Abstract
A reduction in GABAergic neurotransmission has been put forward as a pathophysiological mechanism for human epilepsy. However, in slices of human epileptogenic neocortex, GABAergic inhibition can be clearly demonstrated. In this article we present data showing an increase in the functional lability of GABAergic inhibition in epileptogenic tissue compared with nonepileptogenic human tissue. We have previously shown that the glycolytic enzyme GAPDH is the kinase involved in the glycolysis-dependent endogenous phosphorylation of the α1-subunit of GABAA receptor, a mechanism necessary for maintaining GABAA function. In human epileptogenic cortex obtained during curative surgery of patients with partial seizures, we demonstrate an intrinsic deficiency of GABAA receptor endogenous phosphorylation resulting in an increased lability of GABAergic currents in neurons isolated from this tissue when compared with neurons from nonepileptogenic human tissue. This feature was not related to a reduction in the number of GABAA receptor α1-subunits in the epileptogenic tissue as measured by [3H]flunitrazepam photoaffinity labeling. Maintaining the receptor in a phosphorylated state either by favoring the endogenous phosphorylation or by inhibiting a membrane-associated phosphatase is needed to sustain GABAA receptor responses in epileptogenic cortex. The increased functional lability induced by the deficiency in phosphorylation can account for transient GABAergic disinhibition favoring seizure initiation and propagation. These findings imply new therapeutic approaches and suggest a functional link to the regional cerebral glucose hypometabolism observed in patients with partial epilepsy, because the dysfunctional GABAergic mechanism depends on the locally produced glycolytic ATP.
Keywords: GABAA receptor phosphorylation, GAPDH, human epilepsy, neuronal inhibition
Protein phosphorylation is an important mechanism for the rapid modulation of ion channel properties. Receptor-associated endogenous phosphorylation is required for maintaining the GABAA currents, the principal inhibitory system in the mammalian brain (1, 2). We have identified the kinase of the endogenous phosphorylation as being GAPDH (3), a key glycolytic enzyme. GAPDH is closely associated with the GABAA receptor (GABAAR) macrocomplex at the plasma membrane. GAPDH has a dual role, first as a dehydrogenase in the glycolysis cascade contributing to ATP production and second as a kinase phosphorylating the GABAAR α1-subunit (3). All factors promoting the GAPDH-dependent α1-phosphorylation also favor the maintenance of the receptor in a functional state, thus directly linking GABAergic inhibition with glucose metabolism. The α1-phosphorylation state of the receptor also depends on a membrane-bound phosphatase that is yet to be characterized (4).
A wealth of studies have shown that a decrease, even transient, in the efficacy of the GABAA inhibition induces pathological neuronal synchronization resulting in epileptic seizures (5, 6). A deficiency of endogenous GABAAR phosphorylation may thus play a role in the triggering and/or the propagation of epileptic seizures, particularly under metabolic stress. Here we put forward evidence for a deficiency of α1-subunit phosphorylation and thus GABAAR function in epileptogenic tissue using human cortical tissue removed for strictly therapeutic reasons from epileptic patients and from nonepileptic patients.
Results
The patient population included both epileptic and nonepileptic patients from Rennes, France, and from Paris, France. The epileptic patient population [mostly temporal lobe epilepsy (TLE)] presented very similar clinical features and group distribution in both sites [supporting information (SI) Tables 2 and 3]. The presurgical evaluation and the surgical procedure for the selective resection were identical in both hospital centers: All surgeons involved in this study had undergone neurosurgery training at Sainte-Anne Hospital (Paris, France).
Intrinsic Deficiency of α1-Subunit Phosphorylation.
The α1-subunit specific labeling of GABAAR, respectively, by endogenous phosphorylation and by photoaffinity were measured in membrane fractions prepared from cortical tissue of epileptic and nonepileptic patients (Figs. 1 and 2). To allow direct comparisons, all membrane preparations were diluted to the same protein concentration. Endogenous phosphorylation clearly predominated on an electrophoretic band at 51 kDa (Fig. 1). We have previously demonstrated in purified receptor (7) and in membrane preparations (4) that this band corresponds to the α1-subunit. 33P-labeling of this subunit in cortical membranes prepared from epileptic patients (n = 23) showed a very significant decrease (P = 0.0002 with Student's t test; P = 0.0033 with Wilcoxon rank test) when compared with the surgical control tissues (n = 5) from nonepileptic patients (Fig. 2A).
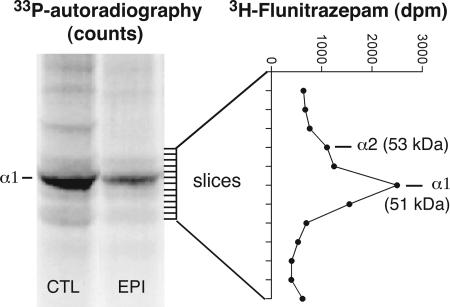
Fig. 1.
Labeling of the GABAAR α1-subunit by endogenous phosphorylation and by [3H]flunitrazepam photoaffinity in membrane fractions prepared from cortical tissue of surgery patients. Shown is the autoradiographic pattern (P-screen scanning) of the polyacrylamide gel after endogenous phosphorylation in a membrane preparation from tissue of a nonepileptic control patient (CTL) and of an epileptic patient (EPI); the predominating 33P-labeling at the 51-kDa band corresponded to the GABAAR α1-subunit as previously demonstrated (4) and was clearly lower in epileptic patients. In a parallel experiment with the same membrane preparations, irreversible [3H]flunitrazepam incorporation was measured in gel slices (as indicated by the dashes), and a labeling profile for the α1 and α2 receptor subunits is shown.
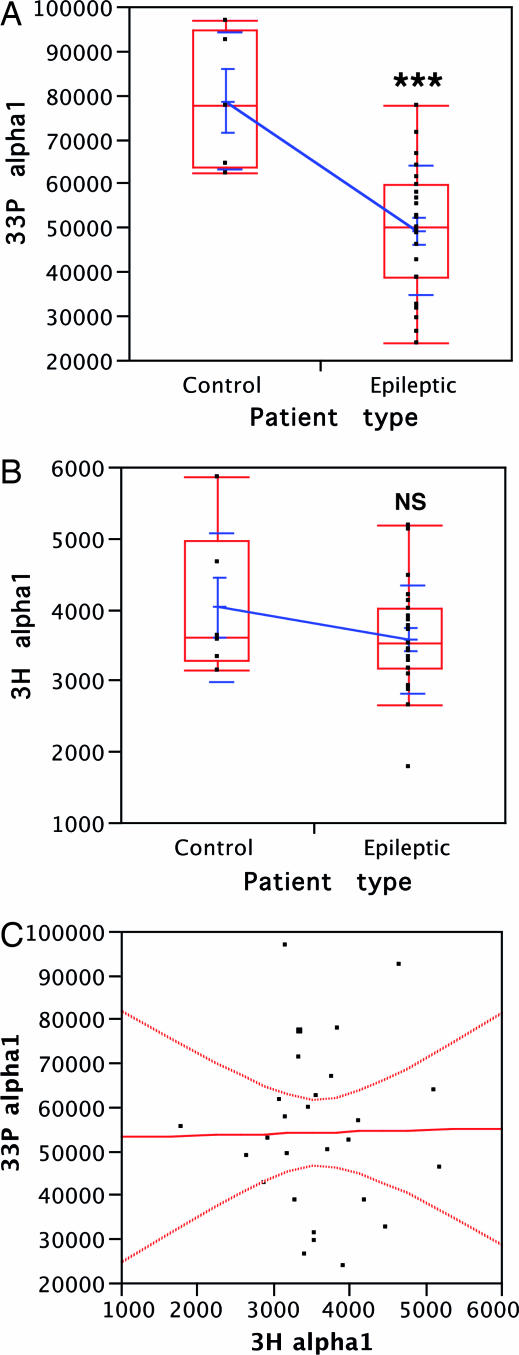
Fig. 2.
The deficiency of GABAAR endogenous phosphorylation in membranes from epileptogenic tissue is intrinsic. (A) 33P-labeled phosphate incorporation (in counts) into α1-subunit by the endogenous phosphorylation compared between nonepileptogenic cortex (n = 5) and epileptogenic cortex (n = 23). The distributions are presented as mean values with SEM and SD (blue) and as median values in quartile boxes (red). ∗∗∗, P < 0.001, Student's t test. (B) 3H-incorporation (in dpm) in α1 by the [3H]flunitrazepam labeling compared between nonepileptogenic cortex (n = 6) and epileptogenic cortex (n = 23). Distributions are presented as in A. NS, nonsignificant (P = 0.24 for the t test). (C) Correlation graph between the individual 33P- and 3H-labeling values of membranes from surgical samples of both epileptic and nonepileptic patients (n = 27). The calculated correlation r = 0.014 and the null leverage indicated the absence of interaction between the two labelings (P = 0.95).
To test whether membranes prepared from epileptic patients retain the capacity to produce glycolytic ATP locally and promote endogenous phosphorylation, we assayed α1-phosphorylation in the presence of 33P-phosphate, ADP, Mg2+, and NAD+. Glyceraldehyde-3-phosphate (G3P) addition increased α1 labeling significantly (P = 0.012) (SI Fig. 5).
To determine whether this decrease in α1-phosphorylation is due to altered subunit content, we performed photoaffinity labeling with the benzodiazepine [3H]flunitrazepam. This irreversible labeling has high affinity for the α-subunits, particularly α1. We measured the quantity of this subunit in the same membrane preparations as those tested for phosphorylation. The values obtained (Fig. 2B) using membrane preparations from epileptic patients (n = 23) and from nonepileptic patients (n = 6) were not statistically different (P = 0.24, t test; P = 0.47, Wilcoxon rank test). This shows that the decreased 33P-phosphorylation in the epileptic patients is not due to a difference in α1-subunit content. We observed some minor degree of 33P and 3H incorporation in a 53-kDa band which corresponds to the α2-subunit (Fig. 1), but this study was limited to the α1-subunit because the α2-labelings were too low for reliable quantification. Cross-comparison between data obtained from membrane preparations used for endogenous phosphorylation and those for benzodiazepine photoaffinity labeling on the α1-subunit indicates that corresponding individual variations are not correlated (Fig. 2C). This lack of correlation corroborates the view that endogenous phosphorylation is indeed deficient in epileptic patients.
Comparisons of Clinical and Biochemical Features.
We tested whether clinical parameters have any effect on the deficiency of endogenous phosphorylation in epileptic patients. Epilepsy duration, age at onset, age at surgery, and postoperative outcome (Engel's classes) were tested by using two-way ANOVA: none had a significant effect (P > 0.05; leverage power < 0.5). For the postoperative outcome, the absence of correlation between surgical outcome and the abnormalities was to be foreseen because 44/49 patients had very good or good results. We found however a near-significant positive effect of age at surgery on the 33P-labeling (P = 0.067), and thus an inverse correlation between age at surgery and phosphorylation deficiency. Among all possible interactions between the examined parameters, we only found a trivial positive cross-effect of epilepsy duration and age at surgery on the 33P-labeling (P = 0.012). Biochemical data were also tested by matching gender (female = 8, male = 15) and seizure onset site (mesiotemporal = 14, lesional–temporal = 6, extratemporal = 2): None of the one-way ANOVA's tests revealed any significant difference.
GABAA Function Deficiency.
Rundown of GABAA currents is a time-dependent response decrease due to a dephosphorylation process (1, 2), that does not require the presence of the receptor agonist. We measured the rundown of these currents in neurons acutely dissociated from the cortex samples of epileptic and nonepileptic patients. These cells presented a pyramidal shape (Fig. 3A). The time course of GABAA currents of a neuron isolated from epileptogenic tissue displayed a very rapid rundown (Fig. 3B). When normalized currents of neurons isolated from epileptogenic tissue (n = 13) and from nonepileptogenic tissue (n = 8) were compared, the rundown of the former was more rapid and more ample (Fig. 4A). This difference was very significant with two-way ANOVA of normalized current and log(time) after the Bonferroni adjustment (P < 0.001), taking in account the time repeats. The difference is already significant for the second application (3 min) when t tests are performed at each time point.
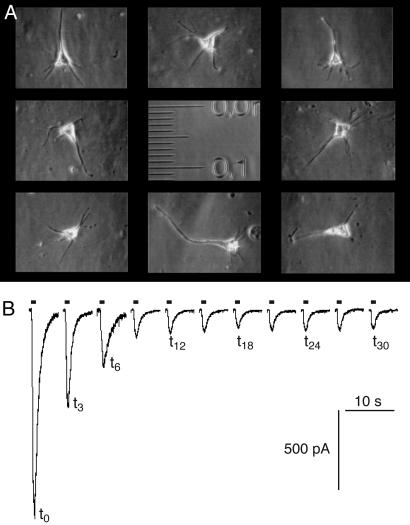
Fig. 3.
Rundown of GABAA currents measured by whole-cell patch clamp on acutely dissociated neurons from cortical tissue of surgery patients. (A) Phase-contrast micrograph of isolated pyramidal neurons. The scale is in millimeters with small graduations of 10 μm. (B) Current recording traces of a neuron from an epileptic patient. GABA (100 μM) was applied for 1 s every 3 min.
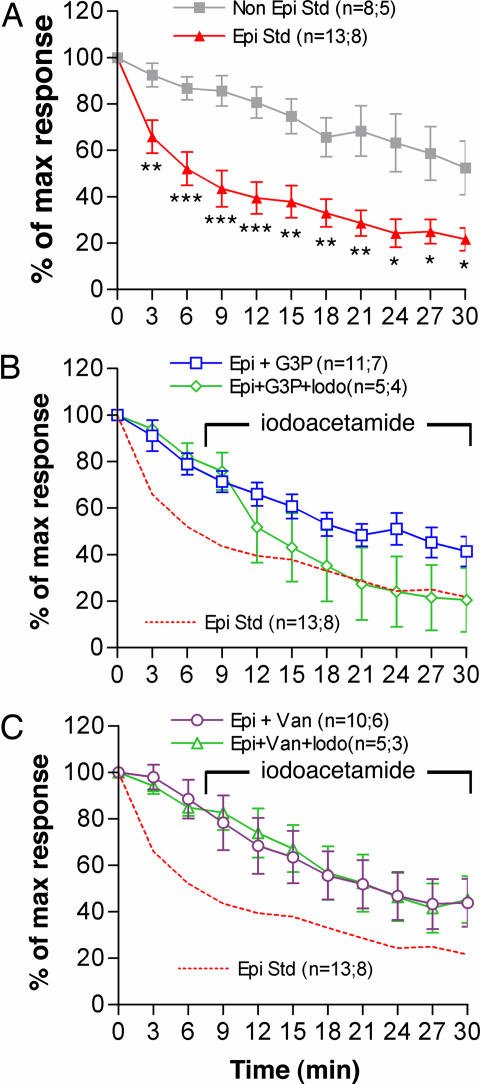
Fig. 4.
Comparison of GABAA current rundown in neurons from epileptogenic and nonepileptogenic cortex and correlation with receptor endogenous phosphorylation. (A) Averaged normalized current in standard condition (Std) for neurons from epileptic (Epi) and nonepileptic (Non Epi) patients. Student's t test at each time comparisons: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (B) The addition of G3P (500 μM) to the pipette milieu markedly reduced rundown in neurons from epileptic patients (Epi+G3P); this effect is counteracted by the addition of iodoacetamide (500 μM) to the bath (Epi+G3P+Iodo). (C) Protective effect against rundown in neurons from epileptic patients of orthovanadate (100 μM) added to the pipette milieu (Epi+Van) and lack of a counteracting effect by iodoacetamide (500 μM) on this protection (Epi+Van+Iodo). Iodoacetamide addition started between GABA application times t = 6 min and t = 9 min and was maintained thereafter (B and C). For each curve, the parentheses indicate, respectively, the number of neurons and the number of corresponding patients. The error bars indicate SEM. Statistical analysis is presented in Table 1.
The addition to the pipette milieu (intracellular) of G3P (500 μM), the GAPDH specific substrate, has a statistically significant positive effect on the maintenance of GABAA currents for the neurons isolated from epileptogenic tissue (P < 0.001 for Bonferroni adjusted two-way ANOVA and Dunnett's post hoc test) (Fig. 4B). This protective effect of G3P against rundown was abolished (P < 0.0007) by the addition to the bath of iodoacetamide (500 μM), an irreversible inhibitor of GAPDH (Fig. 4B). We have shown that a membrane-bound phosphatase responsible for the GABAAR α1-subunit dephosphorylation is inhibited by orthovanadate (4), and that this inhibitor markedly reduces GABAA current rundown in rat neurons (3). The addition of orthovanadate (100 μM) to the pipette milieu also produced a significant reduction of the GABAA currents rundown (P < 0.001) in neurons from epileptic patients (Fig. 4C). In presence of orthovanadate the effect of iodoacetamide was no longer observed, showing that iodoacetamide has no direct effect on the GABAAR and showing that maintenance of the receptor α1-phosphorylation sustains its function. The protective effects of G3P and of orthovanadate addition were observed to allow recovery of rundown to a level close to that of neurons from nonepileptogenic tissue without such an addition (compare A–C in Fig. 4).
Comparisons of Clinical and GABAA Functional Parameters.
We performed statistical analyses to examine the possible influence of clinical parameters on the GABAAR function. The number of cells were sufficient to test several influences, under standard recording conditions (EpiStd; n = 13) and in G3P-treated (Epi+G3P; n = 11) and orthovanadate-treated (Epi+VAN; n = 10) neurons from epileptic patients. The values during the time course from t = 3 min to t = 30 min, and the all times mean value of the normalized GABAA currents were considered. The clinical parameters tested were the age at seizure onset, age at surgery, epilepsy duration and postoperative outcome, using two-way ANOVA. Interestingly, age at surgery has a positive effect in the EpiStd population at the early times: P = 0.024 at t = 3 min and P = 0.061 at t = 6 min; this result parallels the tendency observed for the 33P-phosphorylation of washed membranes. The influence of age at surgery was not observed in the other populations (G3P and VAN), likely because of the rundown reduction with both treatments. There is a positive effect of epilepsy duration significant only at the later times and for G3P-treated cells: P = 0.015 at t = 24 min, P = 0.015 at t = 27 min and P = 0.0083 at t = 30 min. Surgery outcome did not influence current rundown in any neuron population. The search of cross-effects between the clinical parameters reveals a significant interaction between epilepsy duration and age at onset concerning their effects on currents measured at later time points in G3P-treated neurons only: P = 0.027 at t = 24 min, P = 0.031 at t = 27 min and P = 0.033 at t = 30 min. Age at onset influences positively (P = 0.030) the normalized currents only at t = 27 min in the EpiStd population; this may be a fortuitous result. One-way ANOVA were used where ever it was possible to test differences in the GABAA current rundown by matching gender of patients and the localization of the seizure onset area (grouped as following: MT = mesiotemporal, LT = lesional–temporal; ET = extratemporal). The gender (male = 9, female = 4 for EpiStd; male = 6, female = 5 for G3P; male = 9, female = 4 for VAN) has no influence for any of the three neuron series. The influence of seizure onset area location on rundown was assessed among the groups of patients when possible (MT = 8 vs. LT = 3 for EpiStd; MT = 9 vs. ET = 2 for G3P; MT = 7 vs. LT = 3 for VAN); no significant difference could be detected.
Discussion
The analyzed samples included only neocortex, where neuronal loss is reported to be absent in TLE patients (8). In vitro benzodiazepine binding site analysis in TLE patients demonstrated no decrease in lateral temporal gyri, but in mesial structures binding is correlated with neuronal loss (9). Moreover, immunohistochemical studies show multiple GABAA subunit-specific alterations, including the α1- and α2-subunits, in the hippocampus of TLE patients (10). In contrast, no decrease of the benzodiazepine flumazenyl binding, measured in vivo by positron-emission tomography (PET), has been detected outside mesial temporal structures in patients with Ammon's horn sclerosis (11, 12), except for a few TLE cases with Ammon's horn sclerosis where flumazenyl binding was decreased in the temporal pole (13). It was therefore of critical importance to analyze in the neocortical tissue used for this study α1-subunit expression on the same membrane preparations as those used for phosphorylation analysis. 3H-labeling of the α1-subunit was unchanged when samples from epileptic and nonepileptic patients were compared. There was no correlation between the decrease in GABAAR endogenous α1-phosphorylation and α1-subunit expression on membrane preparations. Because ATP concentration was identical for all phosphorylation assays, the observed difference of endogenous α1-phosphorylation could only be due to an intrinsic deficiency. The same line of reasoning can be advanced for functional studies. Any additional transient or permanent deficiency in ATP production would be expected to uncover or worsen the GABAergic dysfunction.
We have previously demonstrated that rundown in rat neurons is counteracted by the endogenous phosphorylation that depends on the glycolytic production of ATP and on the kinase activity of the glycolytic enzyme GAPDH (3). We propose that the increased lability in GABAergic inhibition in the epileptogenic tissue is due to a deficiency of the glycolysis-dependent phosphorylation at the neuronal membrane. Indeed, the effect of the glycolytic GAPDH substrate (G3P) on the GABAA current rundown and its sensitivity to GAPDH inactivation by iodoacetamide confirms in human tissue the findings of our previous study on rat tissue (3). Interestingly, in these patch clamp studies, the glycolytic ATP produced locally from 0.5 mM G3P was preferentially used over the ambient intracellular ATP (7 mM) to sustain GABAA function. G3P addition favored both α1-phosphorylation and recovery of GABAergic function in neurons from epileptic patients. A recovery was also obtained by adding orthovanadate at a concentration known to inhibit a membrane phosphatase responsible for the α1-dephosphorylation (4). Similar effects on GABA responses were reported for phosphatase inhibitors using membrane microsomes from human epileptogenic tissue fused to the plasma membrane of Xenopus oocytes (14).
In vitro studies on slices from epileptogenic temporal cortex in humans show that the GABAergic system is functional (15, 16). Synchronous activity of GABAergic neurons is required to trigger ictal-like discharges in such slices treated with 4-aminopyridine (17), activity which requires powerful GABAergic inhibition. Immunohistochemical studies show that even in the sclerotic hippocampus from TLE patients, the surviving pyramidal cells receive nearly intact inhibitory input (18). Depolarizing GABAergic responses in subiculum neurons may also contribute to interictal activity in TLE patients (19). Our studies support the concept of GAPDH-dependent phosphorylation directly linking glycolysis to the maintenance of GABAergic currents. We hypothesize that any increased load on glucose metabolism produces rapid spatial and/or temporal variations in GABAergic function, allowing cortex to switch over into the epileptic state.
Considering the different clinical profiles in the epileptic patient group (SI Table 2), the homogeneity of the GABAergic functional deficit is noteworthy. When the biochemical and functional results are compared with the clinical data, a few interesting observations can be made. There is a significant positive influence of age at surgery on GABAergic function corroborated by a tendency for improvement of α1-phosphorylation, probably because of the combined effect of age at onset and epilepsy duration. The other clinical parameters did not significantly interact with the level of deficiency. Concerning the functional GABAergic recovery by the addition G3P or orthovanadate, epilepsy duration had a positive influence only on the effect of G3P. This G3P-specific relationship may be accounted for by incomplete metabolic adaptation related to epilepsy duration.
Other studies measuring NAD(P)H fluorescence have shown metabolic dysfunction in relation to mitochondrial membrane potential changes in hippocampus slices from epileptic patients (20), and a correlation has been found between energetic metabolism (phosphocreatine/ATP ratio) and inhibitory postsynaptic potential conductance (21). Interictal glucose consumption is known to be decreased in intractable TLE patients studied by 18fluorodeoxyglucose PET (22–25). Thus, a relationship can be postulated between the deficiency of glycolysis-dependent α1-phosphorylation shown in vitro and the interictal hypometabolism observed in vivo in TLE patients.
It is doubtful that GABAA-R α1-phosphorylation per se contributes significantly to glucose consumption (26). However, a decreased glucose uptake would be expected as a functional consequence of the observed α1-phosphorylation deficiency on postsynaptic inhibition. First, activation of recurrent GABAergic inhibitory circuitry resulted in a marked increase in glucose consumption as evaluated by 14C-deoxyglucose uptake in rat brain hippocampal slices (27). Second, PET studies revealed that administration of a specific GABAA agonist, increased glucose consumption by 17.1% in healthy volunteers and by 24.8% at the hypometabolic side in TLE patients (28, 29). The magnitude of this GABAergic metabolic effect is of the same order than that of hypometabolism in the temporal pole of TLE patients compared with controls (13–17%) (30). On the other hand, an additional primary deficiency in energy metabolism would further affect α1-phosphorylation if glycolysis-dependent ATP production falls locally below a critical level. Indeed both mechanisms may contribute to hypometabolism.
What is causal in the hypometabolism/decreased GABAAR phosphorylation relationship? A decrease in glucose utilization should reflect a decrease in glycolytic ATP production. Because the α1-phosphorylation level is determined by the opposing actions of a kinase and a phosphatase, equilibrium may be reached at a lower α1-phosphorylation level when glycolytic ATP is less used or produced. An intrinsic deficiency of this phosphorylation in epileptic patients and the subsequent decrease in GABAergic inhibition should decrease glucose uptake as observed in the epileptogenic cortex. It would have been useful to quantify the varying degrees of hypometabolism of the examined patients and make a correlation with the observed GABAergic deficiencies. Unfortunately, the acquisition procedure and variability of individual hypometabolism values were not appropriate for a normalization of sufficient quality.
Ictal 18fluorodeoxyglucose PET imaging has demonstrated a restricted area of focal glucose hypermetabolism in severe partial epilepsy (31), in keeping with the ictal focal hyperperfusion systematically observed in a number of single-photon emission computed tomography studies. A relationship between cerebral glucose metabolism and blood flow is evidenced by the observation of physiologically activity-induced increases in glycolysis producing changes in cytosolic NADH/NAD+ ratio that regulates the blood flow in normal human subjects (32). It is however not clear how ictal hypermetabolism influences GABAergic function.
In conclusion, the glycolysis-dependent phosphorylation of α1-subunit responsible for the functional maintenance of the GABAAR is constantly found to be deficient in the human epileptogenic cortex where seizures begin and spread. It is proposed that GAPDH, by fulfilling multiple roles, provides at least in part a direct and molecular link between the GABAAR functional deficiency described here and the interictal glucose hypometabolism regularly observed in human epileptogenic cortex. The enzymes that regulate α1-subunit phosphorylation may therefore be considered as targets for therapeutic research.
Subjects and Methods
Patient Population Selection.
Epileptic patients.
This study was performed on cerebral tissue from 50 patients (32 men and 18 woman) suffering from temporal or frontal pharmacoresistant epilepsy who underwent curative neurosurgery between 1996 and 2005 in the Epilepsy Centers of Sainte-Anne (Paris, France) and Pontchaillou (Rennes, France). The clinical characteristics of the patients are indicated in SI Table 2. Both epileptic patient populations (23 from Rennes and 27 from Paris) presented very similar clinical features and group distribution (SI Table 3). Informed consent was obtained from all patients according to the guidelines of the institutional committees of our respective centers. Presurgical evaluation of patients included clinical examination, EEG video monitoring, and high-resolution MRI with standard T1 and T2 weighted images with or without the fluid attenuated inversion recovery (FLAIR sequence). Interictal–ictal single-photon emission computed tomography with 99mTc-hexamethyl propyleneamineoxine was performed in 19 patients, and [18F]fluorodeoxyglucose PET was performed in another 24 patients. A series of 24 patients underwent stereoelectroencephalography using chronically implanted depth electrodes (33). Details of the surgical procedure have been described elsewhere (33). Postoperative outcome with respect to epileptic seizures was evaluated according to Engel's classification (34). Patients were classified according to the type of epilepsy (SI Table 2): as mesiotemporal lobe epilepsy with hippocampal sclerosis (group 1), mass lesion-related TLE without hippocampal sclerosis (group 2), and extratemporal lobe epilepsy (frontal and occipitotemporal epilepsy) (group 3).
Nonepileptic Patients.
Control cortical tissue was obtained from 11 brain tumor patients in which resection of extralesional tissue was dictated by the technical imperatives of the surgical approach to the tumor (seven women and four men aged from 9 to 80 years; mean of 52 years). The lesion was situated within the temporal (n = 5), frontal (n = 5) or the parietal (n = 1) lobe. The sample tissue was always taken beyond the limits of the apparent lesion. Neuropathological analysis identified several kinds of lesions: glioblastoma (n = 5), meningioma (n = 2), metastasis of melanoma (n = 2), oligodendroglioma (n = 1) and dysembryoplastic neuroepithelial tumor (n = 1).
Cerebral Tissue and Washed Membrane Preparations.
For details see SI Methods.
GABAAR Endogenous Phosphorylation.
The washed membrane P4 fraction (1 mg/ml protein) was incubated for 10 min at 30°C in a 200-μl medium containing 0.33 μM [γ-33P]ATP, 50 mM Hepes·Tris buffer (at pH 7.3), and 1 mM MgCl2. No enzyme or kinase activator was added to the medium. The reaction was stopped by the addition of 500 μl of ice-cold methanol, and the mixture was centrifuged a few seconds at 5,000 × g. At room temperature, chloroform (250 μl) was added and the tube was vortexed and centrifuged again for a few seconds. Water (375 μl) was added, and the tube was vortexed and centrifuged for 1–2 min to clarify the lower-organic from the upper-aqueous phase. Proteins segregated to the phase interface. The upper phase was eliminated with care, and 375 μl of methanol was added. The tubes were thoroughly vortexed for 1 min and centrifuged for 10 min. After eliminating the supernatant, the protein pellet was dried and solubilized in the electrophoresis loading buffer. The samples were then subjected to denaturizing SDS/PAGE. The dried gel was exposed against a phospho-screen for ≈3 days, and the 33P-labeling was detected in a PhosphorImager (GE Healthcare, France). After molecular weight calibration, an integrative counting was made for the band at 51 kDa that corresponds to the α1-subunit of the GABAAR (4).
GABAAR Photoaffinity Labeling.
The washed membrane P4 fraction (1 mg/ml protein) was suspended in 1 ml of a medium containing 10 mM phosphate buffer (pH 7.4) and 10 mM KCl. After addition of 30 nM [3H]flunitrazepam (85 Ci/mmol; Amersham), a photoactivable benzodiazepine, the mixture was preincubated in 2-ml-well culture plates for 60 min at 4°C. Irreversible labeling was then performed by UV-irradiation of the uncovered plates at 365-nm wavelength for 20 min at 4°C under gentle agitation; the source-to-target distance was 4 cm. Aliquots (200 μl) were used for protein precipitation by the methanol/chloroform procedure as described in the previous section. The samples were subjected to SDS/PAGE. After staining, the gel was rehydrated in water for 1 h, then calibrated for the molecular weights. The lanes were cut out from the wet gel and sliced (1 mm) between 46 and 65 kDa. Each individual slice was digested at 60°C for 48 h in a 20-ml stopped vial with 1 ml of 30% H2O2. Radioactivity was counted by liquid scintillation, and the peak at 51 kDa was integrated to measure the α1-specific labeling.
Whole-Cell Patch Clamp on Dissociated Neurons.
The 500-μm thin cortex slices were placed in cold artificial cerebral spinal fluid and gradually warmed to room temperature. Neurons were acutely dissociated from slices by incubation in 3 mg/ml protease type XXIII (Sigma, St. Louis, MO) at 32°C, followed by mechanical dissociation [modified from the method of Kay and Wong (35)]. After washing, the cells were transferred to a Petri dish in a solution containing 135 mM NaCl, 3 mM KCl, 2 mM CaCl2, 10 mM Hepes, 1 mM MgCl2, 7 mM triethylamine chloride, 10 mM d-glucose, and 1 μM tetrodotoxin, adjusted to pH 7.4, and placed on the stage of an inverted microscope (Olympus IMT-2). The neurons were recorded with borosilicate pipettes (resistance of 4–5 MΩ) filled with a solution containing 130 mM CsF, 10 mM CsCl, 4 mM NaCl, 0.5 mM CaCl2, 10 mM Hepes, 5 mM EGTA, and 7 mM Mg-ATP. A whole-cell patch clamp (EPC-7 amplifier; List Electronic) was used to measure the current responses to GABA (100 μM) applied by pressure ejection (10 psi, pulse duration 1 s, every 3 min) from a multichannel glass micropipette placed ≈50 μm from the neuron. Test pulses from another channel containing the extracellular solution were performed to rule out any mechanical artifact. Neurons were held at −80 mV so that GABA application evoked inward current, which reversed at the Cl− equilibrium potential (between −30 and −40 mV). The GABA reversal potential was measured throughout several experiments and remained essentially unchanged. Peak currents were measured for each application and normalized to the maximal response, which was usually obtained 3–6 min after cell penetration. When Mg-ATP was omitted from the pipette solution, the GABA responses declined rapidly and were nearly abolished after 6–9 min. The intrapipette Mg-ATP concentration was maintained at 7 mM throughout the experiments.
Data statistic analysis is presented in SI Methods.
Supplementary Material
Table 1.
Statistical analysis of GABAA current rundown in the different conditions presented in Fig. 4
| Condition |
P values |
|
|---|---|---|
| Two-way ANOVA* (time repeats) | One-way ANOVA† | |
| Epi Std vs. Non Epi Std | <0.001 (10) | 0.0013 (Student) |
| Epi Std vs. Epi+G3P | <0.001 (10) | 0.033 (Dunnett) |
| Epi Std vs. Epi+Van | <0.001 (10) | 0.018 (Dunnett) |
| Epi+G3P vs. Epi+G3P+Iodo | <0.0007 (7) | — |
| Epi+Van vs. Epi+Van+Iodo | NS (7) | — |
Abbreviations are the same as in Fig. 4.
*Normalized current and log[time] with Bonferroni adjustment.
†All times mean current.
Acknowledgments
We are grateful to B. Evrard, D. Toussaint, and J. P. Gagnepain for assistance; to M. Debray for advice on statistics; and to the neurology teams (Drs. M. Bureau, D. Taussig, and J. P. Vignal at Centre Hospitalier Pontchaillou and Drs. F. Chassoux, E. Landré, and M. Mann at Sainte-Anne Hospital).
Abbreviations
- G3P
glyceraldehyde-3-phosphate
- PET
positron-emission tomography
- TLE
temporal lobe epilepsy
- GABAAR
GABAA receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606451104/DC1.
References
- 1.Stelzer A, Kay AR, Wong RKS. Science. 1988;241:339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- 2.Chen QX, Stelzer A, Kay AR, Wong RKS. J Physiol (London) 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laschet JJ, Minier F, Kurcewicz I, Bureau MH, Trottier S, Jeanneteau F, Griffon N, Samyn B, Van Beeumen J, Louvel J, et al. J Neurosci. 2004;24:7614–7622. doi: 10.1523/JNEUROSCI.0868-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minier F, Laschet JJ, Evrard B, Bureau MH. Neurochem Int. 2000;36:499–506. doi: 10.1016/s0197-0186(99)00158-8. [DOI] [PubMed] [Google Scholar]
- 5.Olsen RW, Avoli M. Epilepsia. 1997;38:399–407. doi: 10.1111/j.1528-1157.1997.tb01728.x. [DOI] [PubMed] [Google Scholar]
- 6.Avoli M, Louvel J, Pumain R, Kohling R. Prog Neurobiol. 2005;77:166–200. doi: 10.1016/j.pneurobio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Bureau MH, Laschet JJ. J Biol Chem. 1995;270:26482–26487. doi: 10.1074/jbc.270.44.26482. [DOI] [PubMed] [Google Scholar]
- 8.Babb TL, Brown WJ, Pretorius J, Davenport C, Lieb JP, Crandall PH. Epilepsia. 1984;25:729–740. doi: 10.1111/j.1528-1157.1984.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 9.Burdette DE, Sakurai SY, Henry TR, Ross DA, Pennell PB, Frey KA, Sackellares JC, Albin RL. Neurology. 1995;45:934–941. doi: 10.1212/wnl.45.5.934. [DOI] [PubMed] [Google Scholar]
- 10.Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. J Neurosci. 2000;20:5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koepp MJ, Richardson MP, Labbe C, Brooks DJ, Cunningham VJ, Ashburner J, Van Paesschen W, Revesz T, Duncan JS. Neurology. 1997;49:764–773. doi: 10.1212/wnl.49.3.764. [DOI] [PubMed] [Google Scholar]
- 12.Debets RM, Sadzot B, van Isselt JW, Brekelmans GJ, Meiners LC, van Huffelen AO, Franck G, van Veelen CW. J Neurol Neurosurg Psychiatry. 1997;62:141–150. doi: 10.1136/jnnp.62.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryvlin P, Bouvard S, Le Bars D, De Lamérie G, Grégoire MC, Kahane P, Froment JC, Mauguière F. Brain. 1998;121:2067–2081. doi: 10.1093/brain/121.11.2067. [DOI] [PubMed] [Google Scholar]
- 14.Palma E, Ragozzino DA, Di Angelantonio S, Spinelli G, Trettel F, Martinez-Torres A, Torchia G, Arcella A, Di Gennaro G, Quarato PP, et al. Proc Natl Acad Sci USA. 2004;101:10183–10188. doi: 10.1073/pnas.0403683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartzkroin PA, Haglund MM. Epilepsia. 1986;27:523–533. doi: 10.1111/j.1528-1157.1986.tb03578.x. [DOI] [PubMed] [Google Scholar]
- 16.Avoli M, Louvel J, Drapeau C, Pumain R, Kurcewicz I. J Neurophysiol. 1995;73:468–484. doi: 10.1152/jn.1995.73.2.468. [DOI] [PubMed] [Google Scholar]
- 17.D'Antuono M, Louvel J, Kohling R, Mattia D, Bernasconi A, Olivier A, Turak B, Devaux A, Pumain R, Avoli M. Brain. 2004;127:1626–1640. doi: 10.1093/brain/awh181. [DOI] [PubMed] [Google Scholar]
- 18.Wittner L, Eross L, Czirjak S, Halasz P, Freund TF, Magloczky Z. Brain. 2005;128:138–152. doi: 10.1093/brain/awh339. [DOI] [PubMed] [Google Scholar]
- 19.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 20.Kann O, Kovacs R, Njunting M, Behrens CJ, Otahal J, Lehmann TN, Gabriel S, Heinemann U. Brain. 2005;128:2396–2407. doi: 10.1093/brain/awh568. [DOI] [PubMed] [Google Scholar]
- 21.Williamson A, Patrylo PR, Pan J, Spencer DD, Hetherington H. Brain. 2005;128:1199–1208. doi: 10.1093/brain/awh444. [DOI] [PubMed] [Google Scholar]
- 22.Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Fowler J, Hoffman E, Alavi A, et al. Circ Res. 1979;44:127–137. doi: 10.1161/01.res.44.1.127. [DOI] [PubMed] [Google Scholar]
- 23.Engel J, Jr, Kuhl DE, Phelps ME, Mazziotta JC. Ann Neurol. 1982;12:510–517. doi: 10.1002/ana.410120603. [DOI] [PubMed] [Google Scholar]
- 24.Henry TR, Mazziotta JC, Engel J, Jr, Christenson PD, Zhang JX, Phelps ME, Kuhl DE. J Cereb Blood Flow Metab. 1990;10:748–757. doi: 10.1038/jcbfm.1990.128. [DOI] [PubMed] [Google Scholar]
- 25.Chassoux F, Semah F, Bouilleret V, Landre E, Devaux B, Turak B, Nataf F, Roux RX. Brain. 2004;127:164–174. doi: 10.1093/brain/awh014. [DOI] [PubMed] [Google Scholar]
- 26.Attwell D, Laughlin SB. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Ackermann RF, Finch DM, Babb TL, Engel J., Jr J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peyron R, Le Bars D, Cinotti L, Garcia-Larrea L, Galy G, Landais P, Millet P, Lavenne F, Froment JC, Krogsgaard-Larsen P, Mauguière F. Epilepsy Res. 1994;19:45–54. doi: 10.1016/0920-1211(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 29.Peyron R, Cinotti L, Le Bars D, Garcia-Larrea L, Galy G, Landais P, Millet P, Lavenne F, Froment JC, Krogsgaard-Larsen P, Mauguière F. Epilepsy Res. 1994;19:55–62. doi: 10.1016/0920-1211(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 30.Semah F, Baulac M, Hasboun D, Frouin V, Mangin JF, Papageorgiou S, Leroy-Willig A, Philippon J, Laplane D, Samson Y. Epilepsia. 1995;36:447–456. doi: 10.1111/j.1528-1157.1995.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer CC, Adelson PD, Brenner RP, Crumrine PK, Van Cott A, Schiff DP, Townsend DW, Schreur ML. Epilepsia. 2000;41:193–200. doi: 10.1111/j.1528-1157.2000.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 32.Vlassenko AG, Rundle MM, Raichle ME, Mintun MA. Proc Natl Acad Sci USA. 2006;103:1964–1969. doi: 10.1073/pnas.0510632103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talairach J, Bancaud J. In: Methodology of Functional Stereotaxic Inventigations. Krayenbühl H, Maspes PE, Sweet WH, editors. Basel: Karger; 1973. pp. 297–354. [Google Scholar]
- 34.Engel J, Jr, Van Ness PC, Rassmussen TB, Ojemann LM. In: Surgical Treatment of the Epilepsies. Engel J Jr, editor. New York: Raven; 1993. pp. 609–621. [Google Scholar]
- 35.Kay AR, Wong RK. J Physiol (London) 1987;392:603–616. doi: 10.1113/jphysiol.1987.sp016799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.