Abstract
Relatively little is known about immune evasion during the productive phase of infection by the γ1-herpesvirus Epstein–Barr virus (EBV). The use of a unique system to isolate cells in lytic cycle allowed us to identify a host shutoff function operating in productively EBV-infected B cells. This impairment of protein synthesis results from mRNA degradation induced upon expression of the early lytic-cycle gene product BGLF5. Recently, a γ2-herpesvirus, Kaposi sarcoma herpesvirus, has also been shown to encode a host shutoff function, indicating that host shutoff appears to be a general feature of γ-herpesviruses. One of the consequences of host shutoff is a block in the synthesis of HLA class I and II molecules, reflected by reduced levels of these antigen-presenting complexes at the surface of cells in EBV lytic cycle. This effect could lead to escape from T cell recognition and elimination of EBV-producing cells, thereby allowing generation of viral progeny in the face of memory T cell responses.
Keywords: herpesvirus, HLA, immune escape, RNA degradation
Epstein–Barr virus (EBV), a human herpesvirus, is carried by >90% of the adult world population and is the etiologic agent of infectious mononucleosis. Importantly, EBV is associated with various forms of cancer, including Burkitt's lymphoma, nasopharyngeal carcinoma, and Hodgkin's disease (1). EBV is the most frequent cause of tumors in AIDS patients and transplant recipients undergoing immunosuppressive treatment, supporting a crucial role of antiviral immunity in controlling infection. Accordingly, in healthy individuals, persistent EBV infection is controlled by robust CD4+ and CD8+ T cell responses (2, 3). Nonetheless, EBV replicates continuously in infected individuals (4), indicating that the virus has evolved potent mechanisms to avoid immune eradication.
Successful viral persistence in immunocompetent hosts is facilitated by immune evasion strategies. Exemplary in this context are the herpesviruses. These large DNA viruses are categorized into three subfamilies (α-, β-, and γ-herpesviruses) (5) based on their biological properties and tissue tropism. Besides EBV, the γ-herpesvirus subfamily also includes another human pathogen, Kaposi sarcoma-associated herpesvirus (KSHV). As opposed to studies in α- and β-herpesviruses, few studies have documented strategies used by human γ-herpesviruses in general and EBV in particular to elude the host immune system.
Upon primary infection, EBV establishes a latent infection in B cells, characterized by the expression of only a restricted subset of viral genes with proposed roles both in viral episome maintenance and in conferring a survival advantage on the latently infected cell (1). During strict latency in circulating memory B cells, EBV remains invisible to the immune system. Although distinct from α- and β-herpesviruses in its ability to replicate the viral episome by host DNA polymerases during cell division of latently infected B cells, like all herpesviruses, the EBV lytic cycle generates progeny virions required for transmission to another host. However, in a pool of EBV-infected cells, only a subpopulation of B cells undergoes lytic EBV replication. After reactivation, >80 EBV proteins required for completion of the lytic cycle are synthesized in a strictly regulated order beginning with the immediate-early genes, which transactivate the early genes, and finally culminating with expression of the late genes (1). Thus, during the replicative phase, EBV-infected cells express a large number of viral antigens, creating a wide variety of targets for detection and destruction by the immune system. Abundant memory T cells are generated after primary infection (2, 3), and these will respond within a few hours after reactivation to eliminate cells harboring replicating virus unless counteracted by EBV-encoded immunoevasins. Because EBV persists in the infected immunocompetent host and continuously replicates, the virus has likely evolved highly effective escape mechanisms to elude the host antiviral response.
In the infected cell, herpesviruses use several general evasive approaches, such as blocking the induction of programmed cell death and inhibition of host protein synthesis (5). Host shutoff is a well described feature of α- but not β-herpesviruses and can result in down-regulation of surface expression of MHC class I and II molecules, which normally display peptide fragments of viral antigens for activation of specific T cells (6–8). Herpesviruses exploit various mechanisms to specifically perturb T cell recognition, and the range of genes involved in MHC class I down-regulation varies from one herpesvirus to another, with limited conservation among members of subfamilies (9). By analogy with other herpesviruses, we predict that numerous viral molecules operate to effect both general and specific immune evasion during the lytic phase of infection with the prototypic γ1-herpesvirus EBV. Indeed, we have reported that EBV gp42 specifically thwarts HLA class II-restricted T cell recognition through steric hindrance (10, 11).
Interactions of the immune system with productive EBV infection are poorly understood, largely because of the absence of in vitro models for study. We therefore recently developed a system allowing efficient isolation of EBV+ cells that have entered the lytic cycle (12) and have now used this system to reveal mechanism(s) affecting HLA class I- and II-restricted antigen presentation by productively EBV-infected B cells.
Results
Experimental Strategy.
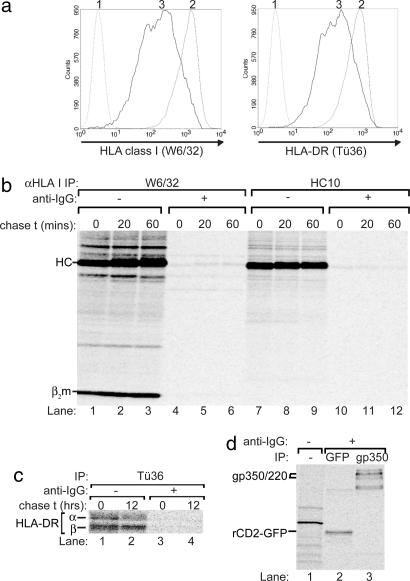
Mechanisms of immune evasion were studied by using our strategy to identify and isolate B cells that undergo EBV lytic replication (12). In brief, the EBV+ Akata Burkitt's lymphoma line was stably transfected (AKBM cells) to express a reporter fusion protein, rat CD2-GFP, in lytically induced cells as occurs after EBV reactivation by cross-linking of surface IgG. Productively infected cells remain viable for 5–7 days, indicating a window for immune evasion. Reporter+ cells in EBV lytic cycle displayed down-regulation of surface HLA class I and II (Fig. 1a) (12, 13), when compared with latently infected cells.
Fig. 1.
HLA class I expression is blocked during productive EBV infection. (a) Flow cytometry analysis of latently infected (rat CD2-GFP reporter−) and lytically infected (rat CD2-GFP reporter+) AKBM cells at 20 h after induction with anti-IgG. Histograms are depicted for latently (2) and lytically (3) EBV-infected cells showing surface staining for HLA class I (mAb W6/32) and HLA class II (mAb Tü36); as a negative control, no primary antibody was added (1). (b) Latently (−anti-IgG) and lytically (+anti-IgG, sorted 20 h after induction) EBV-infected AKBM cells were metabolically labeled for 1 h with [35S]Met and chased for the times indicated. HLA class I molecules were recovered from Nonidet P-40 cell lysates with conformation-dependent mAb W6/32 (lanes 1–6) or, in the presence of SDS, conformation-independent mAb HC10 (lanes 7–12). Samples were analyzed by SDS/PAGE. (c) In another experiment, AKBM cells were labeled for 6 h with [35S]Cys. HLA class II was immunoprecipitated from Nonidet P-40-cell lysates using mAb Tü36. (d) From the lysates in b, proteins induced in AKBM cells upon viral reactivation were precipitated with Abs specific for GFP (for reporter rat CD2-GFP) and gp350 (mAb 72A1).
HLA Class I and II Protein Synthesis Is Blocked During the EBV Lytic Cycle.
Down-regulation of HLA class I at the surface of virus-infected cells can result from specific posttranslational interference with the antigen presentation machinery (9). To examine whether intracellular retention and/or protein degradation is responsible for the down-regulation of HLA class I induced by EBV during its lytic cycle (Fig. 1a) (12), we monitored the biosynthesis and posttranslational processing of HLA class I molecules in EBV-infected cells by pulse–chase analysis (Fig. 1b). AKBM cells were cultured for 20 h with anti-human IgG antibody to induce viral replication, and cells in the lytic cycle were positively selected for surface expression of the reporter (rat CD2-GFP); uninduced latently infected AKBM cells served as controls. Control and reporter+ EBV-producing AKBM cells were pulse-labeled for 60 min with [35S]Met and chased for 0, 20, or 60 min in the absence of radiolabel. Mature HLA-class I/β2m/peptide complexes were recovered from Nonidet P-40-cell lysates with mAb W6/32. For cells in the latent state of EBV infection, newly synthesized HLA class I molecules acquired W6/32 reactivity (Fig. 1b; lanes 1–3). In contrast, W6/32-reactive molecules were barely detectable in reporter+ AKBM cells supporting the EBV replicative cycle (Fig. 1b; lanes 4–6). To determine whether this result represented a loss of mature HLA class I complexes only, additional immunoprecipitations were performed with mAb HC10 that reacts with uncomplexed heavy chains and, under denaturing conditions, detects the total pool of HLA class I molecules. As with mAb W6/32, almost no class I was recovered from B cells in EBV lytic cycle using mAb HC10 (Fig. 1b; compare lanes 10–12 to 7–9). The observation that both immature and mature class I molecules cannot be detected in a pulse-labeling experiment indicates that no new HLA class I proteins are synthesized during productive EBV infection.
To determine whether this was a more general effect, we followed the biosynthesis of a number of other cellular proteins. Metabolically labeled HLA-DR α and β chains (Fig. 1c), transferrin receptors, and actin (not shown) were virtually absent from lytically induced AKBM cells. Thus, EBV appears to impair de novo cellular gene expression.
To exclude the possibility that cells in lytic EBV cycle are defective in their protein synthesis machinery, we studied biosynthesis of EBV-encoded proteins as well as of the rat CD2-GFP reporter in AKBM cells. At 20 h postinduction, reporter+ AKBM cells were pulse-labeled with [35S]Met for 60 min, and Nonidet P-40-cell lysates were prepared for immunoprecipitation. The rat CD2-GFP reporter protein, which is induced early in lytic cycle, and the EBV-encoded gp350/220 glycoproteins, which are induced late in lytic cycle, were readily detected as newly synthesized molecules (Fig. 1d), as were complexes of gH, gp42, and gL (11). Collectively, these results implicate that AKBM cells in lytic cycle are defective for translation of proteins of host cell origin, including HLA molecules.
Lytic EBV Replication Induces a Shutoff of Host Gene Expression.
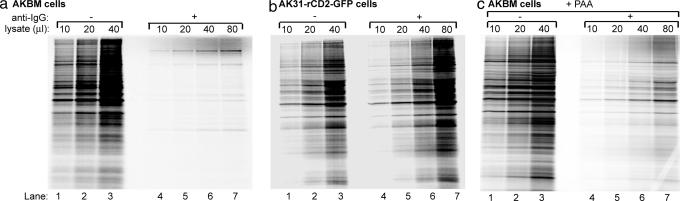
Although EBV-producing B cells were viable (12) and were capable of biosynthesis of virus-encoded proteins (Fig. 1d) (11, 12), there appeared to be a profound effect on host protein synthesis at 20 h after reactivation. To confirm that the shutoff observed for the above cellular proteins similarly occurs for other endogenous proteins, we investigated total host protein expression in lytically EBV-infected cells. Analysis of [35S]Met pulse-labeled AKBM cells showed that, whereas total protein extracts from uninduced latently infected EBV+ cells contained numerous labeled protein bands, these were either absent or considerably weaker for reporter+ cells in lytic cycle (Fig. 2a). To control for the effects of anti-IgG treatment and the MACS sorting procedure, an EBV− Akata subclone constitutively expressing the rat CD2-GFP reporter (AK31-rCD2-GFP) was examined under the same conditions. In these control cells, translation was unaffected upon ligation of the B cell receptor and immunomagnetic sorting (Fig. 2b), indicating that the marked decrease in total cellular protein synthesis in lytically reactivated B cells requires expression of an EBV lytic cycle gene product.
Fig. 2.
EBV lytic cyle protein(s) expressed at early times of infection induce host shutoff. (a and b) EBV+ AKBM cells (a) and control EBV− AK31-ratCD2-GFP cells (b) were cultured with anti-IgG antibody to induce EBV reactivation. At 20 h after induction, AKBM cells in the lytic cycle were positively selected for the expression of the inducible rat CD2-GFP reporter protein; the control AK31 cells constitutively expressing the rat CD2-GFP reporter were also sorted and served as controls. All cell populations were pulse-labeled for 60 (a, lanes 1–7) or 30 min (b, lanes 1–7) with [35S]Met before Nonidet P-40-cell lysis. SDS/PAGE analysis is shown for increasing amounts of total protein extracts denatured in sample buffer. (c) AKBM cells were either left uninduced (−anti-IgG) or were induced overnight (+anti-IgG; sorted for reporter+ cells) in the absence or presence of 300 μg/ml PAA added at the time of induction. Cells were assayed for total cellular protein synthesis by [35S]Met labeling, followed by SDS/PAGE, of titrations of total protein extracts.
Shutoff of Host Cell Protein Synthesis Is an Early Event in the Lytic Program of EBV.
To facilitate identification of the EBV gene responsible, we first determined at what stage in the lytic cycle the generation of newly synthesized proteins is inhibited. Addition of phosphonoacetic acid (PAA) blocks viral DNA synthesis and can be used to discriminate early from late EBV protein expression, the latter depending on replication of the viral genome (6). Because the rat CD2-GFP reporter is expressed by AKBM cells from early through late stages of the replicative cycle (12), this permits immunomagnetic sorting irrespective of late antigen expression. To selectively and completely eliminate late gene expression, AKBM cells were pretreated with PAA before viral reactivation. PAA treatment did not prevent the EBV-induced block in cellular protein synthesis (Fig. 2c). As a control for the effect of PAA on early and late gene expression, immunoprecipitations were performed to detect the rat CD2-GFP reporter, as a marker of early gene expression, and the late gH protein within the labeled lysates. As expected, synthesis of the late gH glycoprotein was prohibited by PAA, whereas no inhibition of rat CD2-GFP expression was observed [supporting information (SI) Fig. 6].
Together, these results imply that an early EBV lytic-cycle gene product is responsible for the generalized block to host gene expression. Host protein synthesis shutoff, to our knowledge, has not previously been reported for EBV.
Shutoff of Host Cell Protein Synthesis Occurs at the mRNA Level.
For α-herpesviruses, diminished synthesis of cellular proteins, a key event early in replication, is mediated by a virion host shutoff (vhs) protein that degrades cellular mRNAs (14). To assess whether the EBV γ1-herpesvirus encodes a nuclease function analogous to the α-herpesviruses, we determined RNA levels for a number of genes expressed at 20 h of viral reactivation. Total amounts of cytoplasmic RNA in each sample were controlled by cell counting and the use of equal cell numbers for RNA isolation from postnuclear Nonidet P-40 cell lysates. cDNA was generated by using random primers and reverse transcriptase; for comparison, genomic DNA was isolated from cell nuclei. This procedure resulted in comparable levels of 18S rRNA in samples representing latently and lytically EBV-infected cells, as determined by quantitative real-time PCR [cycle thresholds (Ct) of 20.6 for cDNA from both latent and lytic cells; variation coefficients for Ct values were <1%). EBV lytic-cycle genes were transcribed in productively infected cells, as evidenced by increased BNRF1 mRNA levels (reflected by a decrease in Ct from 30.3 for cDNA from latent to 22.7 from lytic cells) encoding the major virus tegument protein. In contrast, mRNA levels of the cellular enzyme GAPDH were reduced by approximately half the number of transcripts in EBV-producing cells (i.e., >1 Ct difference: from Ct = 27.1 to 28.4 for cDNA from latent and lytic cells, respectively). We extended this analysis by semiquantitative PCR to also study transcription of other genes (Fig. 3). Consistent with the real-time PCR analysis, genomic DNA levels of GAPDH remained largely unaltered, whereas a ≈50% reduction in GAPDH RNA levels was observed for productively EBV-infected AKBM cells (lanes 3 and 4). Even more pronounced were the reductions in transcripts for β2m (to 43% of the amount in latently infected cells), HLA-DRα (to 24%), and transporter-associated with antigen processing (TAP1) (to 21%) after entry into the replicative cycle (lanes 5–10). In contrast, transcription of the late EBV gene encoding gH (BXLF2) is initiated in isolates from EBV+ lytic-cycle reporter+ B cells (lanes 11 and 12). Collectively, these results reveal that shutoff during EBV lytic cycle occurs as a consequence of reduced mRNA levels.
Fig. 3.
Reduced levels of cellular mRNAs in EBV-producing B cells. Genomic DNA (lanes 1 and 2) and cDNA (lanes 3–12) was prepared from latently (−anti-IgG) and productively (+anti-IgG and sorted) EBV-infected AKBM cells; cDNA was obtained after reverse transcription of total cytoplasmic RNA using random primers. PCR products representing the indicated genes (generated using specific primers) were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining.
EBV BGLF5 Promotes mRNA Degradation.
To identify the viral gene(s) responsible for EBV-induced shutoff, we searched the EBV genome for homologs of α-herpesvirus vhs (UL41), but no obvious homolog was found. Recently, lytic replication of the γ2-herpesvirus KSHV was shown to cause a widespread shutoff of cellular gene expression that occurred by enhanced mRNA turnover (15). A viral protein, encoded by ORF37 and expressed at early times of infection, was found to be responsible for host shutoff in KSHV-infected cells (15–17). The ORF37 gene product is conserved across the entire herpesvirus family, where it is known to function as an alkaline exonuclease (AE) on viral DNA (18–20). However, herpes simplex virus 1 (HSV-1) AE fails to promote RNA turnover (15), and the AE proteins have not been implicated in host shutoff in either α- or β-herpesvirus family members (18, 20). Therefore, the KSHV ORF37-encoded SOX (for shutoff and exonuclease) appears to have acquired a function absent from its α- and β-herpesvirus homologs. The BGLF5-encoded EBV DNase displays a very high degree of homology to KSHV SOX at the amino acid level (52% similarity, 40% identity; SI Fig. 7). The BGLF5 product is an early lytic cycle protein, its expression starting from ≈6 h postreactivation (21), congruent with the onset of host shutoff.
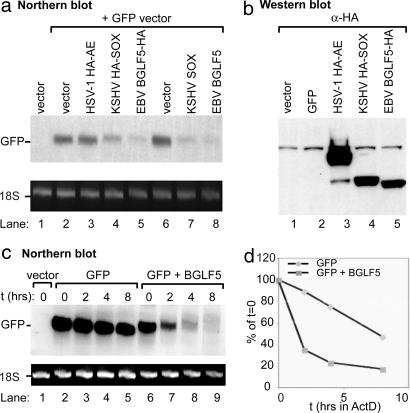
To explore the possibility that EBV BGLF5 also promotes host shutoff, we transiently cotransfected expression vectors containing BGLF5 and a GFP reporter into 293T cells and examined GFP RNA accumulation in Northern blots. Similar to KSHV SOX, both WT and HA-tagged BGLF5 dramatically reduced the levels of GFP mRNA (Fig. 4a) in a dose-dependent manner (not shown), indicating that it likely functions as the EBV host shutoff factor. In contrast, expression of HSV-1 AE had no effect (Fig. 4a), confirming that DNase activity per se does not produce a decrease in mRNA levels. To assess whether the reduction in mRNA is due to BGLF5-induced mRNA instability, we examined the half-life of GFP mRNA in cells in the presence or absence of BGLF5 using the transcriptional inhibitor actinomycin D. When expressed in 293T cells alone, GFP mRNA was quite stable, exhibiting a half-life of ≈8 h (Fig. 4 c and d). By contrast, in the presence of BGLF5, the GFP message half-life decreased to <2 h (Fig. 4 c and d), indicating that BGLF5 promotes mRNA degradation.
Fig. 4.
EBV BGLF5 promotes enhanced GFP mRNA turnover. (a) 293T cells were transfected with either empty vector or cotransfected with plasmids expressing GFP and HA-tagged HSV-1 AE, KSHV SOX, or EBV BGLF5 at a 1:4 ratio. Total RNA was extracted from half of the cells 24 h after transfection, 10 μg of RNA was resolved by agarose-formaldehyde electrophoresis, transferred to a nylon membrane, and Northern-blotted with a [32P]-labeled GFP DNA probe. In all Northern blots, the ribosomal 18S RNA served as a loading control. (b) Protein lysates from the other half of the cells were immunoblotted with an HA-specific antibody to detect expression of the HA-tagged viral proteins. (c) 293T cells were transfected with either empty vector or cotransfected with plasmids expressing GFP and EBV BGLF5 at a 1:3 ratio. Forty-eight hours after transfection, cells were treated with actinomycin D (2 μg/ml) for the indicated amount of time to halt transcription. Total RNA was harvested from each sample and Northern-blotted with a [32P]-labeled GFP DNA probe. (d) The half-life of the mRNAs was determined by phosphorimager-based quantification of the blot depicted in c. Values for each band represent the percentage of the respective t = 0 time-point intensity.
It has previously been shown that the dual functions of KSHV SOX occur in distinct cellular compartments; the DNase activity required for viral genome processing occurs in the nucleus, whereas the host shutoff function occurs in the cytoplasm of cells. In contrast, HSV-1 AE, which possesses only the DNase function, is strictly nuclear (SI Fig. 8) (17, 19). Given the apparent functional similarities between EBV BGLF5 and KSHV SOX, we hypothesized that BGLF5 would similarly localize to both the cytoplasm and nucleus of cells. Indeed, although BGLF5 contains two nuclear localization sequences (22), it was clearly also present within the cytoplasm of 293T cells (SI Fig. 8), which is likely to be the site of its host shutoff activities.
EBV BGLF5 Expression Is Sufficient to Induce Shutoff of Host Gene Expression Including HLA Class I.
To evaluate the impact of BGLF5-induced RNA degradation on total cellular protein synthesis, we expressed BGLF5 in Spodoptera frugiperda (Sf9) insect cells using a recombinant baculovirus. Four days after baculovirus BGLF5 (or control baculovirus EBNA1) infection, substantial amounts of the recombinant proteins were detectable by immunoblot analysis (SI Fig. 9b). Metabolic labeling showed a significant and global reduction in new polypeptide synthesis in BGLF5-expressing cells compared with uninfected cells or cells infected with the control EBNA1-expressing baculovirus (SI Fig. 9a).
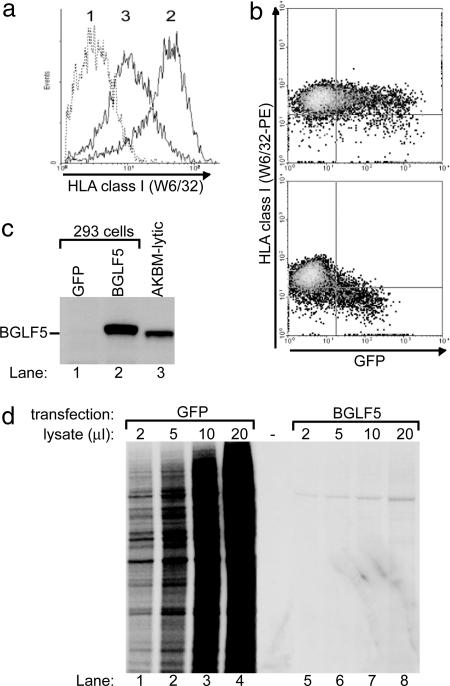
Next, we assessed whether expression of BGLF5 alone in eukaryotic cells would mimic the shutoff and reduced HLA class I surface display we had observed during productive EBV infection. To this end, 293 cells were transfected with a bicistronic vector expressing BGLF5 and GFP (used to identify transfected cells) and, at 48 h after transfection, levels of HLA class I on BGLF5-expressing cells were measured by flow cytometry. BGLF5 clearly reduced HLA class I expression at the cell surface (Fig. 5 a and b). The BGLF5-induced shutoff of protein synthesis was also reflected by a drop in GFP fluorescence intensity in cells transfected with BGLF5 compared with control cells (Fig. 5b). Pure populations of BGLF5-transfected cells were found to express BGLF5 protein at levels comparable to or in excess of those in lytically EBV-infected AKBM cells (Fig. 5c). In a metabolic labeling experiment, BGLF5 expression induced a dramatic reduction in total protein synthesis in the BGLF5-transfected eukaryotic cells (Fig. 5d).
Fig. 5.
HLA class I down-regulation induced by EBV BGLF5. 293 cells were transfected with pcDNA3-BGLF5-IRES-nlsGFP or an empty control vector. (a and b) At 48 h after transfection, HLA class I expression at the surface of transfected cells was visualized by flow cytometry. (c) GFP+ cells were enriched at 24 h after transfection by using a FACS cell sorter. Protein lysates from the transfected and sorted cells were immunoblotted with a BGLF5-specific polyclonal antiserum (rabbit 120). (d) At 48 h, cells were assayed for total protein synthesis by [35S]Met labeling, followed by SDS/PAGE of titrations of total protein extracts.
Thus, we have shown that the phenotype of host shutoff mediated by BGLF5 expressed in isolation (Fig. 5 and SI Fig. 9) exactly duplicates the phenotype of the shutoff observed in authentic lytic replication (Fig. 2). These data implicate EBV BGLF5 as an important viral effector of global mRNA degradation resulting in a severe restriction of cellular gene expression. In addition, our observations raise the possibility that an EBV-induced host shutoff might be a contributing factor affecting antigen presentation.
Discussion
Here, we demonstrate that EBV induces a robust and generalized host shutoff in productively infected cells. EBV-induced shutoff affects biosynthesis of HLA class I and II molecules, thereby likely contributing to viral escape from T cell recognition. We have identified the gene product responsible for EBV-induced shutoff to be encoded by the early lytic-cycle gene BGLF5 whose protein product acts by induction of enhanced mRNA turnover.
Our study describes that the prototypic γ1-herpesvirus EBV impairs de novo host gene expression during the lytic cycle. So far, this issue has remained elusive, because only a small percentage of EBV-infected B cells undergo lytic replication at any given time. Consequently, inhibitory effects on a subpopulation of lytically infected cells are masked by the absence of these effects in the majority of cells. The use of our recently developed in vitro system to examine effects of productive EBV infection (ref. 12 and refs. therein) has permitted the discovery of an EBV-induced shutoff function encoded by BGLF5. Likewise, a novel endothelial cell model of lytic infection with the γ2-herpesvirus KSHV led to the recent identification of a potent host shutoff resulting from expression of the ORF37-encoded product, SOX (15–17). The observation that both proteins are DNase/alkaline exonuclease homologs, are expressed at early times of lytic infection (15, 21), and induce host shutoff by mRNA degradation implicates this function as conserved among members of the γ-herpesvirus subfamily. In line with this interpretation, one report also shows a block in de novo cellular protein synthesis for the γ2-herpesvirus MHV-68 (23), but the gene responsible for this effect has not been elucidated.
The mechanism of γ-herpesvirus-induced mRNA degradation still remains to be resolved in detail. Given their nuclease activity on DNA, it is conceivable that BGLF5 and SOX might also function as RNases. However, neither protein has been observed to exert RNase activity in vitro. Thus, other, most likely cellular, factors are involved in virus-induced mRNA degradation. In view of our demonstration that BGLF5 expression causes a block in protein synthesis even in insect cells (SI Fig. 9), the host machinery required for shutoff appears to be widely conserved.
Interestingly, the gene product responsible for shutoff by at least two γ-herpesviruses is the viral alkaline exonuclease, a protein involved in the maturation of viral DNA and conserved in all herpesviruses. Apparently evolution has endowed both the EBV and KSHV AE homologs with an additional mRNA turnover function not present in their α- and β-herpesvirus homologs (SI Fig. 7). Within the cell, DNA- and RNA-destabilizing functions of EBV BGLF5 and KSHV SOX may in fact be exerted at distinct locations; these proteins are expressed both in the nucleus and the cytoplasm, as opposed to the strictly nuclear localization of HSV-1 AE (SI Fig. 8) (17, 19). The cytoplasmic pool of BGLF5 and SOX would be very well localized for promoting mRNA turnover, especially given that this is the site of most cellular mRNA degradation. This additional shutoff function of AE proteins may represent a discriminatory factor between the lymphotropic γ-herpesviruses and other herpesvirus subfamilies.
The kinetics and gene products that γ-herpesviruses use to modulate host mRNA turnover appear to differ significantly from those used by α-herpesviruses and may well have evolved to suit the specific biology of infection. Members of the α-herpesvirus subfamily carry a conserved vhs protein, encoded by UL41 (6, 7), for which no obvious homolog is present in the genomes of γ-herpesviruses.
The function of UL41 in α-herpesvirus infection appears to be 2-fold: to enable efficient transition from immediate-early to early and late viral proteins synthesis and to block host responses to infection. The current mechanistic model is that, immediately upon infection, the vhs protein is released from the tegument of the virus particle directly into the cytoplasm and acts as an RNase (14, 24). Host rRNA and tRNA are unaffected, whereas viral and host mRNAs are rapidly degraded. Because viral mRNA is transcribed at a higher rate than cellular mRNA, viral mRNAs, and thus viral proteins, are thought to accumulate as a consequence of vhs function. The HSV-1 vhs protein works in concert with the immediate-early protein, ICP27, that is involved in the export of intronless viral mRNAs (25). An ICP27-homolog is present in EBV: the BMLF1 gene codes for the SM or EB2 protein. SM induces cytoplasmic accumulation of a subset of early mRNAs encoding proteins essential to EBV replication and inhibits expression of intron-containing cellular genes (26–28). It is possible that SM acts in concert with BGLF5 to confer specificity to the shutoff, which is preferential for host genes.
The block in host-cell protein synthesis affects a multitude of cellular proteins. Among these are immunologically important molecules, including HLA class I and II (Figs. 1 and 5), and therefore host shutoff potentially contributes to immune evasion by EBV during the viral replicative cycle. This general mechanism is likely to synergize with more specific viral interference mechanisms. This hypothesis is supported by the delayed times at which EBV-induced host shutoff is effective, leaving a window for specific interference with, for instance, T cell activation. Immune evasion involves escape not only from classic CD8+ cytotoxic T lymphocyte (CTL) acting by HLA class I but also from CD4+ CTL acting by class II, the latter being increasingly recognized as a major component in EBV-directed immunosurveillance (29). In the case of HLA class II-restricted antigen presentation, we have recently described that EBV gp42, bound to class II complexes at the cell surface, sterically blocks TCR engagement (10, 11). A BGLF5-induced reduction in class II molecules presented at the surface of productively EBV-infected cells will increase the chances of gp42 covering all remaining class II complexes to avoid CD4+ T helper cell detection. To escape from recognition by CD8+ CTL, the combination of specific interference with antigen processing and inhibition of de novo HLA class I synthesis could similarly result in an efficient block in presentation of lytic antigen-derived peptides. In line with this interpretation, we have recently identified an EBV lytic-cycle gene product that impairs TAP function in lytically EBV-infected B cells, resulting in reduced transport into the endoplasmic reticulum of peptides destined for HLA class I presentation (ref. 12; unpublished results). TAP molecules are very stable, and steady-state levels are not affected by the host shutoff function at 20 h after reactivation of EBV (12). Therefore, it is likely that the inhibition of TAP transport and the host shutoff by BGLF5 represent complementing strategies used by EBV to interfere with the expression of antigen-loaded HLA class I molecules at the cell surface during productive infection.
Other herpesviruses also provide precedents of combined host shutoff and subversion of specific steps of antigen processing. For α-herpesviruses, the vhs protein efficiently reduces the synthesis of new MHC class I molecules but has no effect on the existing pool of class I complexes that will continue to present antigenic peptides to CTL. Peptide transport appears to be a bottleneck in the MHC class I presentation route and is perturbed by two α-herpesvirus gene products (30–32). In doing so, the transport of antigenic peptides derived from newly synthesized (viral) proteins is blocked. The effectiveness of multiple independent evasion mechanisms is clearly illustrated by HSV (8). The presence of either the HSV vhs protein or ICP47 partially inhibits the lysis of infected fibroblasts by CTL. When both proteins are present, they act synergistically and almost completely inhibit lysis by specific CTL clones (8).
The γ2-herpesviruses KSHV and MHV68 combine host shutoff with destabilizing newly synthesized MHC class I molecules [KSHV K3 and K8 (33, 34); MHV68 mK3 (23)]. Interesting to note in this context is the large variety of HCMV-encoded immunoevasins: peptide transport by TAP is blocked (US6), MHC class I molecules are retained in the endoplasmic reticulum (US3), and US2 and US11 target class I heavy chains for proteasomal degradation (9). It is tempting to speculate that the unprecedented rapidity of class I proteolysis imposed by US2 and US11 might replace the lack of a generalized host shutoff in HCMV (35).
Deletion mutant viruses have been instrumental in addressing the biological and immunological aspects of vhs in α-herpesviruses. In particular, the combination with animal models has provided compelling evidence for a role of vhs in interference with host antiviral responses, for instance for HSV (8, 36–39). Likewise, the construction of a mutant EBV from which the host shutoff function has selectively been disabled (without affecting DNase activity) will contribute to establishing the extent to which BGLF5 affects host protein synthesis and immune recognition. Furthermore, comparison of WT and modified EBV, from which the BGLF5 shutoff function has specifically been mutated, will also allow studies on interference with other aspects of the adaptive immune system (e.g., dendritic cell activation) and with innate immune recognition (7). The observation that, in contrast to HSV, EBV BGLF5-induced host shutoff requires de novo protein synthesis, combined with the facts that EBV does not directly result in productive infection and does not infect small animals, however, requires a novel approach of investigation.
In conclusion, we have shown that the EBV early lytic cycle gene product BGLF5 imposes a shutoff of host protein synthesis resulting in reduced surface display of HLA molecules for T cell recognition. Our results indicate that, during the productive phase of infection, EBV appears to exploit multiple strategies to corrupt host T cell and other antiviral responses.
Materials and Methods
To study the productive phase of EBV infection, we used EBV+ AKBM cells, which express a rat CD2-GFP reporter protein in cells induced into lytic cycle. The derivation of the AKBM line and the immunoaffinity isolation of populations in lytic cycle after induction with anti-human IgG antibody have been described (12). Expression of selected viral gene products in epithelial cells was achieved by transient transfections. For Northern blot analysis, 293T cells were transfected by using Fugene 6 (Roche, Indianapolis, IN) (15). For metabolic labeling experiments, the transfection of 293 cells with a bicistronic vector expressing BGLF5 and GFP was achieved by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), and transfected cells were sorted to high purity by flow cytometry on the basis of their expression of GFP. Additional details on plasmids and reagents and molecular biological, biochemical, and immunological assays are described in SI Text.
Supplementary Material
Acknowledgments
We thank Drs. Franca Hartgers and Eric Claas for help with quantitative PCRs and Dr. Sinéad Keating for help with generating the AKBM cell line and initial experiments. We thank collaborators for providing reagents and constructs. These studies have been financially supported by the Dutch Cancer Society (Grant UL 2005-3259), the M. W. Beijerinck Virology Fund of the Royal Academy of Arts and Sciences, the Netherlands Organization for Scientific Research (NWO Vidi Grant 917.76.330), and the Wellcome Trust, London (Grant GR072425).
Abbreviations
- KSHV
Kaposi sarcoma-associated herpesvirus
- PAA
phosphonoacetic acid
- vhs
virion host shutoff
- Ct
cycle threshold
- AE
alkaline exonuclease
- HSV-1
herpes simplex virus 1
- CTL
cytotoxic T lymphocyte
- TAP
transporter associated with antigen processing.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611128104/DC1.
References
- 1.Rickinson AB, Kieff E. In: Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2575–2627. [Google Scholar]
- 2.Amyes E, Hatton C, Montamat-Sicotte D, Gudgeon N, Rickinson AB, McMichael AJ, Callan MF. J Exp Med. 2003;198:903–911. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao QY, Rickinson AB, Epstein MA. Int J Cancer. 1985;35:35–42. doi: 10.1002/ijc.2910350107. [DOI] [PubMed] [Google Scholar]
- 5.Roizman B, Pellet PE. In: Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2381–2397. [Google Scholar]
- 6.Koppers-Lalic D, Rijsewijk FA, Verschuren SB, van Gaans-Van den Brink JA, Neisig A, Ressing ME, Neefjes J, Wiertz EJ. J Gen Virol. 2001;82:2071–2081. doi: 10.1099/0022-1317-82-9-2071. [DOI] [PubMed] [Google Scholar]
- 7.Smiley JR. J Virol. 2004;78:1063–1068. doi: 10.1128/JVI.78.3.1063-1068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tigges MA, Leng S, Johnson DC, Burke RL. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 9.Lilley BN, Ploegh HL. Immunol Rev. 2005;207:126–144. doi: 10.1111/j.0105-2896.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 10.Ressing ME, van Leeuwen D, Verreck FA, Gomez R, Heemskerk B, Toebes M, Mullen MM, Jardetzky TS, Longnecker R, Schilham MW, et al. Proc Natl Acad Sci USA. 2003;100:11583–11588. doi: 10.1073/pnas.2034960100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ressing ME, van Leeuwen D, Verreck FA, Keating S, Gomez R, Franken KL, Ottenhoff TH, Spriggs M, Schumacher TN, Hutt-Fletcher LM, et al. J Virol. 2005;79:841–852. doi: 10.1128/JVI.79.2.841-852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ressing ME, Keating SE, van Leeuwen D, Koppers-Lalic D, Pappworth IY, Wiertz EJ, Rowe M. J Immunol. 2005;174:6829–6838. doi: 10.4049/jimmunol.174.11.6829. [DOI] [PubMed] [Google Scholar]
- 13.Keating S, Prince S, Jones M, Rowe M. J Virol. 2002;76:8179–8188. doi: 10.1128/JVI.76.16.8179-8188.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taddeo B, Zhang WR, Roizman B. Proc Natl Acad Sci USA. 2006;103:2827–2832. doi: 10.1073/pnas.0510712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaunsinger B, Ganem D. Mol Cell. 2004;13:713–723. doi: 10.1016/s1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- 16.Glaunsinger B, Ganem D. J Exp Med. 2004;200:391–398. doi: 10.1084/jem.20031881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glaunsinger B, Chavez L, Ganem D. J Virol. 2005;79:7396–7401. doi: 10.1128/JVI.79.12.7396-7401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knopf CW, Weisshart K. Eur J Biochem. 1990;191:263–273. doi: 10.1111/j.1432-1033.1990.tb19119.x. [DOI] [PubMed] [Google Scholar]
- 19.Reuven NB, Antoku S, Weller SK. J Virol. 2004;78:4599–4608. doi: 10.1128/JVI.78.9.4599-4608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheaffer AK, Weinheimer SP, Tenney DJ. J Gen Virol. 1997;78:2953–2961. doi: 10.1099/0022-1317-78-11-2953. [DOI] [PubMed] [Google Scholar]
- 21.Daibata M, Sairenji T. Virology. 1993;196:900–904. doi: 10.1006/viro.1993.1555. [DOI] [PubMed] [Google Scholar]
- 22.Liu MT, Hu HP, Hsu TY, Chen JY. J Gen Virol. 2003;84:677–686. doi: 10.1099/vir.0.18739-0. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson PG, Efstathiou S, Doherty PC, Lehner PJ. Proc Natl Acad Sci USA. 2000;97:8455–8460. doi: 10.1073/pnas.150240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong AD, Frenkel N. Proc Natl Acad Sci USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandri-Goldin RM. J Virol. 2004;78:4389–4396. doi: 10.1128/JVI.78.9.4389-4396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruffat H, Batisse J, Pich D, Neuhierl B, Manet E, Hammerschmidt W, Sergeant A. J Virol. 2002;76:9635–9644. doi: 10.1128/JVI.76.19.9635-9644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruvolo V, Wang EY, Boyle S, Swaminathan S. Proc Natl Acad Sci USA. 1998;95:8852–8857. doi: 10.1073/pnas.95.15.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruvolo V, Sun L, Howard K, Sung S, Delecluse HJ, Hammerschmidt W, Swaminathan S. J Virol. 2004;78:340–352. doi: 10.1128/JVI.78.1.340-352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heller KN, Gurer C, Munz C. J Exp Med. 2006;203:805–808. doi: 10.1084/jem.20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert PM, Tampe R, Peterson PA, Yang Y. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 31.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 32.Koppers-Lalic D, Reits EA, Ressing ME, Lipinska AD, Abele R, Koch J, Marcondes RM, Admiraal P, van Leeuwen D, Bienkowska-Szewczyk K, et al. Proc Natl Acad Sci USA. 2005;102:5144–5149. doi: 10.1073/pnas.0501463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coscoy L, Ganem D. Proc Natl Acad Sci USA. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishido S, Wang CY, Lee BS, Cohen GB, Jung JU. J Virol. 2000;74:5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mocarski ES, Courcelle CT. In: Fields Virology. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Strauss SE, editors. New York: Lippincott Williams & Wilkins; 2001. pp. 2629–2674. [Google Scholar]
- 36.Geiss BJ, Smith TJ, Leib DA, Morrison LA. J Virol. 2000;74:11137–11144. doi: 10.1128/jvi.74.23.11137-11144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith TJ, Ackland-Berglund CE, Leib DA. J Virol. 2000;74:3598–3604. doi: 10.1128/jvi.74.8.3598-3604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strelow LI, Leib DA. J Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.