] [PubMed] [Google Scholar]
- 33.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Shendure J, Mitra RD, Varma C, Church GM. Nat Rev Genet. 2004;5:335–344. doi: 10.1038/nrg1325. [DOI] [PubMed] [Google Scholar]
- 36.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. EMBO J. 1991;10:123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Proc Natl Acad Sci USA. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He TC, Chan TA, Vogelstein B, Kinzler KW. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yochum GS, Cleland R, McWeeney S, Goodman RH. J Biol Chem. 2007;282:871–878. doi: 10.1074/jbc.M609391200. [DOI] [PubMed] [Google Scholar]
- 40.Krystal GW, Armstrong BC, Battey JF. Mol Cell Biol. 1990;10:4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sancho E, Batlle E, Clevers H. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 42.Rizvi AZ, Wong MH. Stem Cells. 2005;23:150–165. doi: 10.1634/stemcells.2004-0096. [DOI] [PubMed] [Google Scholar]
- 43.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 44.Moser AR, Pitot HC, Dove WF. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 45.Nelson JD, Denisenko O, Sova P, Bomsztyk K. Nucleic Acids Res. 2006;34:e2. doi: 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Wang SY, Nottke AC, Rocheleau JV, Piston DW, Goodman RH. Proc Natl Acad Sci USA. 2006;103:9029–9033. doi: 10.1073/pnas.0603269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.