Abstract
Chemokines and their receptors are key factors in the onset and progression of AIDS. Among them, accumulating evidence strongly indicates the involvement of IL-8 and its receptors, CXCR1 and CXCR2, in AIDS-related conditions. Through extensive investigation of genetic variations of the human CXCR1–CXCR2 locus, we identified a haplotype of the CXCR1 gene (CXCR1-Ha) carrying two nonsynonymous single nucleotide polymorphisms, CXCR1_300 (Met to Arg) in the N terminus extracellular domain and CXCR1_142 (Arg to Cys) in the C terminus intracellular domain. Transfection experiments with CXCR1 cDNAs corresponding to the CXCR1-Ha and the alternative CXCR1-HA haplotype showed reduced expression of CD4 and CXCR4 in CXCR1-Ha cells in human osteosarcoma cells as well as in Jurkat and CEM human T lymphocytes. Furthermore, the efficiency of X4-tropic HIV-1NL4–3 infection was significantly lower in CXCR1-Ha cells than in CXCR1-HA cells. The results were further confirmed by a series of experiments using six HIV-1 clinical isolates from AIDS patients. A genetic association study was performed by using an HIV-1+ patient cohort consisting of two subpopulations of AIDS with extreme phenotypes of rapid and slow progression of the disease. The frequency of the CXCR1-Ha allele is markedly less frequent in patients with rapid disease onset than those with slow progression (P = 0.0003). These results provide strong evidence of a protective role of the CXCR1-Ha allele on disease progression in AIDS, probably acting through modulation of CD4 and CXCR4 expression.
Keywords: AIDS, SNP, chemokine receptor, genotyping
A principal feature of AIDS is progressive depletion of CD4+ cells, leading to multiple immune-related symptoms (1). Rates of CD4+ depletion and subsequent disease progression are highly variable among HIV-1-seropositive individuals (2). A small portion of patients maintains the normal range of CD4+ cell counts and is free of disease symptoms for many years, whereas some others have a contrasting host response characterized by rapid loss of CD4+ and onset of symptoms (3). The role of the chemokine–chemokine receptor system has been extensively investigated after the discovery of the anti-HIV-1 activity of the CC-chemokines RANTES, MIP-1α, and MIP-1β (4) and the identification of CXCR4 and CCR5 as the major coreceptors of HIV-1 (5–7). A genetic variant of CCR5 (delta-32) has been shown to associate with low risk of HIV-1 infection and slow disease progression (SP) in Caucasians (8–10). Although variants in other chemokine and receptor genes may also affect the disease progression, their association with HIV infection and disease progression have not been elucidated.
IL-8 is the best-characterized proinflammatory C-X-C chemokine (11). IL-8 activates and attracts neutrophils, T cells, and basophils and is believed to be a key mediator in inflammatory disorders (12–14). We could not find association of variants of IL-8 with risk of HIV-1 seroconversion or disease progression (A.V., M.L., and F.M., unpublished work). However, dysregulation and elevated IL-8 production (15, 16) and reduced expression of IL-8 receptors, CXCR1 and CXCR2, were reported in HIV-1-infected patients (17). HIV-1 replication was shown to be up-regulated by IL-8 in macrophages and T lymphocytes, and inhibited by IL-8 antagonists and GRO-α (18). Reduced CXCR1 activity upon HIV-1 infection due to cross-receptor-mediated internalisation with the major coreceptors CCR5 and CXCR4 has been shown (19). These observations suggest that CXCR1 and CXCR2 could affect AIDS-related conditions.
Results
Identification and Characterization of the CXCR1 and CXCR2 Polymorphisms.
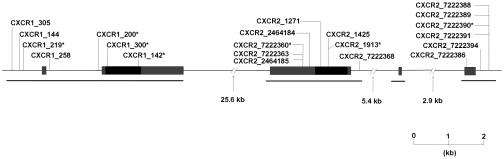
CXCR1 and CXCR2 form a single locus spanning a region of ≈26 kb on chromosome 2q35. By sequencing we determined genetic polymorphisms in 471 French Caucasian volunteers [control (CTR) series] (see Materials and Methods). Among 93 polymorphisms identified, 21 had minor allele frequencies of >1% (Fig. 1 and Table 1). Two of these involved nonsynonymous amino acid substitutions: a change of methionine to arginine at position 31 in the N terminus extracellular domain of CXCR1 (CXCR1_300) and a change of arginine to cysteine at position 335 in the C terminus intracellular domain (CXCR1_142). Strong linkage disequilibrium (LD) was observed across CXCR1-CXCR2. The 21 frequent variants formed 10 haplotypes with estimated frequencies of >1%. In particular, the alleles at the two nonsynonymous variant sites in CXCR1 exhibited complete LD on 942 chromosomes. Four other polymorphisms, CXCR1_200, CXCR1_219, CXCR2_7222390 and CXCR2_7222360, were also in complete LD with these. The minor alleles at these six sites constituted a single haplotype with an estimated frequency of 3.9% (CXCR1–2_H3 in Table 2). Nearly complete LD was also observed with CXCR2_1913.
Fig. 1.
Physical map of the human CXCR1 and CXCR2 genes. Coding and untranslated regions are indicated by black and gray bars, respectively. The location of polymorphisms with frequencies of >1% is indicated. The asterisk corresponds to polymorphisms that are in strong LD. A horizontal line below each gene shows the regions that have been sequenced.
Table 1.
Summary of genetic polymorphisms in the human CXCR1 and CXCR2 genes
| Polymorphisms |
Frequencies A1 |
P values for statistical tests (when <0.01) |
dbSNP ID | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | CNG ID | Position | Location | A1 | A2 | CTR | SP | RP | CTR vs. SP vs. RP | RP vs. NP | |
| CXCR1 | 305 | −2668 | Promoter | G | A | 0.95 | 0.95 | 0.95 | — | — | rs2671222 |
| 144 | −2423 | Promoter | G | A | 0.98 | 0.98 | 0.99 | — | — | rs17838611 | |
| 219 | −2329 | Promoter | C | T | 0.96 | 0.94 | 1.00 | 0.0008 | 0.0003 | rs16858841 | |
| 258 | −1566 | Intron | C | G | 0.94 | 0.95 | 0.95 | — | — | rs3138060 | |
| 200 | −143 | Intron | C | T | 0.96 | 0.94 | 1.00 | 0.0008 | 0.0003 | rs16858816 | |
| 300 | 92 | Exon (Met/Arg) | T | G | 0.96 | 0.94 | 1.00 | 0.001 | 0.0003 | rs16858811 | |
| 142 | 1003 | Exon (Arg/Cys) | C | T | 0.96 | 0.94 | 1.00 | 0.001 | 0.0002 | rs16858808 | |
| CXCR2 | 7222388 | −9203 | Promoter | A | G | 0.57 | 0.59 | 0.55 | — | — | rs3890158 |
| 7222389 | −9191 | Promoter | — | T | 0.57 | 0.59 | 0.56 | — | — | ss69355493 | |
| 7222390 | −9185 | Promoter | T | G | 0.96 | 0.93 | 1.00 | 0.0004 | 0.0003 | rs3890157 | |
| 7222391 | −9179 | Promoter | T | — | 0.52 | 0.59 | 0.57 | — | — | ss69355494 | |
| 7222394 | −8909 | Promoter | T | C | 0.52 | 0.52 | 0.54 | — | — | rs4674258 | |
| 7222386 | −8490 | Exon (5′-UTR) | A | G | 0.57 | 0.59 | 0.55 | — | — | rs4674259 | |
| 7222368 | −270 | Intron | G | A | 0.53 | 0.54 | 0.54 | — | — | ss69355495 | |
| 1913 | 768 | Exon (Val/Val) | C | T | 0.95 | 0.93 | 0.99 | 0.0015 | 0.0006 | rs11574750 | |
| 1425 | 786 | Exon (Leu/Leu) | C | T | 0.52 | 0.51 | 0.51 | — | — | rs2230054 | |
| 1271 | 936 | Exon (Leu/Leu) | C | T | 0.99 | 0.99 | 0.99 | — | — | ss69355496 | |
| 2464184 | 1209 | Exon (3′-UTR) | C | T | 0.57 | 0.58 | 0.51 | — | — | rs1126579 | |
| 7222360 | 1420 | Exon (3′-UTR) | A | G | 0.96 | 0.94 | 1.00 | 0.0004 | 0.0002 | rs13306441 | |
| 7222363 | 1437 | Exon (3′-UTR) | C | T | 0.01 | 0.01 | 0.01 | — | — | ss69355497 | |
| 2464185 | 1441 | Exon (3′-UTR) | G | A | 0.53 | 0.53 | 0.54 | — | — | rs1126580 | |
The position of each SNP was counted on the reference sequence (NT_005403.10) from the first nucleotide of the initiation codon as +1. A1 represents the nucleotide identical to that of the reference sequence. Single Nucleotide Polymorphism Database (dbSNP) IDs are also given for those that are already reported and registered on dpSNP (www.ncbi.nlm.nih.gov/SNP). P values for comparisons of the allele frequencies in the specified series are shown when <0.05. For these comparisons, adjustment for multiple testing (see Methods) gave P <0.01 in all instances except for the CTR vs. SP vs. RP comparison of SNP_7222390 in CXCR2 (P = 0.02). CNG, Centre National de Génotypage.
Table 2.
Haplotypes of CXCR1 and CXCR2 sites that are associated with disease progression
| Haplotype | Polymorphisms |
Haplotype distribution |
P value |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR |
SP |
RP |
CTR vs. SP vs. RP |
SP vs. RP |
||||||||||||
| 219 | 200 | 300 | 142 | 7222390 | 7222391 | 1913 | 7222360 | Counts | Freq | Counts | Freq | Counts | Freq | Corrected | ||
| CXCR1_HA | C | C | T | C | — | — | — | — | 903/942 | 0.958 | 496/526 | 0.943 | 172/172 | 1.000 | 0.00083 | 0.00029 |
| CXCR1_Ha | T | T | G | T | — | — | — | — | 39/942 | 0.041 | 30/526 | 0.057 | 0/172 | 0.000 | 0.00083 | 0.00029 |
| CXCR2_H1 | — | — | — | — | T | — | C | A | 446/940 | 0.474 | 271/524 | 0.516 | 96/170 | 0.565 | 0.053 | 0.290 |
| CXCR2_H2 | — | — | — | — | T | T | C | A | 445/940 | 0.474 | 217/524 | 0.415 | 73/170 | 0.429 | 0.077 | 0.721 |
| CXCR2_H3 | — | — | — | — | G | — | T | G | 39/940 | 0.041 | 31/524 | 0.059 | 0/170 | 0.000 | 0.00064 | 0.00018 |
| CXCR2_H4 | — | — | — | — | T | — | T | A | 10/940 | 0.010 | 5/524 | 0.009 | 1/170 | 0.006 | 0.923 | 1.000 |
| CXCR1–2_H1 | C | C | T | C | T | — | C | A | 447/938 | 0.474 | 272/522 | 0.517 | 97/172 | 0.564 | 0.054 | 0.334 |
| CXCR1–2_H2 | C | C | T | C | T | T | C | A | 444/938 | 0.472 | 218/522 | 0.415 | 74/172 | 0.430 | 0.096 | 0.790 |
| CXCR1–2_H3 | T | T | G | T | G | — | T | G | 37/938 | 0.039 | 29/522 | 0.055 | 0/172 | 0.000 | 0.00108 | 0.00029 |
| CXCR1–2_H4 | C | C | T | C | T | — | T | A | 10/938 | 0.010 | 3/522 | 0.006 | 1/172 | 0.006 | 0.839 | 1.000 |
Freq, frequency.
Reduction of Cell-Surface Expression of CD4 and CXCR4 in CXCR1-Ha Cells.
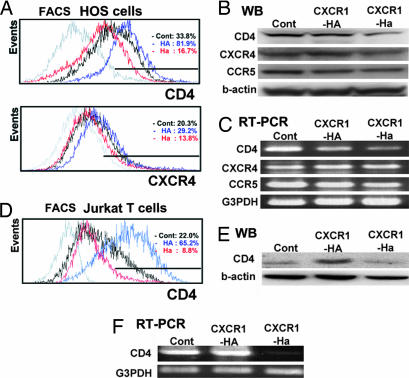
To evaluate biological function of the two CXCR1 nonsynonymous variants, we established transfectants of the CXCR1-HA and CXCR1-Ha haplotypes, in which HA and Ha incorporate the major and minor alleles, respectively, at both the CXCR1_300 and the CXCR1_142 sites (Table 2). Cell-surface expression of the HIV receptor CD4 and coreceptors CXCR4 and CCR5 was assessed on human osteosarcoma (HOS) cells after introduction of CXCR1 variant cDNAs (see Materials and Methods). Flow cytometric analysis showed a reduction of cell-surface expression of CD4 and CXCR4 in CXCR1-Ha cells compared with CXCR1-HA cells (Fig. 2A). Western blotting and RT-PCR showed that the expression of CD4 protein and the amount of CD4 mRNA were markedly lower in CXCR1-Ha compared with CXCR1-HA cells, whereas CCR5 was unaffected (Fig. 2 B and C). Reduced expression of CXCR4 was also confirmed at a protein level by Western blotting, although no clear difference of mRNA amount was detected by RT-PCR. (Fig. 2 B and C). We then examined whether variant cDNAs also affects levels of endogenous CD4 and CXCR4 molecules by transfection experiments using Jurkat human T lymphocytes. Reduced expression of CD4 was observed in Jurkat CXCR1-Ha cells compared with Jurkat CXCR1-HA cells by flow cytometry, Western blotting and RT-PCR (Fig. 2 D–F). Endogenous CD4 and CXCR4 showed similar patterns in another T lymphocyte cell line, CEM cells, transfected with variant cDNAs (results not shown).
Fig. 2.
Expression of HIV receptor/coreceptor on HOS cells (A–C) or Jurkat cells (D–F). (A) Flow cytometric analysis of HIV receptor CD4 and coreceptor CXCR4. (B and C) Western blot analysis (B) and RT-PCR analysis (C) of HIV receptor CD4, and coreceptors CXCR4 and CCR5. (D) Flow cytometric analysis of HIV receptor CD4. (E and F) Western blot analysis (E) and RT-PCR analysis (F) of HIV receptor CD4. A representative result from three independent experiments is shown.
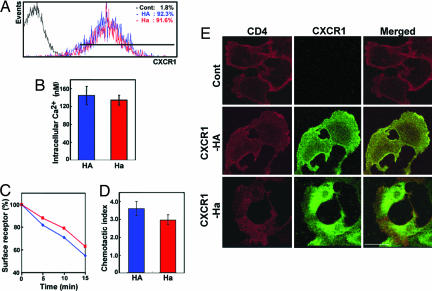
The cell-surface expression levels of CXCR1-HA and CXCR1-Ha were found to be similar (Fig. 3A). The intracellular [Ca2+] mobilization, receptor endocytotic activity, and chemotactic activity were slightly reduced in CXCR1-Ha cells as compared with CXCR1-HA cells (Fig. 3 B–D). Biological consequences of these phenomena still remain to be investigated. Immunohistochemical analysis revealed that the distribution of CD4 at the plasma membrane coincided with CXCR1-HA, but not with CXCR1-Ha (Fig. 3E).
Fig. 3.
CXCR1 expression and cellular responses to its cognate ligand IL-8 by using HOS cells. (A) Flow cytometric analysis of CXCR1 expression. (B) Intracellular Ca2+ mobilization. (C) Receptor internalization. (D) Chemotactic activity. (E) Confocal fluorescence microscopic images showing the subcellular distribution of CD4 (left column, green) and CXCR1-HA or CXCR1-Ha (middle column, red) on each transfected HOS cells. Overlaid green and red images show the colocalization between CD4 and CXCR1 (right column, yellow).
We then examined whether the CXCR1-Ha haplotype correlates lower CD4 expression under physiological conditions. Of ≈2,800 healthy Thai volunteers, we chose 8 subjects heterozygous for the CXCR1 allele (CXCR1-HA/Ha) and 11 wild-type homozygotes (CXCR1-HA/HA). Because of a low frequency (5.3%) of the CXCR1-Ha allele in Thai population, we couldn't include individuals homozygous for the CXCR1-Ha allele. Expression of the cell surface CD4 was performed by using flow cytometry by measuring the mean fluorescence intensity (MFI) of CXCR1+/CD4+ fraction of peripheral blood leukocytes. Although a tendency of lower CD4 expression levels was observed in individuals carrying CXCR1-HA/Ha (MFI: 224.57 ± 41.13) than in those with CXCR1-HA/HA (MFI: 234.82 ± 29.53), the difference was not statistically significant, probably due to a limited number of individuals examined. Future examinations employing individuals who are homozygous for the CXCR1-Ha allele, a rare subset of the population, will provide a conclusive answer to this issue.
CXCR1-Ha Has an Inhibitory Effect on HIV Infection.
It is of interest to examine whether CXCR1 variability influences the efficiency of HIV-1 infection in vitro. We first analyzed the infection efficiency of X4-tropic HIV-1NL4–3 strain to HOS CXCR1-HA and CXCR1-Ha transfectants by using HIV p24 expression as a marker. After HIV-1NL4–3 exposure, CXCR1-Ha cells showed lower HIV p24 expression compared with control cells (transfected only with empty vector), whereas CXCR1-HA cells exhibited enhanced p24 levels (Fig. 4A). There was no significant difference in p24 levels between CXCR1-Ha and CXCR1-HA cells when R5-tropic HIV was used (data not shown). These results were further confirmed by a series of experiments with HIV-1 clinical isolates obtained from AIDS patients with or without syncytia formation in MT-2 cells (termed S.I. or N.S.I., respectively). CXCR1-Ha transfectants showed significantly lower p24 expression compared with CXCR1-HA transfectants in all of the isolates (Fig. 4), demonstrating an inhibitory effect of the Ha-variant allele on HIV-1 infection. Interestingly, p24 levels in CXCR1-HA cells were consistently similar to control cells in the experiments with HIV-1 isolates from patients without syncytia formation (Fig. 4C), whereas they were consistently increased in HIV-1 isolates from patients with syncytia (Fig. 4B) and X4-tropic HIV-1NL4–3 strain (Fig. 4A). This finding may reflect the content of X4-tropic HIV-1 in isolates; S.I. isolates are from patients in later stages of disease and thus richer in X4-tropic HIV-1. Increased cell-surface CXCR4 expression in CXCR1-HA cells may result in higher p24 levels. When using N.S.I. isolates with a lesser content of X4-tropic HIV-1, the difference of p24 expression became smaller between control cells and CXCR1-HA cells. The possibility of the involvement of R5-tropic HIV-1 in reduction of infection efficiency in CXCR1-Ha cells is also conceivable. In all instances, CXCR1-Ha cells had lower p24 expression than control cells.
Fig. 4.
The efficiency of X4-tropic HIV infection in HOS cells transfectants by using HIV-1NL4–3 strain (A), S.I. clinical isolates (B), and N.S.I. clinical isolates (C). S.I. represents an isolate with CXCR4 predominance, and N.S.I. represents and isolate with CCR5 predominance. Red, blue, and black lines show the means from three three-replicate assays for CXCR1-Ha (red lines), CXCR1-HA (blue lines) and control cells transfected with empty vector (black lines). Vertical bars indicate the range of the results obtained for each set of measurements. The amount of p24 protein in culture medium is indicated as ng/ml. Differences between CXCR1-Ha and CXCR1-HA for day 8 are significant in all instances as evaluated by a t test: NL, P = 0.002; S.I.-1, P = 0.001; S.I.-2, P = 0.01; S.I.3, P = 0.008; N.S.I.-1, P = 0.003; N.S.I.-2, P = 0.006; N.S.I.-3, P = 0.03.
Predominant Role of CXCR1_142 Over CXCR1_300.
Additional transfection experiments were undertaken with HOS cells by using artificial constructs of CXCR1 cDNA that carry a single variation, either at CXCR1_142 or CXCR1_300. These experiments allowed us to map the effect on CD4 expression on the cell surface to the CXCR1_142 site and exclude a major effect of CXCR1_300 (results not shown). Other data also support a predominant role for CXCR1_142 over CXCR1_300. The minor allele at CXCR1_142 introduces a cysteine residue in the C terminus intracellular domain, which is a target of palmitoylation in most chemokine receptors. Palmitoylation influences receptor trafficking and signal transduction by altering interaction with signaling and regulatory proteins (20). Moreover, extensive site-directed mutagenesis studies have failed to demonstrate significance of the methionine substitution at the CXCR1_300 position for IL-8 binding and calcium flux (21).
Association of CXCR1 and CXCR2 SNPs with AIDS Progression.
The relationship of the 21 variants of CXCR1 and CXCR2 to AIDS progression was evaluated by genotyping of the GRIV cohort (22), consisting of 253 asymptotic HIV-1 seropositive individuals (SP series) and 84 patients with rapid disease progression (RP series). To validate the cohort, we examined the CCR5 delta-32 polymorphism. The results are compatible with previous studies in which CCR5 delta-32 has been shown to restrict infection in homozygous individuals and to reduce disease progression in the heterozygous state in Caucasians (8–10, 23). We found no individuals homozygous for the CCR5 delta-32 allele in GRIV compared with two individuals in the CTR series. The frequency of CCR5 delta-32 was 12% in the SP series, 7% in the CTR series, and 2% in the RP series (P = 0.0002), confirming its association with progression.
The frequencies of the common CXCR1–CXCR2 polymorphisms in the CTR, SP, and RP series are shown in Table 1. Significant differences in allele frequencies among the groups were found for seven SNPs (P < 0.001). Of particular interest, only the two sites involving nonsynonymous amino acid substitutions (CXCR1_300 and CXCR1_142) and the others in strong LD with these (CXCR1_219, CXCR1_200, CXCR2_7222390, and CXCR2_7222360) showed significant frequency differences. At these six sites, the minor alleles were absent in the RP series, whereas the corresponding frequencies of these alleles in the SP and CTR series were ≈6% and ≈4%, respectively. The most extreme allele frequencies in the SP and RP series, with the CTR intermediate, is similar to those in CCR5 and compatible with association to progression. The haplotype carrying the minor alleles at the six strongly associated sites (designated Ha) was absent in the RP series and has significantly higher frequencies in SP and CTRs (P = 0.001 for SP vs. CTR vs. RP; P = 0.0003 for SP vs. RP; see Table 2). To determine if these associations could be due to variants in neighboring genes in LD with a CXCR1–CXCR2 locus, we performed SNP identification of the two flanking genes, ARPC2 (50-kb telomeric to CXCR1) and FLJ46536 (35-kb centromeric to CXCR2). None of the polymorphisms detected exhibited significant LD with CXCR1 or CXCR2 variants, demonstrating that the disease-associated haplotype does not extend to the neighboring genes.
Discussion
Our results afford strong biological and genetic evidence of a protective role of CXCR1 variants on HIV-1 disease progression. As discussed above, the CCR5 delta-32 variant is also known to be associated with resistance to CD4+ cell depletion, but different mechanisms are likely to underlie between the involvement of CCR5 and CXCR1 in disease progression. CCR5 delta-32 gives rise to a truncated CCR5 molecule that forms heterocomplexes with intact CCR5 in the endoplasmic reticulum, leading to reduced CCR5 cell-surface density and contributing to slower disease progression in heterozygotes (8–10). The major effect of the reduced CCR5 density appears to be modulation of virus replication cycle in early stages, particularly of reverse transcription (24).
In contrast, the inhibitory effects of variant CXCR1 on the X4-tropic HIV-1 infection are likely to be a consequence of the suppressed expression of CD4 and CXCR4. The importance of CXCR1 in disease progression may be related to the Th1-to-Th2 shift observed in the later stages of AIDS. In atopic patients, this shift has been associated with an increase of CXCR1+CD4+ cells (25). The lower expression of CD4 and CXCR4 in CXCR1-Ha cells demonstrated in our study could be caused by unknown CXCR1-mediated signaling that regulates expression and intracellular trafficking of CD4 and CXCR4. Coexpression of CCR5 and CXCR4 interferes with HIV-1 entry under low density of cell surface CD4 (26), and the appearance of CXCR4 tropic HIV-1 variants in later stages of infection is associated with a decline in CD4+ T cell counts and adverse clinical prognosis of AIDS (25). Direct involvement of reduced CXCR1-mediated signals in HIV-1 replication is also possible (18) through palmitoylation of CXCR1-Ha at the cysteine introduced in the C-terminal intracellular domain. Indeed, palmitoylation of C-terminal cysteine in CCR5 is known to modify receptor trafficking and activation of intracellular signaling pathways (27). Genetic association of the CXCR1-Ha haplotype with chronic obstructive pulmonary disease and asthma was reported (28). It is interesting to examine functional roles of the variant CXCR1 molecule in inflammatory response.
Materials and Methods
Patients and Control Subjects.
The GRIV cohort was established in 1995 in France to generate a large collection of DNAs for genetic studies on candidate polymorphisms associated with rapid and slow progression of AIDS (22). This cohort consists of two subpopulations with extreme phenotypes selected from a pool of ≈25,000 French HIV-1+ individuals: 253 asymptomatic individuals with a CD4+ cell count >500/mm3 for 8 or more years after seroconversion (SP series) and 84 patients with rapid progression showing a drop in their CD4+ cell count <300/mm3 in <3 years after the last seronegative test (RP series). We estimated that the RP and SP series represented the 1% extremes of seropositive patients seen in the participating clinics in France (22). Members of the cohort were diagnosed with a seropositive test before 1996, and all are of French Caucasian origin. All patients enrolled in the study gave informed consent. The data on 471 CTR subjects of the same ethnic origin with unknown HIV status were obtained as part of the Epidemiological Study on the Genetics and Environment of Asthma (EGEA) study of asthma (29). DNA was obtained from fresh peripheral blood mononuclear cells or from EBV-transformed cell lines.
SNP Identification and Genotyping.
Oligonucleotide primers were designed to amplify the exon-containing DNA fragments and the promoters by PCR [see supporting information (SI) Table 3]. Nucleotide sequencing was performed by the dye terminator method with an ABI PRISM 3700 DNA analyzer (Applied Biosystems, Foster City, CA). Results were aligned to NT_005403.10 and analyzed for SNP discovery and genotyping with the software Genalys (30). Genotypes of SNPs that showed statistical significance were reconfirmed by Taqman technology (Applied Biosystems). Information about all of the SNPs, including those with a frequency of <1%, is also available from the Centre National de Génotypage (CNG) web site (SI Table 4).
Statistics.
Differences in the allele frequencies of individual polymorphisms among the three groups and between the SP and RP groups were examined by using a Fisher's exact test on the resulting 2 × 3 or 2 × 2 tables of counts. P values were also adjusted by performing a randomization test in which phenotype status (RP, SP, or CTR) was reassigned to the different individuals, conserving the multilocus genotypes to preserve LD. The adjusted P value at a specific locus was calculated as frequency over 50,000 replicates of obtaining a result at any locus as or more extreme than that observed at the specific locus. Haplotype frequencies using all polymorphisms for each gene were estimated with an EM algorithm. Differences between haplotype frequencies were examined in an analogous way to that used for the individual polymorphisms, except that within each series, expected haplotype numbers were computed from the estimated marginal haplotype probability distribution. These numbers were rounded to the nearest integer, and P values were computed by using Fisher's exact test.
Cell Culture, Transfection, and Flow Cytometry.
HOS/CD4.CCR5 (HOS), Jurkat, and CEM cells were maintained in DMEM or RPMI medium 1640 supplement with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 mg/ml streptomycin. The coding sequences of CXCR1 corresponding to amino acids nos. 2–350 were amplified by PCR using specific oligonucleotide primers (SI Table 3) and were inserted into the EcoRI site of the pRc/CMV-tag vector. The constructs were checked by nucleotide sequencing for the ORF, orientation and Taq errors. Transfections were performed by using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA). Geneticin (800 μg/ml)-resistant cells were cloned by fluorescence-activated cell sorter system EPICS ELITE ESP (Beckman Coulter, Hialeah, FL). For flow cytometric analysis, transfected HOS cells were incubated with anti-CXCR1, anti-CD4, anti-CXCR4, or anti-CCR5 antibody (DAKO, Carpinteria, CA) in PBS (pH 7.3) with 0.1% FBS for 60 min at 4°C. The cells were then washed, and analyzed for cell-surface expression of the receptor by using a flow cytometry EPICS-XL (Beckman Coulter) fitted with a single 15-mW argon ion laser providing excitation at 488 nm. FITC and phycoerythrin, respectively, were monitored through 525- and 575-nm bandpass filters.
For the expression analysis of CD4 in peripheral blood leukocytes, blood samples were collected in Na2EDTA tubes and stained within 6 h. Fifty microliters of blood was gently mixed with 1 ml of red blood cell lysis buffer and incubated at room temperature for 30 min. The sample was then centrifuged at 340 × g for 5 min at 4°C. The leukocyte pellet was washed twice with cold PBS. The supernatant was discarded, leaving 10 μl of fluid to resuspend the cell pellet. Five microliters of anti-CXCR1 and anti-CD4 antibodies were added to the cell suspension, and the staining reaction was incubated on ice for 1 h. After centrifugation at 340 × g for 5 min at 4°C, the cells were washed twice with cold PBS and analyzed by a FACS machine.
RT-PCR, Western Blot Analysis, and Immunohistochemical Analysis.
RT-PCR was performed according to the standard procedures with 35 cycles by using specific primers for human CXCR1, CD4, CXCR4, and CCR5. At the same time, constitutively expressed GAPDH mRNA was amplified as an internal standard. For Western blot analysis, HOS cells (5 × 105) were suspended in loading buffer [50 mM Tris (pH 7)/3% SDS/10% glycerol/5% 2-ME] and applied on polyacrylamide gel. After electrophoresis, proteins were electrotransferred to nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ), and blotted by using each primary antibody [goat anti-CD4 (C-18; Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-CCR5 (CKR5; C-20; Santa Cruz Biotechnology), rabbit anti-CXCR4 (fusin; H-118; Santa Cruz Biotechnology), or mouse anti-actin antibody (C-4; Chemicon, Temecula, CA) in accordance to the manufacturer's instructions. Blots were developed with ECL reagent (Amersham Pharmacia Biotech). For immunohistochemical analysis, HOS cells were fixed with 2% paraformaldehyde in PBS, rinsed with PBS, and then treated with 0.2% Triton X-100 in PBS. The cells were incubated with fluorescence-labeled anti-CD4 and anti-CXCR1 antibodies. Images were collected with a confocal microscope (Olympus, Melville, NY).
HIV-1 Infectivity Assay.
Clinical HIV-1 isolates were obtained from the plasma derived from HIV-1-infected individuals by using MAGIC-5 cells. The ability to induce syncytia formation of clinical isolates was examined in MT-2 cells. All transfected HOS cells (5 × 103) were exposed to 100 blue-cell-forming units (measured by MAGIC-5 cells) of HIV-1NL4–3 or an HIV-1 clinical isolate in 500 μl of medium for 2 h. Infected cells were washed twice with PBS and cultured in 1 ml of medium. On days 2, 4, 6, and 8 of infection, 10-μl aliquots of culture supernatants were filtered and stocked for measurements of p24 antigen concentration. The concentration of p24 in each supernatant was determined by chemiluminescence enzyme immuno-assay (CLEIA) kit (Fuji-Rebio, Tokyo, Japan). Assays were performed in triplicate.
Intracellular [Ca2+] Measurement and Chemotaxis Assays.
For the calcium influx study, HOS cells (106) were washed with Tyrode's salt solution (TSS) (Sigma, St. Louis, MO) and incubated with 4 μM Fluo-3 acetoxymethyl ester in DMSO containing 20% Pluronic F-127 (Molecular Probes, Eugene, OR) at room temperature in the dark for 1 h. After washing with the same buffer, the cells were suspended in TSS containing 0.1% BSA buffer and transferred to 96 black well plates (Nunclon) for reading. Recombinant human IL-8 (final concentration, 100 nM) was added to each sample for stimulation, and fluorescence was monitored for 3 min with the microfluorescence recorder Fluoroskan Ascent system (Labsystems, Chicago, IL). Intracellular calcium concentrations were computed as described in the manufacturer's instructions. Background stabilization and probe levels were determined for each sample. Chemotaxis of all transfected Jurkat cells in response to 10-nM recombinant IL-8 was evaluated in triplicate by using a 96-well microchemotaxis chamber (Neuro Probe, Gaithersburg, MD) and a 3-μm polycarbonate membrane. RPMI medium 1640 supplemented with 10% FBS, 0.1% BSA, and 10-mM Hepes was used for the assay. Migrated cells in the lower chamber were counted by a flow cytometer EPICS-XL (Beckman Coulter).
Supplementary Material
Acknowledgments
We thank all patients and medical staff who were concerned with the establishment of the GRIV cohort and the Epidemiological Study on the Genetics and Environment of Asthma (EGEA) cooperative group, who allowed us access to data on the EGEA study, which is partly supported by an Institut National de la Santé et de la Recherche Médicale/Merck Sharp & Dohme convention. We also thank M. Alizon for his help in the construction of the vectors for transfection experiments and M. Matsuoka for valuable suggestions. V.P., P.A., and T.S. are research members of the Core University Program, which is supported by the Japan Society for the Promotion of Science. The Centre National de Génotypage is supported by the Ministere de la Recherche et des Nouvelles Technologies. The work was supported in part by Core Research for Evolutional Science and Technology, Solution Oriented Research for Science and Technology, the Japan Science and Technology Agency, the Japan Science Foundation, Agence Nationale de Recherche sur le SIDA, and the AIDS–Cancer Vaccine Development Foundation.
Abbreviations
- CTR
control
- HOS
human osteosarcoma
- LD
linkage disequilibrium
- N.S.I.
nonsyncytia-inducing
- RP
rapid disease progression
- S.I.
syncytia-inducing
- SP
slow disease progression.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the National Center for Biotechnology Information dbSNP database (ID nos. ss69355493–ss69355497).
This article contains supporting information online at www.pnas.org/cgi/content/full/0611670104/DC1.
References
- 1.McCune JM. Nature. 2001;410:974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 2.Taylor JM, Tan SJ, Detels R, Giorgi JV. AIDS. 1991;5:159–167. doi: 10.1097/00002030-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, Pantaleo G, Fauci AS. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 6.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y, Broder CC, Kennedy PE, Berger EA. Science. 1996;272:872–877. [Google Scholar]
- 8.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 9.Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 11.Baggiolini M, Dewald B, Moser B. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 12.Schroder JM, Mrowietz U, Morita E, Christophers E. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- 13.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto T, Miike T, Nelson RP, Trudeau WL, Lockey RF, Yodoi J. Clin Exp Immunol. 1993;93:149–151. doi: 10.1111/j.1365-2249.1993.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meddows-Taylor S, Martin DJ, Tiemessen CT. Clin Diagn Lab Immunol. 1999;6:345–351. doi: 10.1128/cdli.6.3.345-351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meddows-Taylor S, Martin DJ, Tiemessen CT. J Infect Dis. 1998;177:921–930. doi: 10.1086/515232. [DOI] [PubMed] [Google Scholar]
- 18.Lane BR, Lore K, Bock PJ, Andersson J, Coffey MJ, Strieter RM, Markovitz DM. J Virol. 2001;75:8195–8202. doi: 10.1128/JVI.75.17.8195-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson RM, Tokunaga K, Marjoram R, Sata T, Snyderman R. J Biol Chem. 2003;278:15867–15873. doi: 10.1074/jbc.M211745200. [DOI] [PubMed] [Google Scholar]
- 20.Qanbar R, Bouvier M. Pharmacol Ther. 2003;97:1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 21.Leong SR, Kabakoff RC, Hebert CA. J Biol Chem. 1994;269:19343–19348. [PubMed] [Google Scholar]
- 22.Hendel H, Cho YY, Gauthier N, Rappaport J, Schachter F, Zagury JF. Biomed Pharmacother. 1996;50:480–487. doi: 10.1016/s0753-3322(97)89278-5. [DOI] [PubMed] [Google Scholar]
- 23.Rappaport J, Cho YY, Hendel H, Schwartz EJ, Schachter F, Zagury JF. Lancet. 1997;349:922–923. doi: 10.1016/S0140-6736(05)62697-9. [DOI] [PubMed] [Google Scholar]
- 24.Lin YL, Mettling C, Portales P, Reynes J, Clot J, Corbeau P. Proc Natl Acad Sci USA. 2002;99:15590–15595. doi: 10.1073/pnas.242134499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Lapham CK, Chen H, King L, Manischewitz J, Romantseva T, Mostowski H, Stantchev TS, Broder CC, Golding H. J Virol. 2000;74:5016–5023. doi: 10.1128/jvi.74.11.5016-5023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanpain C, Wittamer V, Vanderwinden JM, Boom A, Renneboog B, Lee B, Le Poul E, El Asmar L, Govaerts C, Vassart G, et al. J Biol Chem. 2001;276:23795–23804. doi: 10.1074/jbc.M100583200. [DOI] [PubMed] [Google Scholar]
- 28.Stemmler S, Arinir U, Klein W, Rohde G, Hoffjan S, Wirkus N, Reinitz-Rademacher K, Bufe A, Schultze-Werninghaus G, Epplen JT. Genes Immun. 2006;6:225–230. doi: 10.1038/sj.gene.6364181. [DOI] [PubMed] [Google Scholar]
- 29.Kauffmann F, Dizier MH, Pin I, Paty E, Gormand F, Vervloet D, Bousquet J, Neukirch F, Annesi I, Oryszczyn MP, et al. Am J Respir Crit Care Med. 1997;156:S123–S129. doi: 10.1164/ajrccm.156.4.12tac9. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M, Matsuda F, Margetic N, Lathrop M. J Bioinform Comput Biol. 2003;1:253–265. doi: 10.1142/s021972000300006x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.