Abstract
The ligands for programmed cell death 1 (PD-1), an immunoinhibitory receptor belonging to CD28/cytotoxic T lymphocyte antigen 4 family, are PD-1 ligand 1 and 2 (PD-Ls). Recent reports suggest that the aberrant expression of PD-Ls on tumor cells impairs antitumor immunity, resulting in the immune evasion of the tumor cells. Although an inverse correlation between the expression level of PD-Ls and patients' prognosis has been reported for several malignant tumors, the follow-up period was limited because of the lack of the antibody (Ab) applicable to paraffin-embedded specimens. Here we generated a new Ab against PD-1 ligand 1 (PD-L1) and analyzed the expression level of PD-Ls in human ovarian cancer using paraffin-embedded specimens. Patients with higher expression of PD-L1 had a significantly poorer prognosis than patients with lower expression. Although patients with higher expression of PD-1 ligand 2 also had a poorer prognosis, the difference was not statistically significant. A significant inverse correlation was observed between PD-L1 expression and the intraepithelial CD8+ T lymphocyte count, suggesting that PD-L1 on tumor cells directly suppresses antitumor CD8+ T cells. Multivariate analysis showed the expression of PD-L1 on tumor cells and intraepithelial CD8+ T lymphocyte count are independent prognostic factors. The PD-1/PD-L pathway can be a good target for restoring antitumor immunity in ovarian cancer.
Keywords: costimulation, tumor immunity, immunohistochemistry
Recent developments in treatment modalities including wide resection-based surgery and new chemotherapy regimens have achieved a marked improvement in the short-term survival of patients with ovarian cancer (1–3). Nevertheless, the long-term prognosis in advanced cases remains unsatisfactory, requiring a new paradigm in the treatment strategy. Various prognostic factors have been proposed and used clinically to predict the clinical course and to aid clinical decision-making in ovarian cancer patients. Currently, the clinical staging system of the International Federation of Gynecology and Obstetrics, which focuses on the extent of the disease, is extensively used to predict the prognosis of the patients (4). However, even using this system, prediction of the long-term prognosis, especially of late recurrence after remission, remains difficult, suggesting a need to introduce other parameters such as sensitivity to chemotherapy and the strength of antitumor immune response. Several reports have shown the significance of tumor-infiltrating lymphocytes (TILs) as a prognostic factor in malignant tumors such as cutaneous melanoma, colorectal cancer, esophageal cancer, renal cancer, and ovarian cancer, suggesting that immunological parameters are significant and useful in assessing the prognosis of cancer patients (5–9). However, there are few reports suggesting that a specific molecule can represent the extent of the host–tumor immunity.
It is usually assumed that, although tumors are persistently exposed to host immune attack, they evade this attack via an immunological phenomenon termed as “tumor immune escape.” A unique feature of this phenomenon is that tumors frequently use physiological immunosuppressive mechanisms to escape from host immunity. For example, tumors secrete various immunosuppressive factors such as transforming growth factor β and the soluble MHC class I chain-related molecule (10–12), express immunosuppressive molecules such as Fas ligand (13), or induce the expression of cytotoxic T lymphocyte antigen 4 on T cells (14, 15). Recently, programmed cell death 1 (PD-1), an immunoinhibitory receptor belonging to the CD28 family, has been found to play a critical role in the tumor immune escape (16, 17). PD-1 expressed on activated T and B cells inhibits their activation by recruiting protein tyrosine phosphatase SHP-2 (18–20). Two ligands for PD-1, PD-1 ligand 1 (PD-L1; B7-H1) and PD-1 ligand 2 (PD-L2; B7-DC), have been identified based on the similarity to other B7 family molecules (21–24). PD-L1 is expressed on T cells, B cells, macrophages, dendritic cells, and some nonimmune cells and is up-regulated after their activation. PD-L2 is regulated more tightly and is expressed mainly on activated macrophages and dendritic cells. PD-1 ligand 1 and 2 (PD-Ls) expressed on antigen-presenting cells have been shown to induce T cell anergy or apoptosis via PD-1 on T cells, whereas PD-L1 expressed on peripheral tissues directly suppresses self-reactive lymphocytes (16, 17).
It was recently reported that PD-Ls are also expressed in several malignant tumors including carcinomas of the esophagus, kidney, lung, and brain. Moreover, the expression levels of these molecules have been shown to correlate with the prognosis of the patient in some cases (25–29). However, most of the studies have been conducted on frozen specimens because of the lack of an appropriate anti-human PD-L1 antibody (Ab) that can stain PD-L1 on formalin-fixed, paraffin-embedded specimens, which is essential for the long-term follow-up. Thompson et al. (30) examined the expression of PD-L1 on paraffin-embedded specimens of renal cell carcinoma using an original antigen retrieval method. However, the percentage of patients with PD-L1-positive tumors is significantly lower than that reported in their previous study on frozen specimens, suggesting that their result may not reflect the actual expression of PD-L1 (28, 30). Although it is also reported that PD-L1 is expressed in ovarian cancer using a small number of cases (25, 31), its clinical significance as a prognostic factor has not yet been explored. Moreover, there is little information on PD-L2 expression in tumors, except for esophageal and lung cancer (26, 32).
In the present study we generated a new Ab against human PD-L1 that strongly recognizes human PD-L1 protein on formalin-fixed, paraffin-embedded specimens and examined the long-term prognostic value of PD-L1 expression in ovarian cancer. In accordance with former studies on other cancers using frozen specimens, patients with PD-L1-positive tumors had a significantly poorer prognosis. A significant inverse correlation was found between PD-L1 expression and intraepithelial CD8+ T lymphocyte count, indicating that PD-L1 on tumor cells inhibit the host–tumor immunity and facilitate the immune evasion of the tumors. These results suggest that the PD-L1 expression is a reliable prognostic parameter in ovarian cancer.
Results
Clinical Profiles of the Patients.
The average age of the patients was 55 years (range, 26–78; SD, 11.18 years). Of 70 patients, 27 (38.6%) were diagnosed as International Federation of Gynecology and Obstetrics stage I, 4 (5.7%) were diagnosed as stage II, 26 (37.1%) were diagnosed as stage III, and 13 (18.6%) were diagnosed as stage IV. Histological subtypes of the tumor comprised 28 (40.0%) cases of serous, 22 (31.4%) cases of clear cell, 11 (15.7%) cases of endometrioid, 2 (2.9%) cases of mucinous, and 7 (10.0%) cases of other types [supporting information (SI) Table 2].
Toward the end of the study, 29 (41.4%) patients had died of their disease, and 41 (58.6%) were alive. The mean follow-up period was 5.19 years (range, 0.07–11.36; SD, 3.0).
PD-Ls Expression and Patient Prognosis.
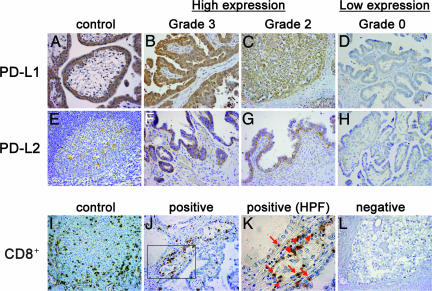
Among 70 tissue samples, the PD-L1 expression level was scored as 0, 1, 2, and 3 in 8 (11.4%), 14 (20.0%), 36 (51.4%), and 12 (17.1%) cases, respectively (Fig. 1 B–D and SI Table 2). PD-L2 expression was scored as 0, 1, 2, and 3 in 32 (45.7%), 12 (17.1%), 24 (34.3%), and 2 (2.8%) cases (Fig. 1 F–H). Accordingly, the proportion of high expression (scores 2 and 3) was 68.6% for PD-L1 and 37.1% for PD-L2 (Fig. 1 B, C, F, and G and SI Table 2).
Fig. 1.
Immunohistochemical staining of human ovarian cancer tissues using anti-PD-L1, PD-L2, and CD8+ Abs. (A–H) Representative staining patterns of serous adenocarcinomas with grade 3 (B and F), grade 2 (C and G), and grade 0 (D and H) expression of PD-L1 (B–D) and PD-L2 (F–H) are shown. Placenta (A) and tonsil (E) were used for positive control of PD-L1 and PD-L2, respectively. (I–L) Representative staining patterns of clear cell carcinomas with (J and K) or without (L) CD8+ TILs are shown. Arrows and arrowheads in K indicate intraepithelial and stromal CD8+ TILs, respectively. In I, spleen was used for positive control of CD8+. (Original magnification: ×200 for A–J and L, ×400 for K.)
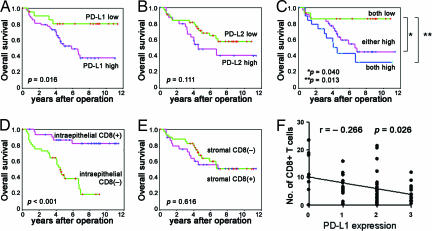
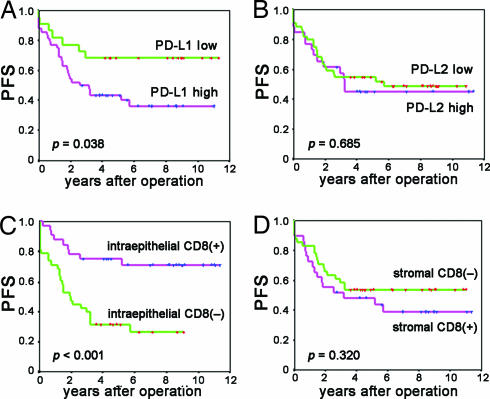
The Kaplan–Meier curve and log rank test showed that the 5-year survival rate of patients with high expression of PD-L1 was significantly worse than that with low expression (Figs. 2A and 3A and SI Table 3) (overall survival rate ± SD, low vs. high: 80.2 ± 8.9% vs. 52.6 ± 7.7%, P = 0.016; progression-free survival, low vs. high: 68.2 ± 9.9% vs. 43.5 ± 7.2%, P = 0.038). The overall survival period (years, mean ± SD) in the PD-L1 low vs. high group was 9.56 ± 0.82 vs. 6.48 ± 0.62, and the progression-free survival period was 6.12 ± 0.72 vs. 5.92 ± 0.99.
Fig. 2.
Overall survival analyses of patients with ovarian cancer according to the expression of PD-Ls and the presence of tumor-infiltrating CD8+ T lymphocytes. (A and B) Kaplan–Meier curves according to high or low expression of PD-L1 (A) and PD-L2 (B). (C) Kaplan–Meier curves according to combination of expressions of PD-L1 and PD-L2. (D and E) Kaplan–Meier curves according to positive or negative intraepithelial (D) and stromal (E) tumor-infiltrating CD8+ T cells. (F) Correlation between PD-L1 expression and intraepithelial CD8+ T lymphocytes.
Fig. 3.
Progression-free survival analyses of patients with ovarian cancer based on the expression of PD-Ls and the presence of tumor-infiltrating CD8+ T lymphocytes. (A and B) Kaplan–Meier curves according to expressions of PD-L1 (A) and PD-L2 (B). (C and D) Kaplan–Meier curves according to intraepithelial (C) and stromal (D) CD8+ T cells. The y axes represent progression-free survival (PFS).
The 5-year survival rate in patients with high PD-L2 expression was worse than in those with low PD-L2 expression; however, the difference was not statistically significant (Figs. 2B and 3B and SI Table 3) (overall survival rate ± SD, low vs. high: 68.4 ± 7.4% vs. 48.4 ± 10.7%, P = 0.111; progression-free survival, low vs. high: 54.6 ± 7.5% vs. 45.1 ± 9.9%, P = 0.685). The overall survival period (years, mean ± SD) of patients with low vs. high PD-L2 expression was 8.13 ± 1.01 vs. 5.13 ± 0.70, and the progression-free survival period was 6.12 ± 0.72 vs. 5.92 ± 0.99.
The overall survival rate of patients with low expression of both PD-L1 and PD-L2 was significantly better than the others, as shown in Fig. 2C (5-year survival rate of both-low group vs. both-high group: 86.2% vs. 43.2%, P = 0.013; both-low group vs. either-high group: 86.2% vs. 59.2%, P = 0.040). These data clearly demonstrate the strong inverse correlation between PD-L1 expression and the prognosis of the patients with ovarian cancer.
Correlation Between PD-Ls Expression and Clinicopathological Factors.
Among the various clinicopathological factors, primary tumor status [risk ratio (RR), 7.90; 95% confidence interval (C.I.), 2.73–22.83; P < 0.001], lymph node metastasis (RR, 3.24; 95% C.I., 1.50–7.00; P = 0.003), distant metastasis (RR, 2.28; 1.01–5.16; P = 0.047), and residual tumor status (RR, 4.54; 95% C.I., 2.17–9.50; P < 0.001) were significantly unfavorable factors in univariate analysis, whereas the age of the patient, histology, and adjuvant chemotherapy showed no correlation (SI Table 3).
Neither PD-L1 nor PD-L2 expression had any statistically significant correlation with various clinicopathological factors such as age, primary tumor status, lymph node metastasis, distant metastasis, histological type, residual tumor status, and chemotherapy (SI Table 2).
Multivariate Analysis.
Multivariate analysis using the Cox hazard model revealed that PD-L1 was an independent poor prognostic factor for both overall and progression-free survival (Table 1; RR, 4.26; 95% C.I., 1.39–12.94; P = 0.011 and RR, 2.57; 95% C.I., 1.11–5.93; P = 0.027), whereas PD-L2 expression was not. Other factors contributing to overall poor survival (Table 1) were tumor status (RR, 4.05; 95% C.I., 1.06–15.50; P = 0.041), lymph node metastasis (RR, 2.98; 95% C.I., 1.13–7.83; P = 0.027), and residual tumor status (RR, 8.83; 95% C.I., 2.74–28.48; P < 0.001), whereas other factors contributing to poor progression-free survival (Table 1) were lymph node metastasis (RR, 3.37; 95% C.I., 1.59–7.15; P = 0.002) and residual tumor status (RR, 5.99; 95% C.I., 2.92–12.27; P < 0.001). Taken together with the result of univariate analysis, PD-L1 expression is an independent poor prognostic factor.
Table 1.
Cox multivariate analysis demonstrating the independent risk factors for the cancer-specific death of the patients with ovarian cancer (n = 70)
| Variable | n | Overall survival, multivariate risk ratio | P value | Progression-free survival, multivariate risk ratio | P value |
|---|---|---|---|---|---|
| Analysis with PD-L1 expression | |||||
| PD-L1 expression | 0.011 | 0.027 | |||
| Low | 22 | 1 | 1 | ||
| High | 48 | 4.26 (1.39–12.94) | 2.57 (1.11–5.93) | ||
| Tumor status | 0.041 | ||||
| pT1 + pT2 | 31 | 1 | |||
| pT3 | 39 | 4.05 (1.06–15.50) | |||
| LN metastasis | 0.027 | 0.002 | |||
| pN0 | 56 | 1 | 1 | ||
| pN1 | 14 | 2.98 (1.13–7.83) | 3.37 (1.59–7.15) | ||
| Residual tumor | <0.001 | <0.001 | |||
| Optimal | 49 | 1 | 1 | ||
| Suboptimal | 21 | 8.83 (2.74–28.48) | 5.99 (2.92–2.27) | ||
| Analysis with intraepithelial CD8+ TIL | |||||
| Intraepithelial CD8+ TIL | <0.001 | 0.001 | |||
| Negative | 38 | 7.62 (2.76–21.08) | 3.75 (1.74–8.07) | ||
| Positive | 32 | 1 | 1 | ||
| LN metastasis | 0.040 | 0.001 | |||
| pN0 | 56 | 1 | 1 | ||
| pN1 | 14 | 2.72 (1.05–7.08) | 3.43 (1.61–7.34) | ||
| Residual tumor | 0.006 | <0.001 | |||
| Optimal | 49 | 1 | 1 | ||
| Suboptimal | 21 | 3.37 (1.42–7.99) | 5.47 (2.69–11.09) | ||
Numbers in parentheses represent 95% C.I.
Tumor-Infiltrating CD8+ T Lymphocyte Count and Prognosis.
Tumor-infiltrating CD8+ T lymphocytes were evaluated separately first in cancer cell nests (intraepithelial) and then in cancer stroma (stromal) (Fig. 1K). Intraepithelial and stromal CD8+ T lymphocytes were positive in 32 (45.7%) and 29 (41.4%) of the 70 tumors, respectively (Fig. 1 J–L and SI Table 2). The average numbers of intraepithelial and stromal CD8+ T cells were 6.43 (range, 0–56.8; SD, 8.32) and 7.29 (range, 0–60.6; SD, 8.42) per 0.0625 mm2.
The Kaplan–Meier curve and log rank test showed that patients positive for intraepithelial CD8+ T lymphocyte infiltration had significantly better overall and progression-free survival than patients negative for intraepithelial CD8+ T lymphocyte infiltration [5-year survival rate ± SD, positive vs. negative: 86.9 ± 6.1% vs. 39.0 ± 8.7%, P < 0.001 (Fig. 2D and SI Table 3) and 75.0 ± 7.7% vs. 31.6 ± 7.5%, P < 0.001 (Fig. 3C)]. The overall survival period (years, mean ± SD) for positive vs. negative intraepithelial CD8+ infiltration was 9.6 ± 0.6 vs. 4.7 ± 0.5. Progression-free survival in positive vs. negative intraepithelial CD8+ infiltration was 8.9 ± 0.8 vs. 3.5 ± 0.6. On the other hand, stromal CD8+ T cells had no significant impact on either overall or progression-free survival (Figs. 2E and 3D: P = 0.616 and P = 0.320) (SI Table 3).
Multivariate analysis indicated that intraepithelial CD8+ T lymphocyte count was an independent poor prognostic factor for both overall and progression-free survival (Table 1) (RR, 7.62; 95% C.I., 2.76–21.08; P < 0.001 and RR, 3.75; 95% C.I., 1.74–8.07; P = 0.001, respectively). These data are in agreement with previous report by others, confirming the contribution of the immune system in the eradication of ovarian cancers (33–35).
Correlation Between Tumor-Infiltrating CD8+ T Lymphocyte Count and Other Factors, Including PD-Ls Expression.
No correlation was found between intraepithelial CD8+ T lymphocyte count and various clinicopathological factors, suggesting that intraepithelial CD8+ T lymphocyte count is an independent prognostic factor (SI Table 2). In contrast, there was a significant inverse correlation between intraepithelial CD8+ T lymphocyte count and PD-L1 expression (Fig. 2F) (r = −0.266, P = 0.026). PD-L2 expression was not associated with intraepithelial CD8+ T lymphocyte count (r = −0.025, P = 0.835). Stromal CD8+ T lymphocyte count was not correlated with any other factors. Taken together, it is likely that PD-L1 on tumor cells prohibits the intraepithelial invasion of tumor-specific CD8+ T cells, resulting in the tumor evasion of the immune system.
Discussion
An appropriate Ab that can be applied to paraffin-embedded specimens is especially valuable in evaluating historical specimens with known outcome and routinely processed clinical specimens. Most of the previous reports on PD-Ls expression were performed by using frozen specimens (25–29, 32). Thompson et al. (30) examined the expression of PD-L1 on paraffin-embedded specimens of renal cell carcinoma using the same Ab that they used in the previous study on frozen section and reported that there was a significant difference in the incidence of PD-L1 expression between paraffin-embedded and frozen specimens. In this study we generated a new Ab against human PD-L1 that strongly recognizes human PD-L1 protein on formalin-fixed, paraffin-embedded specimens. Because positive incidence of PD-L1 expression in our study using this Ab is similar to the previous report on frozen specimen of ovarian cancers using other Abs (25), it appears that our Ab adequately evaluates the expression of PD-L1 on paraffin-embedded specimens and should be useful in the analysis of various cancers.
The frequency of PD-L1 expression is highest among various malignancies including carcinoma of the lung (50%), esophagus (44%), stomach (42%), breast (34%), and kidney (37%), suggesting that PD-L1 expression is more prevalent in ovarian cancer than in other malignancies and may play an important role in the suppression of host–tumor immunity against highly immunogenic tumors (26, 28, 32, 36, 37). In contrast, expression of PD-L2 was less frequent, being found in approximately one-half of the ovarian cancers. This is the first report of PD-L2 expression in ovarian cancer, and the incidence is similar to that seen in lung and esophageal cancer (26, 32).
Analysis of PD-Ls expression in correlation with survival revealed that patients with high PD-L1 expression had a significantly poorer outcome than patients with low/negative expression. Notably, χ2 test revealed no significant correlation between PD-L1 expression and various clinicopathological factors such as clinical stage and histological subtype of the tumor. Multivariate analysis also showed that PD-L1 expression was an independent prognostic factor, along with the well established factors including primary tumor status, lymph node metastasis, and residual tumor. PD-L2 showed a similar tendency to PD-L1, but without statistical significance. Patients negative for both PD-L1 and PD-L2 had a far better prognosis than those positive for either or both PD-L1 and PD-L2. These data suggest that PD-L1 expression in tumor tissue is a novel independent prognostic factor and possibly reflects the interruption of the antitumor immunity of the host. High expression of PD-L1 has also been shown to be an independent prognostic factor in renal cancer and gastric cancer (30, 36). Both PD-L1 and PD-L2 were reported to be independent prognostic factors in esophageal cancer (32). In contrast, neither was correlated with prognosis in a report on lung cancer (26). Therefore, the involvement of PD-L1 and PD-L2 in the tumor immune escape seems to differ depending on the organs or tumor types.
PD-Ls on tumor cells are found to suppress the effector function of CD8+ T cells (25, 38). To elucidate whether PD-L1 expression indeed reflects host–tumor immunity, we evaluated CD8+ TILs because the presence of particular TIL subsets has been shown to correlate with better prognosis in cutaneous melanoma, colorectal cancer, esophageal cancer, renal cancer, and ovarian cancer (5–9, 33–35). Our results clearly support the findings that intraepithelial CD8+ TILs are a strong prognostic factor in ovarian cancer. In addition, intraepithelial CD8+ TILs predict not only overall survival but also progression-free survival of patients with ovarian cancer, which was not clear in former studies. Moreover, CD8+ TILs are not associated with known clinicopathological factors such as tumor stage. Thus, the number of intraepithelial CD8+ TILs is a significant prognostic parameter predicting the clinical course of ovarian cancer irrespective of other conventional parameters.
We found a significant inverse correlation between tumor PD-L1 expression and intraepithelial CD8+ TIL count (correlation index, −0.266, P = 0.026) (Fig. 2F), whereas PD-L2 expression was not significantly correlated with CD8+ TIL count. These results contrast with a previous report on esophageal cancer, in which inverse correlation was observed between CD8+ TIL count and PD-L2 but not PD-L1 expression (32). This discrepancy may reflect the differential involvement of PD-L1 and PD-L2 depending on organs or cancer types. Correlation between tumor PD-L1 expression and intraepithelial CD8+ TIL count indicates that tumor PD-L1 expression is indeed relevant to host–tumor immunity and thus reflects patient outcome. However, the reduction of CD8+ TILs may not be the only mechanism by which PD-L1 promotes tumor immune escape because their correlation is not so strong. It may be possible that PD-L1 on tumor cells induces functional impairment of tumor-specific T cells without reducing their number as reported for antiviral T cells (39, 40).
Taken together, our data indicate that PD-L1 expression by tumor cells is a significant prognostic factor in ovarian cancer and is inversely correlated with intraepithelial CD8+ TIL count, a known immunological prognostic indicator. Both PD-L1 expression and CD8+ TIL count are independent factors among the established prognostic factors, suggesting that immunological status is not adequately evaluated by the conventional clinicopathological prognostic factors. Examination of the PD-L1 expression on tumor cells can be an appropriate method of evaluating host–tumor immunity that is fundamental for the better management of ovarian cancer.
Finally, current findings indicate that most ovarian cancers evade the host immune system and accelerate tumor growth by expressing PD-L1, suggesting that the PD-1/PD-L pathway may be a potential target for immunotherapy of ovarian cancer. Indeed, PD-1/PD-L blockade has been shown to facilitate tumor eradication in various experimental systems including Ab blockade of PD-1 or PD-L1, expression of the extracellular region of PD-1 by local gene therapy, and PD-1-deficient mice (27, 31, 38, 41). PD-1 blockade has also been shown to suppress tumor metastasis in melanoma and colon cancer cell lines in mice (42). The clinical efficacy of PD-1 blockers may appropriately be evaluated in ovarian cancer patients.
Materials and Methods
Patients and Samples.
The subjects for the present study were a consecutive series of 70 women who had been diagnosed with and undergone primary operation for ovarian cancer at the Department of Obstetrics and Gynecology of Kyoto University Hospital (Kyoto, Japan) between 1993 and 2001. The relevant clinical data were collected by retrospective review of the patients' files. Patients with nonepithelial-type neoplasia, patients treated before operation, and patients who were not treated surgically were excluded. Borderline tumors of the ovary were also excluded from this study. The patients provided written informed consent under the approval of the Ethics Committee of Kyoto University Hospital.
Immunohistochemistry.
We generated monoclonal Abs specific for human PD-L1 that can detect PD-L1 on formalin-fixed, paraffin-embedded specimens by a standard protocol. Briefly, BALB/c mice were immunized with a recombinant human PD-L1 extracellular domain (A18 to T239), which was expressed in Escherichia coli, refolded in arginine-based buffer with a glutathione redox system, and purified by consecutive column chromatographies on Q Sepharose HP and Superdex 200 (GE Healthcare Bio-Sciences, Piscataway, NJ). The animals were killed, and the splenocytes were fused with SP2/0. We obtained 48 hybridomas that produced Abs specific for human PD-L1 on flow cytometry analysis, one of which, 27A2, was used for immunohistochemical staining in this study.
Immunohistochemical staining was performed by the streptavidin–biotin–peroxidase method. Formalin-fixed, paraffin-embedded specimens were cut into 4-μm-thick sections. The tissue sections were deparaffinized in xylene (3 × 10 min) and dehydrated through graded alcohols (99%, 99%, and 70%) to water. Antigens were retrieved by the following methods. For PD-L1 staining, the samples were boiled in citrate buffer (pH 6.0) by microwaves. For CD8+ staining, the samples were heated in Tris-EDTA buffer (pH 9.0) at 95°C for 40 min. To block endogenous peroxidase activity, all of the sections were treated with 100% methanol containing 0.3% H2O2 for 15 min. Nonspecific binding of IgG was blocked by using normal rabbit serum (Nichirei, Tokyo, Japan). The sections were incubated with mouse anti-PD-L1 monoclonal Abs (clone 27A2), goat anti-PD-L2 polyclonal Abs (R & D Systems, Minneapolis, MN), and mouse anti-CD8+ monoclonal Abs (Nichirei; clone C8/144B) overnight at 4°C. Then, they were incubated with biotinylated rabbit-anti-mouse secondary Abs (Nichirei) for PD-L1 and CD8+ stainings and with biotinylated rabbit-anti-goat secondary Abs for PD-L1 staining, followed by the incubation with streptavidin–peroxidase complex solution for 30 min. Signals were generated by incubation with 3,3′-diaminobenzidine. Finally, the sections were counterstained with hematoxylin and observed under the microscope.
Evaluation of the Specimens.
Two independent gynecological pathologists examined the immunohistochemical slides without any prior information on the clinical history of the patients. The expression of PD-L1 and PD-L2 was evaluated according to the intensity of the staining and scored as follows: 0, negative; 1, very weak expression; 2, moderate expression but weaker than placenta (PD-L1) and tonsil (PD-L2); and 3, equivalent to or stronger expression than placenta or tonsil. Cases with scores 0 and 1 were defined as the low-expression group, and cases with scores 2 and 3 were the high-expression group.
CD8+ staining was evaluated according to the previous reports (33, 35). Briefly, tumor-infiltrating CD8+ T lymphocytes were counted separately by their localization as intraepithelial or stromal. CD8+ T lymphocytes infiltrating into cancer cell nests, designated intraepithelial CD8+ T lymphocytes, were counted with a microscopic field at ×200 (0.0625 mm2). Three areas with the most abundant infiltration were selected, and the average count was calculated. The result was interpreted as negative when fewer than five cells per 0.0625 mm2 and as positive when more than or equal to five cells per 0.0625 mm2. CD8+ T lymphocytes detected within the cancer stroma, designated stromal CD8+ T lymphocytes, were evaluated in the same way.
Statistical Analysis.
Fisher's exact test and the χ2 test were used to analyze the associations between PD-Ls expression and tumor-infiltrating CD8+ T lymphocyte count and various clinicopathological factors. Comparisons of tumor-infiltrating CD8+ T lymphocyte counts were carried out by using Student's t test and ANOVA. Spearman's coefficient rank test was used to evaluate the correlation between PD-Ls expression and tumor-infiltrating CD8+ T lymphocyte count. Univariate analysis for overall and progression-free survival was performed and evaluated with the log rank test, and Kaplan–Meier curves were generated. A multivariate Cox proportional-hazard model was used to evaluate the independency of PD-Ls expression and tumor-infiltrating CD8+ T cell count as prognostic factors among other variables such as pTNM stage, histology, residual tumor state, and adjuvant chemotherapy. All P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to Ms. Michiko Muraoka for technical assistance. This work was supported in part by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation and by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations
- PD-1
programmed cell death 1
- PD-L1
PD-1 ligand 1
- PD-L2
PD-1 ligand 2
- PD-Ls
PD-1 ligand 1 and 2
- TIL
tumor-infiltrating lymphocyte
- C.I.
confidence interval
- RR
risk ratio.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611533104/DC1.
References
- 2.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Semin Oncol. 1996;23(Suppl 12):40–47. [PubMed] [Google Scholar]
- 2.Vaidya AP, Curtin JP. Semin Oncol. 2003;30:401–412. doi: 10.1016/s0093-7754(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Benedet JL, Bender H, Jones H, III, Ngan HY, Pecorelli S. Int J Gynaecol Obstet. 2000;70:209–262. [PubMed] [Google Scholar]
- 5.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Jass JR. J Clin Pathol. 1986;39:585–589. doi: 10.1136/jcp.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 8.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 9.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 10.Groh V, Wu J, Yee C, Spies T. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 11.Berzofsky JA, Ahlers JD, Belyakov IM. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 12.Pardoll DM. Nat Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 13.Lang K, Entschladen F, Weidt C, Zaenker KS. Cancer Immunol Immunother. 2006;55:749–760. doi: 10.1007/s00262-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers CA, Kuhns MS, Egen JG, Allison JP. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 15.Collins M, Ling V, Carreno BM. Genome Biol. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwald RJ, Freeman GJ, Sharpe AH. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki T, Honjo T. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Ishida Y, Agata Y, Shibahara K, Honjo T. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. Proc Natl Acad Sci USA. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong H, Zhu G, Tamada K, Chen L. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 22.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 24.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 26.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 27.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 28.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, et al. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 30.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 31.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 32.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 34.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 35.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, et al. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 40.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 41.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 42.Iwai Y, Terawaki S, Honjo T. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.