Abstract
HIV infection of the central nervous system can result in neurologic dysfunction with devastating consequences in AIDS patients. NeuroAIDS is characterized by neuronal injury and loss, yet there is no evidence that HIV can infect neurons. Here we show that the HIV-encoded protein tat triggers formation of a macromolecular complex involving the low-density lipoprotein receptor-related protein (LRP), postsynaptic density protein-95 (PSD-95), N-methyl-d-aspartic acid (NMDA) receptors, and neuronal nitric oxide synthase (nNOS) at the neuronal plasma membrane, and that this complex leads to apoptosis in neurons negative as well as positive for NMDA receptors and also in astrocytes. Blockade of LRP-mediated tat uptake, NMDA receptor activation, or neuronal nitric oxide synthase significantly reduces ensuing neuronal apoptosis, suggesting that formation of this complex is an early step in tat toxicity. We also show that the inflammatory chemokine, CCL2, protects against tat toxicity and inhibits formation of the complex. These findings implicate the complex in HIV-induced neuronal apoptosis and suggest therapeutic targets for intervention in the pathogenesis of NeuroAIDS.
Keywords: glutamate, dementia, HIV-1, NeuroAIDS, excitotoxicity
HIV enters the CNS early after infection. Viral persistence within the CNS can produce cognitive impairment, HIV encephalitis, and, in some cases, dementia. NeuroAIDS is characterized by neuronal damage and loss and cognitive and motor deficits and can have devastating consequences in a significant number of individuals with AIDS. As HIV-infected individuals live longer on antiretroviral therapy, the prevalence of cognitive impairment is increasing, and the study of the pathogenesis of NeuroAIDS becomes even more critical (1, 2).
Although HIV infection of the CNS causes neuronal cell damage and loss, the virus cannot directly infect neurons. Rather, HIV-associated damage is thought to be due to an indirect mechanism whereby virally infected, as well as uninfected, cells elaborate neurotoxins. Candidate toxins include cytokines, glutamate, and virally encoded proteins such as the HIV transactivator protein, tat (3). Tat potentiates glutamate-induced excitotoxicity (4, 5) and promotes neuronal apoptosis (6–8). Antagonists of the N-methyl-d-aspartic acid (NMDA) receptor (NMDAR) protect against tat-induced apoptosis (5, 7), implicating NMDARs in this process.
The low-density lipoprotein receptor-related protein (LRP) is expressed by many cells in the CNS, including neurons and astrocytes (9, 10). LRP is a receptor for at least 16 endogenous ligands and also for the viral protein tat, and mediates uptake of these ligands into endosomes in various cells, including neurons (9). Tat and some other LRP ligands can activate NMDARs and mediate calcium signaling in neurons (4, 11, 12). Tat, in contrast to other LRP ligands, escapes from the endosomal/lysosomal compartment (9) and, by mechanisms still poorly known, induces apoptosis in both neurons and astrocytes.
This study was undertaken to examine mechanisms by which tat promotes NMDAR-dependent apoptosis of neurons and astrocytes. Postsynaptic density protein-95 (PSD-95) is a candidate factor, as a scaffolding protein that acts through its PDZ-binding domains to interact with a number of postsynaptic proteins (13) including NMDARs (14–16) and LRP (17). A critical signaling protein downstream of NMDARs and implicated in apoptosis is neuronal nitric oxide synthase (nNOS) (18). Activation of nNOS depends on its association with PSD-95 and NMDAR-mediated calcium influx (19). We show here that tat treatment of cultures of human cells highly enriched in neurons promotes the formation of a macromolecular complex involving LRP, PSD-95, NMDARs, and nNOS, leading to the generation of NO and subsequent apoptosis in NMDAR-negative as well as -positive neurons and, although to a lesser extent, in astrocytes. These findings implicate this complex in the pathogenesis of NeuroAIDS.
CCL2 is elevated in the brain and cerebrospinal fluid of HIV-positive individuals and in macaques with simian immunodeficiency virus (SIV) encephalitis (20–23). CCL2 is a chemoattractant for monocytes, inducing their entry into the CNS, promoting inflammation, and thereby contributing to neuronal and glial damage. However, elevated CCL2 levels in infected macaques early in pathogenesis do not correlate well with inflammatory damage (24), suggesting additional actions of this chemokine. Consistent with this finding, we found that CCL2 protects against tat-induced apoptosis in vitro (7). Now we demonstrate that CCL2 treatment of neuronal cultures inhibits tat-mediated formation of the LRP–PSD–95–NMDAR–nNOS complex, providing a mechanism for its neuroprotective properties.
Results
The LRP Receptor Is Essential for Tat-Induced Apoptosis in Cultures of Human Neurons and Astrocytes.
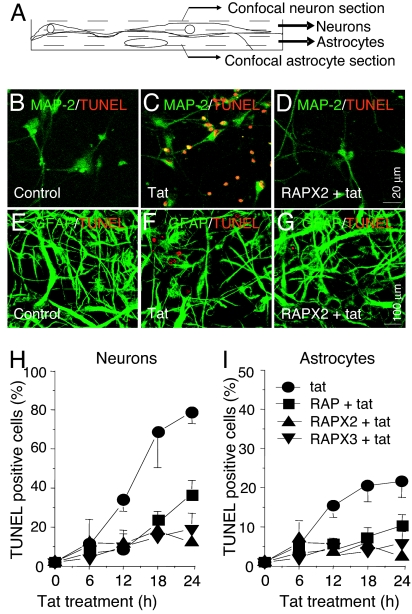
Addition of tat (10 ng/ml) to human mixed cultures (60–80% neurons, 20–40% astrocytes) resulted in extensive apoptosis by 24 h: ≈70% of neurons and ≈20% of astrocytes were TUNEL positive (Fig. 1B, C, and H for neurons and Fig. 1 E, F, and I for astrocytes; see ref. 7). Similar data were obtained for Annexin-5 labeling [supporting information (SI) Fig. 5]. Although only 25–35% of the neurons were NMDAR positive (7), the NMDAR blockers MK801 (7) and AP-5 (see Fig. 4 B and C, third and fourth bars from the left) almost completely inhibited apoptosis in both neurons and astrocytes (#, P < 0.005 vs. tat alone). These findings suggest that NMDAR-positive neurons are crucial for initiating tat-induced apoptosis. Although some NMDAR-positive cells become apoptotic and most NMDAR-negative cells also die, we do not know whether the relative mortality is the same for both types of cell. Similarly, we do not understand the basis of the limited mortality of astrocytes.
Fig. 1.
LRP is necessary for tat-induced apoptosis. (A) Schematic illustrating cocultures, with neurons growing on top of astrocytes; neurons were imaged in a more superficial optical section. (B–D) Double staining for TUNEL (apoptosis) and MAP-2 (neurons). (E–G) Staining for TUNEL and GFAP (astrocytes). There was minimal apoptosis in untreated cultures (B and E), increased apoptosis after 24 h of tat treatment (C and F), and reduced apoptosis when the LRP inhibitor, RAP, was applied 15 min before and 6 h after tat (D and G). (H and I) Percentage of TUNEL-positive cells after different durations of treatment with tat alone (●) or with tat plus RAP. Control cells without treatment did not show increased apoptosis (data not shown). Addition of RAP once (15 min before tat treatment, RAP, ■), twice (a second application 6 h after tat treatment, RAPX2, ▴), or three times (at 6 and 12 h after tat treatment, RAPX3, ▾) reduced tat-induced apoptosis in neurons and astrocytes (P < 0.05 for all treatments vs. control, n = 7, no significant difference between RAP treatments).
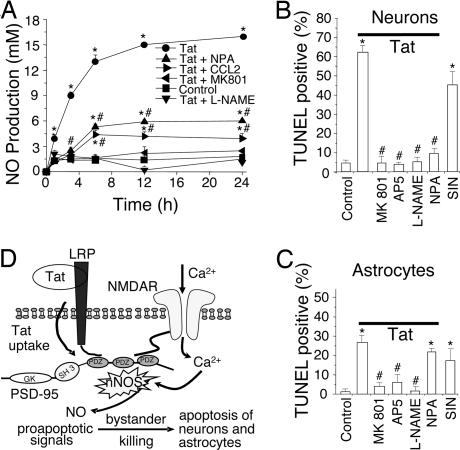
Fig. 4.
Tat induces NO production, primarily through nNOS activation, resulting in neuron and astrocyte apoptosis. (A) Cultures were treated with tat (●) or tat plus NPA (▴), CCL2 (▶), MK801 (◀), or L-NAME (▾). At different time points, medium was collected and NO production was measured by the Griess reaction. Tat treatment induced significant NO production over the untreated condition (■; P < 0.001). MK801 or L-NAME abolished tat-induced production of NO (P < 0.001 vs. tat alone). NPA or CCL2 reduced tat-induced production of NO (P < 0.001) but not to basal levels, suggesting a source of NO in addition to nNOS. The addition of MK801, L-NAME, NPA, or CCL2 alone did not change basal NO production (data not shown). ∗, P < 0.001 vs. control; #, P < 0.001 for a treatment compared with tat alone (n = 4). (B and C) Summary data of apoptosis in neurons and astrocytes after 24-h treatment. Tat induced a high percentage of apoptosis compared with control (first two bars on the left). The NMDA blockers, MK801 and AP5, provided substantial protection (third and fourth bars). The general NOS antagonist, L-NAME, blocked apoptosis in both cell types. The nNOS-specific blocker, NPA, greatly inhibited neuronal apoptosis with little effect on astrocyte apoptosis (sixth bar). The NO donor, SIN, alone (seventh bar) induced almost as much apoptosis as tat [∗, P < 0.001 vs. control conditions and #, P < 0.001 compared with tat treatment (n ≥ 6)]. (D) Proposed mechanism of tat-induced apoptosis. Tat induces formation of a macromolecular complex of LRP, PSD-95 [protein with three PDZ domains, an SH3 and guanylyl kinase (GK)-like domain], NMDAR, and nNOS. The formation of this complex generates proapoptotic signals, such as NO, that can be transmitted to cells lacking NMDARs, resulting in extensive apoptosis.
Internalization of tat by neurons is LRP-dependent (9) and occurs within 1 h of tat treatment. However, it was not known whether LRP is required for tat-induced apoptosis. The LRP inhibitor protein, receptor-associated protein (RAP), is an endoplasmic chaperone for LRP. When applied extracellularly, RAP tightly binds to LRP, blocking uptake of LRP ligands, including tat (9, 25). RAP added to cultures 15 min before tat treatment resulted in a significant reduction in the number of TUNEL-positive neurons and astrocytes compared with tat treatment alone (Fig. 1 H and I; P < 0.05 at 12, 18, and 24 h). Because LRP is subject to recycling as well as internalization and degradation (25), we also tested multiple additions of RAP to maintain an effective extracellular concentration over time. When RAP was added 15 min before and 6 h after tat (RAPX2+Tat, Fig. 1 D and G–I) or 15 min before and 6 and 12 h after tat (RAPX3+Tat, Fig. 1 H and I), the number of TUNEL-positive neurons was also reduced but not to a significantly greater extent than with a single application. Treatment of neuronal cultures with RAP alone did not affect basal apoptosis (data not shown).
To determine whether LRP-mediated apoptosis was tat-specific, cultures were treated with three other LRP ligands, lactoferrin (50 units/ml), α2-macroglobulin (α2M, 500 nM), or ApoE4 (200 nM), and TUNEL positivity was analyzed at 12 and 24 h. Only ApoE4 induced a significant increase in the number of TUNEL-positive neurons and astrocytes over baseline but to a significantly lower extent than that observed for tat (†, P < 0.001 vs. tat; see Table 1). These findings demonstrate that, of the LRP ligands, tat is unique in its ability to induce high levels of apoptosis in neurons and astrocytes, and that blocking of tat–LRP interaction by RAP significantly reduces apoptosis, suggesting that this interaction is required for tat-induced cell death in both cell types.
Table 1.
LRP ligands other than tat do not induce high levels of apoptosis after 24 h of treatment
| Condition | Neuronal apoptosis, % | Astrocyte apoptosis, % |
|---|---|---|
| Control | 2.8 ± 1.4 | 1.6 ± 0.4 |
| Tat | 61.2 ± 9.7* | 18.7 ± 5.8* |
| Lactoferrin | 3.2 ± 0.23 | 1.5 ± 0.6 |
| α2macroglobulin | 1.8 ± 0.8 | 1.1 ± 0.7 |
| ApoE4 | 7.8 ± 1.7*† | 3.3 ± 0.9*† |
Values represent mean ± SD (n = 5).
*, P < 0.005 compared with control conditions.
†, P < 0.001 compared with tat conditions.
Tat Induces the Formation of a Macromolecular Complex at the Surface of Neurons.
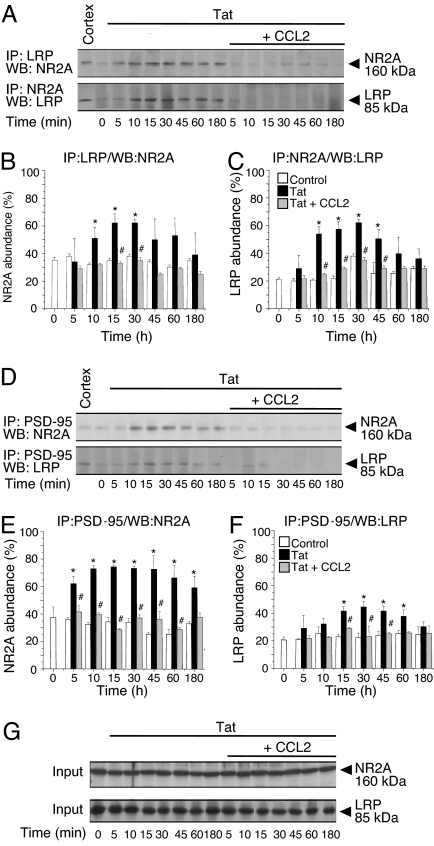
The observation that tat-induced apoptosis is both NMDAR- and LRP-dependent is consistent with a physical interaction between these two receptors. To examine this question, we applied tat to mixed neuron and astrocyte cultures, and because tat is found in the nucleus within 1 h (9), prepared cell lysates at early time points after tat treatment (0–180 min); we then performed coimmunoprecipitation (CoIP) experiments with antibodies to NMDAR subunit 2A (NR2A) and LRP. Reciprocal CoIPs for LRP and NR2A and Western blotting for NR2A and LRP, respectively, demonstrated that LRP and NMDAR interact in cells treated with tat (Fig. 2A). Significant interaction between NR2A and LRP occurred at 10–45 min after tat treatment (Fig. 2 A–C; *, P < 0.05). At later times the amount of protein CoIP decreased, and by 180 min, it had returned to near control levels (Fig. 2 A–C). The NR2B subunit was not detected in the LRP immunoprecipitate (data not shown).
Fig. 2.
NR2A and LRP form a complex after tat treatment, and PSD-95 may mediate complex formation. (A) LRP or NR2A antibodies were used for immunoprecipitation (IP) from lysates of control (time 0) cultures or cultures treated with tat or tat plus CCL2 for 5, 10, 15, 30, 45, 60, and 180 min. Samples were analyzed by Western blotting (WB) with the antibody indicated; input samples were used as loading controls. Human cortex lysate (Cortex) was used as a positive control for NR2A and LRP. (B and C) Densitometric analysis of the CoIP protein compared with the amount in the input (n = 5). Tat treatment increased association of NR2A and LRP maximally at 10–45 min (∗, P < 0.05 vs. control). The association was largely blocked by CCL2 (#, P < 0.05 vs. tat alone). (D) PSD-95 was immunoprecipitated from lysates of control cultures (time 0) or cultures treated with tat or tat plus CCL2 and analyzed by WB with antibodies to NR2A and to LRP. (E and F) Quantification of these data (n = 5). Tat treatment increased association of PSD-95 with LRP and with NR2A (∗, P < 0.05 vs. control). These effects were blocked by CCL2 (#, P < 0.05 vs. tat treatment). (G) The total input protein was used as a loading control for each IP (Input and data not shown).
PSD-95, a member of the MAGUK family, contains PDZ domains that bind many proteins localized to the synaptic area (26). Although PSD-95 can interact with NMDARs (14–16, 27) and with LRP (17), the impact of tat on these interactions was not known. Tat treatment markedly increased the association between PSD-95 and NR2A (Fig. 2 D and E) and between PSD-95 and LRP (Fig. 2 D and F), consistent with the possibility that tat induces the formation of a complex involving LRP, PSD-95, and NMDARs. Reciprocal CoIPs between NR2A/PSD-95 and LRP/PSD-95 showed similar levels of interaction (data not shown).
Because the chemokine CCL2 protects neuronal cultures from tat-induced apoptosis (7), we determined whether complex formation was altered in the presence of CCL2. In CoIP experiments, CCL2 and tat cotreatment resulted in significantly less complex formation than did tat treatment alone (Fig. 2 A–F, #, P < 0.05). Changes in complex formation were not due to changes in the overall amount of protein because no differences were detected in the total amount of LRP, PSD-95, or NMDAR in lysates from control, tat, or CCL2 plus tat-treated cultures (Fig. 2G and data not shown).
Confocal analyses of the neuronal surface (SI Fig. 6) confirmed the CoIP data. We counted pixels over neurons for NR1 and LRP fluorescence above a threshold value, as set by the background fluorescence of isotype-matched nonspecific IgG control, and determined the percentage of total NR1 pixels that were LRP positive and the percentage of total LRP pixels that were NR1 positive. In control conditions, only ≈30% of NR1 pixels were LRP positive, but the percentage more than doubled after tat treatment (SI Fig. 6 B–G and M; *, P < 0.05 vs. control, n = 5). Similarly, the percentage of LRP pixels with NR1 increased from ≈50% to >90% after tat treatment (SI Fig. 6 B–G and N; *, P < 0.05 vs. control, n = 5). We also found that total LRP and NR1 labeling per neuron did not differ in control, tat-treated, or CCL2 plus tat-treated cultures (SI Fig. 6O). Colocalization of LRP and NR1 in CCL2 plus tat-treated cultures was not significantly different from that in control conditions (SI Fig. 6 H–J, M, and N; #, P < 0.05 vs. tat alone, n = 5); thus, CCL2 prevented tat-induced association of LRP and NR1. Treatment with CCL2 alone did not detectably alter localization of either of the two proteins (data not shown). These findings demonstrate that tat enhances colocalization of NMDARs and LRP at the surface of neurons, an action that could be mediated by the synaptic scaffolding protein PSD-95, and that is blocked by the chemokine CCL2.
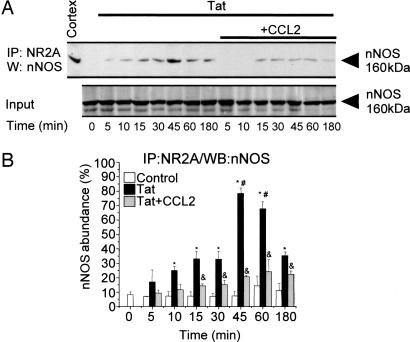
Tat Treatment Causes Activation of nNOS Through Association with NMDARs.
Apoptosis can be induced by nNOS activation and subsequent nitric oxide (NO) production (28). In ischemic models, activation of nNOS by calcium influx through NMDARs requires association of nNOS with PSD-95 and NMDARs (19, 29). In our CoIP experiments, tat treatment of cultures induced association of nNOS and NR2A (Fig. 3; P < 0.05, n = 5), an interaction that might be mediated through PSD-95. Maximal association of nNOS and NR2A occurred between 30 and 60 min (Fig. 3B; *, P < 0.05 vs. control; #, P < 0.05 vs. 10–30 min tat treated; n = 5). Association was blocked by cotreatment with CCL2 (Fig. 3 A and B; &, P < 0.05 vs. tat alone, n = 5).
Fig. 3.
Tat treatment enhances interaction between NR2A and nNOS. (A) NR2A was immunoprecipitated (IP) from lysates of control, untreated cultures (time 0), or cultures treated with tat or tat plus CCL2 for 5, 10, 15, 30, 45, 60, and 180 min, and precipitates were analyzed by Western blotting (WB) for nNOS interaction. Total protein lysate was used as a loading control (Input). (B) Densitometric analysis (n = 3) of nNOS abundance in the CoIP relative to input. No or low interaction between NR2A and nNOS was found in control cells. Tat treatment resulted in nNOS association with NR2A that was maximal between 30 and 60 min after tat treatment (∗, P < 0.05 vs. control; #, P < 0.05 vs. 10–30 min tat treated). CCL2 cotreatment significantly inhibited tat-induced NR2A/nNOS complex formation (&, P < 0.05 vs. tat alone).
Analysis of tangential confocal sections demonstrated that tat treatment increased the percentage of NR1 pixels that were nNOS positive from ≈15% to ≈45% and the percentage of nNOS pixels that were NR1 positive from ≈70% to ≈90% (SI Fig. 7 B–G and K; P < 0.05 vs. control for both parameters, n = 14). Tat induced an increase in colocalization of nNOS and NR1, which was blocked by CCL2 (SI Fig. 7 H–L; #, P < 0.05 vs. tat alone, n = 14). Tat increased the number of nNOS-positive pixels (Fig. 7M; *, P < 0.05 vs. control). These data are in agreement with the NR1/nNOS CoIP data (Fig. 3) and support the hypothesis that tat leads to localization of nNOS to where it can be activated by Ca2+ influx through NMDARs.
Because tat likely leads to recruitment of nNOS to the PSD-95/NMDAR complex (Fig. 3 and SI Fig. 7), we tested our cultures for tat-induced NO generation. Addition of tat induced NO generation in a time-dependent manner (Fig. 4A; *, P < 0.0001 vs. control). Tat increased the amount of NO in the medium for as long as 24 h, the latest time point tested. Tat-induced NO generation was completely blocked with the nonselective NOS inhibitors, L-NAME (3 mM; Fig. 4A, P < 0.0001 vs. tat alone) or L-NMMA (3 mM; data not shown). The addition of a highly selective inhibitor of nNOS, Nω-propyl-l-arginine (NPA; 3 mM) (30), with tat significantly reduced tat-induced NO production (Fig. 4A; #, P < 0.0001 for tat plus NPA vs. tat alone), but NO levels were still elevated relative to controls (Fig. 4A; *, P < 0.0001 for tat plus NPA vs. control). These results suggest that there was an additional source of NO, most likely astrocytes, that was insensitive to nNOS-specific inhibitors. However, tat treatment does not cause apoptosis in pure cultures of astrocytes (7). The stimulus for astrocyte-derived NO in these cultures must have come from the NMDAR-positive neurons because the NMDA channel blockers, MK801 (Fig. 4A) or AP-5 (data not shown), reduced tat-induced NO production to control levels. The addition of CCL2 to tat-treated cultures also reduced NO levels to near control values (Fig. 4A).
Tat-Induced Activation of nNOS Participates in Neuronal Apoptosis, but Astrocyte Apoptosis Requires an Additional Source of NO.
To examine whether NO production is necessary for tat-induced apoptosis in both neurons and astrocytes, cultures were treated with tat in the absence or presence of the NOS enzyme inhibitors, L-NAME and L-NMMA. The addition of L-NAME with tat to cultures resulted in complete block of the tat-induced apoptosis in both neurons and astrocytes (Fig. 4 B and C, fifth bar from left; P < 0.0001 vs. tat for both neurons and astrocytes, not significant vs. control). Similar results were found with L-NMMA treatment (data not shown). These findings strongly suggest that NO production is necessary for tat-induced apoptosis in both cell types. However, the highly selective inhibitor of nNOS, NPA, added to cultures along with tat resulted in marked reduction of apoptosis in neurons but not in astrocytes (Fig. 4B, sixth bar from left; #, P < 0.0001 for tat plus NPA vs. tat alone, tat plus NPA vs. control, n.s., n = 8; Fig. 4C; *, P < 0.05 vs. control; n.s. vs. tat, n = 6). This finding suggests that although NMDAR-positive neurons contribute to the induction of astrocyte apoptosis, as evidenced by the protective effect of NMDAR blockers, a signal distinct from that resulting from activation of nNOS is necessary for apoptosis in astrocytes. The complete block of astrocyte apoptosis by L-NAME (fifth bar from left as already noted) indicates that NO participates in astrocyte apoptosis, although its source is not nNOS. This alternative source may be from up-regulation of iNOS in astrocytes treated with tat (31), which sensitizes the cells to an additional but unknown proapoptotic factor from NMDAR-positive neurons that is necessary for astrocyte apoptosis. To determine whether NO is sufficient to mediate apoptosis in our cultures, cells were treated with the NO donor, SIN-1 (SIN, Fig. 4 B and C, rightmost bar). SIN-1 (which generated ≈2 × as much NO as tat alone, not illustrated) caused apoptosis in neurons (Fig. 4B) and astrocytes (Fig. 4C) to about the same extent as did tat itself (determined after 24 h). These findings demonstrate that nNOS activation by tat treatment in NMDA neurons and subsequent NO production elicit extensive apoptosis in neurons; and that an additional factor, possibly NO, that is not dependent on nNOS is required for apoptosis of astrocytes.
Discussion
HIV infection of the CNS is characterized by neuronal injury and loss despite the inability of HIV to infect neurons. Here we show that treatment of mixed cultures of neurons and astrocytes with the HIV protein, tat, induces the formation of a protein complex of LRP, PSD-95, NMDAR, and nNOS in the plasma membrane of NMDAR-positive neurons. We further show that interaction among these proteins is critical to activation of nNOS and generation of NO, promoting apoptosis in two-thirds of the neurons and one-quarter of the astrocytes (see model in Fig. 4D). Addition of the inflammatory chemokine CCL2 inhibits formation of the macromolecular complex and protects both neurons and astrocytes. This mechanism of tat toxicity shows how a soluble factor, tat, released by HIV-infected cells can kill neurons and suggests a mechanism by which tat induces cognitive impairment in individuals with CNS infection. Our data also emphasize an alternative, noninflammatory, neuroprotective role for CCL2 during HIV neuropathogenesis.
Tat is a viral protein released from HIV-infected cells and taken up by cells (3). Extracellular tat has been detected in the brains of individuals with HIV dementia and encephalitis (32, 33) and is toxic to neurons (6–8). Because neurons are not directly infected, tat-induced neuronal and astrocytic apoptosis is an indirect mechanism by which CNS HIV infection leads to neuronal and glial loss during the course of NeuroAIDS. Uptake of tat by neurons is mediated primarily by LRP (9), indicating that tat-induced apoptosis is a specific process involving participation of cellular proteins. LRP and its ligands regulate diverse effects including cytokine production and synaptic plasticity (25) and are not usually associated with cell death. However, our data indicate that LRP-mediated binding of tat is required for cell death because the specific LRP blocker, RAP, prevented tat-induced neuronal and astrocyte apoptosis. Unlike other LRP ligands, tat escapes from the endosome/lysosome degradation pathway and localizes to the nucleus, where it has been found to alter transcription of some cellular genes (9). Nuclear localization and tat transcriptional effects as well as the complex formation described in this report may be contributing to neuronal apoptosis. Although NMDA-negative neurons express LRP (9) and presumably take up tat, its action is not sufficient to trigger apoptosis because NMDA blockers protect all of the neurons.
The role of NMDARs in tat-induced neurotoxicity has not been well characterized. The data that MK801 and AP-5 almost completely block tat-induced neuronal and astrocyte apoptosis, although only 25–35% of the neurons in our cultures express NMDARs, indicate that NMDARs play a key role in triggering apoptotic signals that affect not only the NMDAR-expressing cells but also are transmitted to other cells. NMDARs are one of the major glutamate-activated ionotropic receptors in the brain and are permeable to sodium, potassium, and calcium. Compromised neuronal viability due to excessive NMDAR activation has been described in several neurodegenerative diseases (34, 35) including HIV dementia (36). We demonstrate the contribution of activated NMDARs as part of the tat–LRP–PSD-95–NMDAR–nNOS macromolecular complex to tat-induced apoptosis, and identify NO as a major downstream effector.
The interaction of PSD-95 with NMDARs results in increased probability of NMDAR channel opening, delivery, and insertion of new channels into the membrane, potentiated currents, and a decreased internalization rate of NMDARs, (15), suggesting that this interaction is critical to maintenance/enhancement of NMDAR activity. Based on these observations, formation of the LRP–PSD-95–NMDAR–nNOS macromolecular complex initiated by tat might be expected to stabilize NMDARs on the cell surface and/or potentiate currents through the NMDARs. This could lead to greater signal generation by the NMDAR, including those signals that become toxic to neighboring cells.
Because the majority of cells in this culture system do not express NMDARs, and because we demonstrated that the NMDAR-positive cells initiate the apoptotic process, there must be a mechanism(s) to induce apoptosis in NMDAR-negative neurons and astrocytes. Two possibilities are: (i) activation of NMDARs resulting in calcium overload and calcium-dependent release of second messengers, such as IP3, and ATP and glutamate; and (ii) activation of nNOS, resulting in NO production and release, and subsequent mitochondrial damage and second messenger signaling. We found that tat treatment activates nNOS and NO production in our human neuronal cultures. NO, because of its diffusibility, is a good candidate for inducing apoptosis in NMDAR-negative cells.
NO was generated in our cultures after tat treatment. Although only approximately one-third of the neurons express NMDARs, MK801 totally abolished NO production and blocked apoptosis in neurons and astrocytes, further underscoring the importance of the NMDAR-positive cells in generating the NO signal. The specific nNOS inhibitor, NPA, reduced NO production and prevented neuronal, but not astrocyte apoptosis, indicating that NO from neurons is important for neuronal, but not astrocyte, cell death. NPA also did not reduce NO production to basal levels in our cultures, although more general NOS blockers did, suggesting that there is an additional source of NO, likely iNOS from astrocytes that has been shown to be induced by tat (31). We hypothesized that iNOS activity is triggered at least in part by NMDAR-positive neurons, as indicated by our MK801 experiments. Also in support of a role for the NMDAR-positive neurons in tat-induced astrocyte apoptosis, previously published data indicate that pure cultures of astrocytes treated with tat do not undergo apoptosis (7). The specific signals from the NMDAR-positive neurons to astrocytes are not yet known. However, they require NMDAR activation but not nNOS activation.
We established that tat induces NO production through interaction and activation of nNOS by NMDARs and that this NO is crucial for apoptosis in neurons. The activation of nNOS in models of ischemia depends on the interactions among NMDARs, PSD-95, and nNOS, as well as on Ca2+ from NMDAR activation (19). The exact mechanism of NO-induced neuronal apoptosis in our system has not been determined. NO activates the p38 apoptotic pathway in the context of glutamate-induced neuronal apoptosis (37) and may be contributing to apoptotic signaling in our system through this mechanism. Alternatively, high NO production leads to the generation of free radicals and mitochondrial damage (18), which also may be occurring in response to tat.
Although nitric oxide is a necessary signal for tat-induced apoptosis, it may not be the only signal, particularly at later time points in the apoptotic process. For example, extracellular glutamate could be playing a role. We previously demonstrated that tat (12–24 h) causes release of glutamate (7). Thus, at later time points, tat-induced glutamate release may also contribute to calcium overload and excitotoxicity through overactivation of NMDARs and other glutamate receptors mediated by high levels of extracellular glutamate (7) and by dysregulation of glutamate metabolism in astrocytes. However, these processes must be initiated by NMDAR-positive neurons because blocking NMDARs prevents tat-induced apoptosis in all cell types.
Studies suggest that CCL2 facilitates the progression of NeuroAIDS by recruiting monocytes to the CNS (38), thus increasing inflammation and providing cells for viral replication; however, CCL2 levels do not always correlate with inflammation (39, 40). We propose that CCL2 has an alternative role and is neuroprotective during some stages of the disease. CCL2 prevents tat-induced complex formation and thereby interferes with the early events in tat-induced apoptosis and protects neurons and astrocytes from tat toxicity. CCL2 may further interfere with the apoptotic process through activating survival signaling cascades and by regulating glutamate receptor trafficking, extracellular levels of glutamate (7), and proapoptotic signaling.
The characterization of this tat-regulated protein complex in NMDAR-positive neurons provides a mechanism for the early steps of tat-induced apoptosis in neurons and astrocytes using the synaptic machinery already expressed by CNS cells (see model Fig. 4D). An understanding of the early mechanisms of tat toxicity as well as the role for CCL2 as a mediator of neuroprotection may lead to new interventional strategies to limit the progression of NeuroAIDS.
Materials and Methods
Human fetal cortical tissue was used and cultured under a protocol approved by the Albert Einstein College of Medicine (41) (see SI Methods for details and sources of reagents). Tat protein was expressed from the tat gene derived from the HIV-BRU strain inserted into an Escherichia coli vector. Tat (10 ng/ml) was added to neurons in the absence or presence of CCL2 (100 ng/ml). Apoptosis was assayed by a TUNEL reaction kit (Roche, Mannheim, Germany). Anti-GFAP and anti-MAP2 were used to identify astrocytes and neurons, respectively. Other primary antibodies were anti-LRP, anti-NR1, anti-NR2A, anti-nNOS, anti-PSD-95, and anti-Annexin-5.
Colocalization of proteins at the surface of neurons was analyzed with confocal microscopy by counting the number of pixels labeled for either or both of two proteins per unit of area in superficial sections.
CoIP with an appropriate antibody was followed by PANSORBIN, pelleting, and resuspension in sample buffer and analysis by SDS/PAGE and Western blotting.
NO was detected by using a kit (Cayman Chemical, Ann Arbor, MI) to detect the end products of NO production, nitrite, and nitrate.
Differences were evaluated by nonparametric Kruscal–Wallis analysis, a Bonferonni–Dunn multiple-comparison test, or a two-tailed, paired t test.
Supplementary Material
Acknowledgments
We are grateful to Dr. Brad Poulos and The Fetal Tissue Repository, the Analytical Imaging Facility at the Albert Einstein College of Medicine (AECOM), and the Center for AIDS Research (CFAR) Immunology/Pathology Core. This work was supported by the National Institutes of Health (NIH) Grants MH052974, MH070297, MH075679, NS11920 (to E.A.E., J.E.K., T.M.C., and J.W.B.), NS20752 (to R.S.Z.), and NS45287 (to M.V.L.B.); AECOM CFAR Grant AI-051519 and Medical Scientist Training Program Training Grant 5 T32 GM007288 (to J.E.K.), and NIH/National Center for Research Resources/Centers of Biomedical Research Excellence Grant P20RR015592 (to A.N.). M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience and Distinguished Professor at the Albert Einstein College of Medicine.
Abbreviations
- NMDAR
N-methyl-d-aspartic acid receptor
- nNOS
neuronal nitric oxide synthase
- iNOS
inducible NOS
- CoIP
coimmunoprecipitation
- NPA
Nω-propyl-l-arginine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611699104/DC1.
References
- 1.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 2.Ghafouri M, Amini S, Khalili K, Sawaya BE. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 4.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Barks JD, Silverstein FS. Neuroscience. 1999;88:585–597. doi: 10.1016/s0306-4522(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 6.Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- 7.Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 8.Kruman II, Nath A, Mattson MP. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- 10.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 11.Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. Proc Natl Acad Sci USA. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu Z, Crutcher KA, Hyman BT, Rebeck GW. Neuroscience. 2003;122:291–303. doi: 10.1016/j.neuroscience.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Kim E, Sheng M. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 14.Carroll RC, Zukin RS. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niethammer M, Kim E, Sheng M. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ, Herz J. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang Y, Kim P, Choi B, Chung H, Ha K, Kwon Y, Kim Y. In Vivo. 2004;18:367–376. [PubMed] [Google Scholar]
- 19.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 20.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 22.McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Am J Pathol. 2000;156:1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- 24.Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- 25.Herz J, Strickland DK. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng M, Sala C. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Gardoni F, Schrama LH, Kamal A, Gispen WH, Cattabeni F, Di Luca M. J Neurosci. 2001;21:1501–1509. doi: 10.1523/JNEUROSCI.21-05-01501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaffrey SR, Snyder SH. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- 29.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. J Med Chem. 1997;40:3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Jana M, Dasgupta S, Koka S, He J, Wood C, Pahan K. J Biol Chem. 2002;277:39312–39319. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiley CA, Baldwin M, Achim CL. Aids. 1996;10:843–847. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- 34.Nishizawa Y. Life Sci. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- 35.Ringheim GE, Szczepanik AM. Curr Pharm Des. 2006;12:719–738. doi: 10.2174/138161206775474215. [DOI] [PubMed] [Google Scholar]
- 36.King JE, Eugenin EA, Buckner CM, Berman JW. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Cao J, Viholainen JI, Dart C, Warwick HK, Leyland ML, Courtney MJ. J Cell Biol. 2005;168:117–126. doi: 10.1083/jcb.200407024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zink MC, Clements JE. J Neurovirol. 2002;8(Suppl 2):42–48. doi: 10.1080/13550280290101076. [DOI] [PubMed] [Google Scholar]
- 40.Zink MC, Spelman JP, Robinson RB, Clements JE. J Neurovirol. 1998;4:249–259. doi: 10.3109/13550289809114526. [DOI] [PubMed] [Google Scholar]
- 41.Eugenin EA, Berman JW. Methods. 2003;29:351–361. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.