Abstract
Objective
Studies have indicated that there is a development of generalized immune dysfunction following septic insult. However, the mechanisms responsible for these changes remain unclear. Recently, accumulating evidence shows that several lymphocyte subpopulations, such as NKT-, CD4+-Th2-T-, CD8+-T-, γδ-T- and CD4+CD25+-T-regulatory cells, are capable of actively contributing to inducing septic immune suppression. Thus, our aim was to investigate the contribution of CD4+CD25+ cells to the immune dysfunction seen in sepsis. To study this, C57BL/6J, C57BL/6-Il6tm1Kopf (IL-6-/-) and -Il10tm1Cgn (IL-10-/-) mice were subjected to cecal ligation and puncture (CLP) or sham operations. 24hr later, blood was collected and splenocytes were isolated with magnetic microbeads and assessed for phenotypic expression of CD4/CD25 by FACS, cell proliferation [presented as Prolifer. Index = (with anti-CD3)/(without anti-CD3)] and immune suppressive capacity by in vitro add-back experiments. The results indicate a marked elevation in CD4+CD25+ cell levels and their Prolif. Index after sepsis in background mice. CD4+CD25− cells from sham and CLP mice proliferated equally. However, co-culture of CD4+CD− with CD4+CD25+ cells suppressed their proliferation in both sham and CLP mice. Alternatively, in vivo depletion of CD25+ cells prior to CLP markedly restored proliferative capacity and Th1 cytokine release, while not altering plasma pro-inflammatory cytokine levels. Subsequently, IL-6-/- and IL-10-/- mice were used to elucidate the possible mediator(s) regulating those changes seen after sepsis. The increase in septic mouse CD4+CD25+ cells was blunted in IL-10-/- mice but not in IL-6-/- mice. Conversely, while the proliferation of CD4+CD25+ cells from septic C57BL/6J and IL6-/- mice increased, it was blunted in IL-10-/- mice. Surprisingly, depletion of CD25+ cells prior to inducing sepsis did not alter septic mortality. Together, these findings suggest that while CD4+CD25+-T-regulatory cells induced by IL-10 appear to contribute to aspects of sepsis induced lymphoid immune suppression, the oblation of CD25+ cells does not provide a survival advantage or disadvantage.
Keywords: Lymphocyte, mouse, Th1 vs. Th2, cecal ligation and puncture
INTRODUCTION
Sepsis and multiple organ failure remains the leading cause of death in the surgical intensive care unit despite significant advances in patient care, such as the application of activated protein C, low-dose steroid administration and the tight control of blood glucose levels. On the cellular level, studies have shown that following sepsis there is a generalized immune dysfunction in T-/B-cell function [1,14]. These alterations along with changes in the phagocytic arm of the immune response have been associated with increased morbidity and mortality in septic patients [4]. Several groups have consistently implicated the role of an active lymphoid suppressor cell populations [1]. However, the details of this suppression are not understood. In this regard, recent studies have suggested that several lymphocyte subpopulations (CD4+CD25+-T-regulatory-cell [10,18,24], NK-T-cells [29], and CD8+T-cells [33]) may have the capacity to actively suppress an adaptive immune response and they may potentially be involved in septic immune dysfunction. In particular, a class of T-regulatory cells that co-express the cell differentiation (CD) markers CD4 and CD25 appear to exhibit this immune suppressive potential. Though CD4+CD25+ cells make up only a small fraction of the T-lymphocyte population in the immune system, recent research has indicated that they are potent effectors: inhibitors of immune responsiveness [10]. Animal studies have established that regulatory T cells are important in the defense against infection and control of autoimmune diseases [22]. Clinical studies have also shown that inhibition of CD4+CD25+ followed by immunization potentiates the developing adaptive immune responses [7]. Cytokines such as IL-10, TGF-β and IL-6 have been reported to affect the differentiation and function of CD4+CD25+ cells [10,11]. Recent research has specified that systemic over-expression of IL-10 induces a boost in CD4+CD25+ cell populations[10].
Based on these observations, we have hypothesized that CD4+CD25+-T-regulatory cells contribute to the immune dysfunction seen following the onset of sepsis, and that this suppression is mediated by IL-10. Our initial goal was therefore to determine if there was an increase in the number of cells in septic mice that exhibit a T-regulatory cell phenotype (CD4+CD25+) and to establish the extent to which T-regulatory cells, isolated from septic mice, could exhibit suppressor function, by suppressing normal T-cell responses. Our secondary goal was to explore if and to what extent this immune suppression is mediated by IL-6 and IL-10. We then investigated the potential of CD4+CD25+ T-cells to stunt expansion/proliferation of non-CD4+CD− T-cells. Lastly, the effect of CD25+ cells on mouse survival was examined by depletion of this cell population using antibody.
METHODS AND MATERIALS
Animals
Male inbred C3H/HeN (Charles River, Wilmington, MA), C57BL/6J (Charles River), C57BL/6-Il6tm1Kopf (IL-6-/-) and -Il10tm1Cgn (IL-10-/-) mice (Jackson Laboratories, Bar Harbor, ME), 8–12 week-old were used. The studies were carried out in accordance with the National Institutes of Health Guidelines on Laboratory Animals and were approved by the Rhode Island Hospital Committee on Animal Use and Care.
Sepsis model induced by cecal ligation and puncture (CLP)
Polymicrobial sepsis was produced as previously described by Ayala et al. [5,28] with minor modifications. In brief, mice were lightly anaesthetized using isoflorane (1-chloro-2, 2, 2-trifluorethyl difluoromethyl ether) and a midline incision (1–2 cm) was made just caudal to the diaphragm, exposing the internal organs. The cecum was ligated and punctured with a 22-gauge needle in two places to induce sepsis. Following the return of the cecum to the peritoneal cavity, the muscle layer and epidermal layer were sutured, and 0.6 ml of lactated Ringer’s solution was administered subcutaneously. Animals were then sacrificed at either 24 hr after CLP (a period when overt suppression of lymphoid immune response is evident [2]) or their survival were monitored for up to 10 days post-CLP.
To examine the effect of the lack of CD25+ cell for some experiments, animals were randomized to receive 200 μg of either endotoxin-free purified anti-mouse CD25 antibody (rat IgG1, λ, clone: PC61.5; e-Bioscience, San Diego, CA) or rat IgG1 as a control in 0.2 ml of saline, i.p., 48 hr prior to performing CLP.
Blood Leukocyte and Splenocyte isolation
At 24 hr after surgery, blood was taken by cardiac puncture in a heparinized tube till processing for cell phenotyping or to obtain plasma. Subsequently, splenocytes were isolated aseptically from these animals according to the methods previously described [2]. Following hypotonic lysis of the residual erythrocytes, splenocytes were washed twice and suspended in phosphate buffered saline (PBS) or RPMI-1640 supplemented with 5% fetal bovine serum. Splenocyte viability and total cell yield were determined by trypan blue exclusion.
Cell phenotyping and flow cytometric analysis
To correlate the changes in the frequency of CD4+CD25+ cells as well as FOXP3 expression in the T-cell compartment following the onset of sepsis we utilized flow cytometric approach. A number of monoclonal antibodies and their isotype controls were used for phenotyping: either fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-Cy7 (PE-Cy7) or allophycocyanin (APC) labeled anti-mouse CD25, anti-mouse-CD4, anti-FOXP3 or their isotype controls were obtained from either BD Pharmingen (San Diego, CA), e-Biosource, Inc., (San Diego, CA) or Caltag, Inc., (Burlingame, CA). Splenocytes (before or after treatment of animals with anti-mouse CD25) or blood leukocytes were processed and stained as previously described by Ayala et al [3]. For intracellular FOXP3 detection, after staining the surface markers (CD4 or CD25), cells were fixed/permeabilized using cytofix/cytoperm kit according to manufactory’s instruction (BD Biosciences) and stained with anti-FOXP3 [6]. Fluorescent assessment was carried out with either a BD-FACSvantage or BD-FACSArray™ bioanalyzer. Thresholds for positive phenotypic expression were delineated with the respective isotype controls as previously described [3] using WinMidi version 2.0 software (Scripps Inst., La Jolla, CA).
Cell Separation Protocol
In order to determine the suppressive capacity of CD4+CD25+ T-cells following sepsis, as well as those potential cytokines that effect this, CD4+CD25+ T-cells from C57B/6J, IL-6-/-, and IL-10-/- mice were isolated using magnetic cell sorting in a two-step procedure (Miltenyi Biotec. Inc., Auburn, CA). First, CD4+ T cells were pre-enriched by depletion of unwanted cells. Then, CD25 cells were positively selected from the enriched CD4+ T cell fraction.
In brief, non-CD4 T cells were depleted by indirect magnetic labeling with a cocktail of biotin-conjugated antibodies (10 μl/107 cells, 10 min. at 4°C) then incubated with anti-biotin microbeads (20μl/107 cells) and CD25-PE (10 μl/107 cells) for additional 15 min. in the dark at 4°C. The magnetically labeled cell suspension was loaded onto a column placed in the magnetic field of the AutoMACS© Separator (Miltenyi Biotec.). The flow-through CD4+ cells were collected and centrifuged. In order to isolate CD4+CD25+ cells, the CD25+ PE-labeled cells in the enriched CD4+ T cell fraction were incubated with anti-PE microbeads (10 μl/107 cells) for 15 min. in the dark at 4°C. The magnetically labeled CD4+CD25+ cells were collected after loaded onto the AutoMACS© Separator. Typically this produced a cell population that was 90±5 % CD4+CD25+ cells.
Cell Culture
In order to investigate the nature of cytokine mediators that contribute to the regulatory effects of CD4+CD25+ seen in sepsis, the effect of IL-6 and IL-10 was examined in mice deficient in either IL-6 or IL-10. 4 × 105 cells were incubated with or without plate-bound 10 μg anti-mouse CD3/ml as a T cell stimulant, for a three-day period [26]. CyQUANT© cell proliferation assay kit (Molecular BioProbes, OR) was used to determine the extent of proliferation of cells in culture with green fluorescent CyQUANT© GR dye according to methods in Ding et al [8]. Cells were lysed by addition of 100 ul CyQUANT© GR dye buffer; and the extent of fluorescence DNA dye incorporation was then measured directly with a microplate fluorescence plate reader (FLx800, Bio-Tek Instruments, Winooski, VT). Proliferation of cells is presented as Prolif. Index (cell fluorescent intensity [in arbitrary units] with anti-CD3/cell fluorescent intensity [in arbitrary units] without anti-CD3).
For some studies the change in proliferative capacity was confirmed by following the dilution of carboxyfluoroscein succinimidyl ester (CFSE) over 72hr by flow cytometry. In brief, 1 × 107cells/ml were labeled with 2 μM CFSE (Molecular Probes, Eugene, OR) for 15 min. at 37°C, washed twice with PBS, stimulated/cultured in the presence or absence of anti-CD3 for 72 hr, then harvested, washed and analyzed on a BD-Facsarray™ bioanalyzer by flow cytometer to establish the % of CD4+ cells that where proliferating.
Co-Culture Studies
Twenty-four hr post-operation, splenocytes were harvested from C57B/6J mice. As before, the Miltenyi AutoMACS© Magnetic Bead Separator was utilized to isolate CD4+CD25+ T-cells. CD4+ T cells were pre-enriched by depletion of unwanted cells, as described above. Then, CD25+ cells were positively selected from the enriched CD4+ T cell fraction.
After isolation, cells were cultured in the following 6 groups in triplicates with or without anti-mouse CD3: (1) 5 × 104 Sham CD4+CD25+ T cells, (2) 1 × 105 Sham CD4+CD25− T cells, (3) 5 × 104 CLP CD4+CD25+ T cells, (4) 1 × 105 CLP CD4+CD25− T cells, (5) 5 × 104 Sham CD4+CD25+ (+) 1 × 105 Sham CD4+CD25− T-cells, and (6) 5 × 104 CLP CD4+CD25+ (+) 1 × 105 Sham CD4+CD25− T-cells. Cells were incubated for three days and cell density was read using the CyQUANT© cell proliferation assay.
Cytometric Bead Array
Mouse cytokine/chemokine levels were determined in plasma or cultured supernatants using cytometric bead array technique (BD™ Cytometric Bead Array Mouse Inflammation Kit, BD Biosciences, San Diego, USA) and samples were analyzed on a BD-Facsarray™ bioanalyzer (BD Biosciences, San Diego, USA) according to the manufacturers instruction [19].
Presentation of Data and Statistical Analysis
The data are presented as mean ± SEM for each group. Differences in data were considered to be significant if p<0.05, as determined using the Mann-Whittney U-test. Differences in survival curves were considered significant by the Log-rank survival test at p<0.05 (SigmaStat® Ver. 3.0; SPSS Inc., Chicago, IL).
RESULTS
Experimental Sepsis Induces Up Regulation of the Frequency of CD4+CD25+/T-Regulatory Cells in both the Spleen and Blood
Figure 1a–d illustrates the result of a typical flow cytometric analysis of the mixed splenocyte and blood leukocyte populations present in the sham vs. CLP mice. While the overall percentage of CD4+CD25+ splenocytes is quite small, the cells harvested from either male C57BL/6 or C3H/HeN mice 24 hr following the onset of CLP nonetheless consistently exhibited a higher frequency of CD4+CD25+ T-cell levels (Figure 1e). We also noted a consistent increase in the splenic CD4+ population of the CD4+CD25+ T-cell activation intracellular marker FOXP3 in septic mice (57.5±1.8% FOXP3+ in sham CD4+ cells vs. 74.1±2.1%* in CLP mouse CD4+ cells [*at P<0.05, n=3 mice/group]). Further, while the percentage of total splenocytes was small they made up 15 to 25% of the total CD4+ cell population and this represented ~20–25% increase (Figure 1f). Alternatively, while the frequency CD4+CD25+ T-cells present in the circulating blood leukocyte population did not appear to change (Figure 1e), because the total percentage CD4+ did decline in the CLP mice, the percent of CD4+CD25+ T-cells actually increased (Figure 1f).
Figure 1a–d.

Typical flow cytograms illustrating the results of two-color flow cytometric analysis of CD4 vs. CD25 cell fluorescent intensity for a cell suspensions obtained from spleen (a,c) or the blood (b,d) 24 hr following sham (a,b) and CLP (c,d). Summary data of 4–5 repeat experiments on samples derived from either C57BL/6J (B6) or C3H/HeN (HeN) mice indicating the number of CD4+CD25+ cells as either a percentage of the total mixed splenocyte or blood leukocyte population (e) or a percentage of the CD4+ cell population alone (f), are provide in the bottom two panels of this figure. Statistically significant differences indicated by * at p<0.05 vs. sham, Mann-Whittney U test, Mean ± SEM; n=4–5 mice/group.
The Increase of Frequency as well as the Proliferative Capacity of CD4+CD25+ Cells from Septic Mice Are Altered by the Absence of IL-10 but not IL-6
In an effort to understand the nature of the mediators that might be regulating the expression/expansion of CD25+CD4+, the splenocytes of sham or septic C57BL/6J, IL-6-/-, and IL-10-/- mice was examined 24 hr following the onset of CLP. We found that percentage of CD4+CD25+ T-cells was significantly increased in septic C57BL/6J background and IL-6-/- mice, whereas in IL-10-/- mice the frequency of CD4+CD25+ T-cells remained unchanged when compared with sham (Figure 2a). Figure 2b shows that CD4+CD25+ cells in the spleen harvested from C57BL/6J and IL-6-/- mice 24 hr after CLP proliferate to a greater extent (increased Prolif. Index) when stimulated with the anti-mouse CD3 as opposed to those cells isolated from IL-10-/- septic mice.
Figure 2a–b.

Average levels of CD25+ cells out of total CD4+ cell population in the spleens from C57BL/6J, IL-6-/-, and IL-10-/- mice (a). Proliferation Index of CD4+CD25+, CD4+CD25− cells in the spleens from C57BL/6J, IL-6-/-, and IL-10-/- mice (b). Statistically significant differences indicated by * at p<0.05 vs. their equivalent sham, Mann-Whittney U test, Mean ± SEM; n=4 mice/group.
Co-Culture with CD4+CD25+ Markedly Decrease the Proliferative Capacity of CD4+CD25− Cells
Figure 3 confirms that CD4+CD25+ cells harvested from C57BL/6J 24 hr following the onset of CLP proliferate to a greater extent when stimulated with anti-CD3. Concomitantly, while CD4+CD25− cells showed higher Prolif. Index and proliferated equally in both sham and CLP mice, there was a general decrease in proliferative capacity when co-cultured with CD4+CD25+ cells, however, the proliferation was further inhibited after septic insult. This suggests that CD4+CD25+ cells can actively inhibit CD4+CD25− cell proliferation.
Figure 3.

Proliferation of purified splenic CD4+CD25+, CD4+CD25− or the mix of CD4+CD25+ with CD4+CD25− cells from either C57BL/6J sham or CLP mice. Statistically significant differences indicated by * at p<0.05 vs. sham CD4+CD25+ alone, Mann-Whittney U test, Mean ± SEM; n=3–4 mice/group.
Treatment of Mice with Antibody against CD25 Markedly Improves the Proliferative Capacity and Th1 Cytokine Release of Septic Mouse Splenocytes, but Had Little Effect on the Systemic Pro-inflammatory Response or Overall Septic Survival
Figure 4a–d illustrates that when animals received anti-mouse CD25 monoclonal antibody i.p. 48 hr prior to CLP, the percentage of purified CD4+ cell population that was CD4+CD25+ was reduced typically from ~11% of the population to just ~0.25% (~44 fold/~98% decrease). When mice were subjected to CLP, it was noted that the loss in splenic CD4+ cell proliferative capacity and IFN-γ/IL–2 release in response to T-cell receptor stimulation was attenuated in the anti-mouse CD25 treated animal’s cells (Figure 4e and 5a). The effect on proliferation was further supported by the observation of an increase in the percentage of cells dividing, as determined by the dilution of CFSE, assessed by flow cytometric analysis (Figure 4f). Interestingly, while there was a trend towards a decline of IL-2 release capacity in CD4+ cell taken from the IgG pre-treated CLP mice, this was not improved by antibody depletion of CD25 cells prior to the onset of sepsis (Figure 5b). Anti-mouse CD25 pre-treatment also showed a trend towards a decline of IL-10 release capacity in mouse CD4+ cells, but this was seen in both sham or CLP treated mice (Figure 5b). Surprisingly, antibody depletion of CD25+ cells prior to sepsis had no effect on the rise in plasma pro-/anti-inflammatory cytokine/chemokine levels (Figure 6).
Figure 4.

Typical flow cytograms illustrating the results of two-color flow cytometric analysis of CD4 vs. CD25 cell fluorescent intensity for a cell suspensions obtained from spleen of sham (a,b) or CLP (c,d) mice that have either been pretreated with IgG (a,c) or anti-mouse CD25 (b,d) 24 hr after surgical procedure. Proliferation (as determined by Prolif. Index [e] or dilution of CFSE fluorescen intensity [% Proliferation][f]) of CD4+ splenocytes purified from sham or CLP mice pre-treated with either IgG or anti-CD25. Statistically significant differences indicated by * at p<0.05 vs. the IgG sham or # vs. the IgG CLP, Mann-Whittney U test, Mean ± SEM; n=3–5 mice/group.
Figure 5.
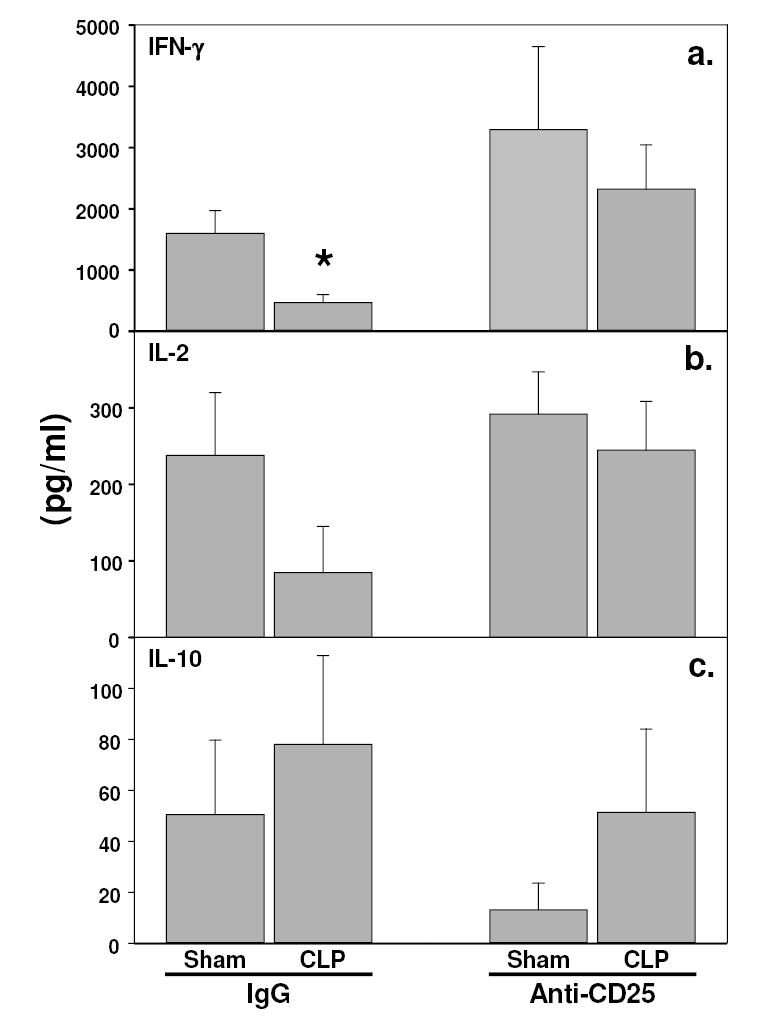
Changes in the capacity of CD4+ splenocytes purified from sham or CLP mice pre-treated with either IgG or anti-CD25 to produce either IFN-γ (a), IL-2 (b) or IL-10 (c) in response to plate-bound anti-CD3. Statistically significant differences indicated by * at p<0.05 vs. the IgG sham or # vs. the IgG CLP or @ vs. CLP anti-CD25, Mann-Whittney U test, Mean ± SEM; n=5 mice/group.
Figure 6.

Changes in plasma IL-6, IL-10, TNF-a and MCP-1 levels from sham or CLP mice pre-treated with either IgG or anti-CD25. Statistically significant differences indicated by * at p<0.05 vs. sham (CLP “-“) IgG treated group or # vs. the sham (CLP “-“) anti-CD25 treated group, Mann-Whittney U test, Mean ± SEM; n=5 mice/group.
With this in mind we attempted to determine what the effect of antibody depletion of these CD25+ cells would be on the mouse capacity to survive following CLP. Here again, mice were pre-treated with anti-mouse CD25 antibody 48 hrs to the induction of sepsis and then their survival was assessed for 10 days. The results indicated that there were no marked differences in the frequency of mortality seen with or without anti-CD25 antibody treatment (Figure 7).
Figure 7.

Depletion of CD25+ cells prior to CLP in does not markedly alter the survival of male C57B/6J mice subjected to septic insult (CLP, cecal ligation and a single puncture with a 22 gauge needle). n=15 mice per group.
DISCUSSION
The aim of this study was to determine whether sepsis induced a change in the frequency of T-regulatory cells (CD4+C25+) and to establish the extent to which T-regulatory cells isolated from septic (CLP) mice had the capacity to suppress mouse CD4+CD25− T-cell responses. The results presented here demonstrate a significant increase in CD4+C25+ T-cells following polymicrobial sepsis in both the spleen and the blood. This latter finding is in keeping with the observations recently made in the clinical setting of septic patients that an increase in the frequency of CD4+C25+ T-cells was detected [17,31]. However, because of the difficulty encountered in isolating sufficient number of CD4+C25+ T-cells from mouse blood, the remainder of the studies done here centered on spleen derived T-cells. The observation that such changes occur in both C3H/HeN as well as C57BL/6J suggests this is not simply an aberration of mouse inbreeding. However, as we have restricted our studies to 8–12 week old male animals we do not know whether this sepsis induced effect is consistent with increasing age and/or over female gender.
Although CD25 expression has been used as one of the surface markers (others are the cytotoxic T lymphocyte-associated antigen-4, CTLA-4 and glucocorticoid-induced TNF receptor-related protein, GITR) for identifying T-regulatory cell population, it does not tell us whether these T-regulatory cells are functional. Recently, FOXP3 (forkhead box P3) protein, a transcriptional factor that belongs to the large family of forkhead box proteins, has been associated with the regulation of development and function of CD4+CD25+ T-regulatory cells [9,11]. Here we confirmed that the increased CD4+CD25+ population seen after CLP is indeed associated with an increase in FOXP3 protein expression, implying that this is an increase in functionally active T-regulatory cells in the septic mice.
Analysis of phenotypic staining and cell proliferative capacity was utilized to examine the roles of IL-6 and IL-10 as mediators of this increase in T-regulatory cell frequency after sepsis. Our results indicate that consistently with background mice, in IL-6-/- CLP mice upregulation of CD4+CD25+population in the spleen was also observed and such increase was two fold higher than background mice, whereas CD4+CD25+ levels in IL-10 -/- mice remain unchanged in response to septic insult. In this regard, IL-6 has been reported to be able to overcome the suppressive effect mediated by of CD25+CD4+ T-regulatory cells [18], however, whether the absence of IL-6 (in the IL-6-/- mice) supports CD4+CD25+ cell expansion following the onset of polymicrobial sepsis remains further exploration. It has been reported that CD25+CD4+ T-cells are anergic, at least in vitro, upon TCR stimulation. They do not proliferate or secrete effecter cytokines (IL-2, IFN-γ or IL-4) [13,21,24]. Our findings are in keeping with the suggestion that while splenic CD4+CD25+ cells isolated from all sham animals proliferated poorly in response to plate-bound anti-CD3, the proliferation was increased in septic background and IL-6-/- mice. Interestingly, while we observed only ~30% increase in proliferation in these septic, background and IL-6-/- mouse cells, there was almost a 3-fold increase in the frequency/accumulation of cells in CLP IL-6-/- mouse spleen. Alternatively, the loss of IL-10 appears to not only stunt the expansion of CD4+CD25+ splenocytes but had no effect on proliferation seen in septic mice when compared background control IL-6-/- mice. This supports IL-10’s potential role in increasing the percentage of active T-regulatory cells in the septic mouse [10].
Results from the co-culture experiment indicate a noticeable decline in non-T-regulatory cell levels when stimulated in the presence of CD4+CD25+ cells. These data are very intriguing, as they suggest a two-fold effect: 1) increased CD4+CD25+ frequency following septic challenge and 2) the CD4+CD25+ T-cells exhibit an enhanced capacity to inhibit CD4+CD25− T-cell immune responsiveness during sepsis. In support of this data we found that conversely when mice were pre-treated with antibody against mouse CD25, so as to deplete these cells, the proliferative capacity of CD4+ splenocytes derived from septic mice was restored to sham levels. However, identify the nature of the T-regulatory effect on CD4+ T-cell responsiveness is not complete as we found that only IFN-γ production was restored and not IL-2 in anti-CD25 treated mice. The extent to which this is mediated by direct cell-cell interaction and/or soluble factors in a paracrine fashion remain to be examined.
Studies have indicated that CD4+CD25+ cells may mediate the immune suppression in other chronic disease states, like diabetes, etc [10]. In this regard, we have documented here that not only is there an upregulation of CD4+CD25+ cells but also a decrease in the CD4+CD25− T-cell proliferation upon TCR stimulation when co-cultured with CD4+CD25+cells. IL-10 appears to be essential to the effect associated with this activity, which is in keeping with not only prior observations made in this laboratory [2,27] but in others [12,16,30]. Together, these findings suggest that CD4+CD25+-T-regulatory cells induced by IL-10 should not only play an important role in sepsis induced immune suppression, but this should contribute to an increased susceptibility to septic mortality. Conversely, we speculated that the depletion of these cells might be protective. Surprisingly, when animals were pretreated with antibodies that depleted the CD25+ cell population we found no effect on septic survival. The initial conclusion might be that the CD4+CD25+ and the associated immune suppressive effect are not essential to these animals survival of CLP. However, such a conclusion is not so simple, since CD4+CD25+ T-regulatory cells are not the only cells that express the CD25+ receptor [20,23]. Thus, such a depletion approach may have removed both the detrimental effects of CD4+CD25+ T-regulatory cells as well as CD25+-non-regulatory T cells, e.g., most activated T-cells, which might beneficial to a functional innate/adaptive immune response to septic challenge. Addressing this may be difficult at present since there is currently, to our knowledge, a lack of other highly specific cell surface markers for T-regulatory cells. One can only speculate that expression of modestly specific intracellular transcription factors within activated T-regulatory cells, like FOXP3 (as we have observed here, appears to change in expression coincident with changes in T-regulatory cell frequency in septic mice) or GITR [15,25], might provide more suitable future targets for therapies like siRNA [32]or membrane-permeable pepdite/protein antagonists. However, these studies remain to be done. Thus, while CD4+CD25+ T-regulatory cells appears to play some role in the development of aspects of lymphoid immune dysfunction, the inability of CD25+ antibody depletion to mitigate the systemic pro-inflammatory response as well as alter septic mouse survival implies more studies will be needed to clarify the significance of this cell population’s expansion to the developing septic morbidity.
Acknowledgments
We would like to thank Mrs. Sarah Spangenberger at the Core Research Laboratories at Rhode Island Hospital/Lifespan for her technical support on the flow cytometer. Support for this project was provided from a N.I.H RO1-GM46354 (to A.A.).
References
- 1.Ayala, A., Chung, C. S., and Song, G. Y. Lymphocyte Activation, Anergy, and Apoptosis in Polymicrobial Sepsis. Marshall, J. C. and Cohen, J. Immune Response in the Critically Ill. (31), 227–245. 1999. Berlin, Springer. Update in Intensive Care and Emergency Medicine. Vincent, J. L.
- 2.Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. JSurgRes. 1994;56:579–585. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- 3.Ayala A, Karr SM, Evans TA, Chaudry IH. Factors responsible for peritoneal granulocyte apoptosis during sepsis. JSurgRes. 1997;69:67–75. doi: 10.1006/jsre.1997.5027. [DOI] [PubMed] [Google Scholar]
- 4.Ayala A, Lomas JL, Grutkoski PS, Chung CS. Pathological aspects of apoptosis in severe sepsis and shock? IntJBiochem& Cell Biol. 2003;35:7–15. doi: 10.1016/s1357-2725(02)00099-7. [DOI] [PubMed] [Google Scholar]
- 5.Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophage to release inflammatory mediators (IL-1, IL-6 and TNF) CircShock. 1992;36:191–199. [PubMed] [Google Scholar]
- 6.Ayala A, Xu YX, Chung CS, Chaudry IH. Does Fas ligand or endotoxin contribute to thymic apoptosis during polymicrobial sepsis? Shock. 1999;11:211–217. doi: 10.1097/00024382-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. JImmunol. 2000;165:4848–4853. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, Ayala A. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22:137–144. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nature Immunology. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 10.Goudy KS e a. Systemic expression of IL-10 induces CD4+ CD25+ cell populations in vivo and ameliorates type 1 diabetes in nonobese diabetic mice in a dose-dependent fashion. JImmunol. 2003;171:2270–2278. doi: 10.4049/jimmunol.171.5.2270. [DOI] [PubMed] [Google Scholar]
- 11.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes and Infection. 2004;6:745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Kalechman Y, Gafter U, Gal R, Rushkin G, Yan D, Albeck M, Sredni B. Anti-IL-10 therapeutic strategy using immunomodulator AS101 in protecting mice from sepsis-induced death: dependence on timing of immunomodulating intervention. JImmunol. 2002;169:384–392. doi: 10.4049/jimmunol.169.1.384. [DOI] [PubMed] [Google Scholar]
- 13.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. NatImmunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 14.Mannick, J. A.trauma, sepsis, and immune defects. In Faist, E., J. L. Meakins, and F. W. Schildberg, eds., Host Defense Dysfunction in Trauma, Shock and Sepsis: Mechanisms and Therapeutic Approaches. Berlin, Springer-Verlag. 1993, 15–21.
- 15.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 16.Miller-Graziano CL, De AK, Kodys K. Altered IL-10 levels in trauma patients’ macrophages and T lymphocytes. JClinImmunol. 1995;15:93–104. doi: 10.1007/BF01541737. [DOI] [PubMed] [Google Scholar]
- 17.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 18.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 19.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas-but not caspase-8 in lung epithelial cells ameliorates experimental acute lung injury. AmJPathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. AnnuRevImmunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. ImmunolRev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 22.Shevach EM. Regulatory T cells in autoimmunity. AnnuRevImmunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 23.Shevach EM. Certified professionals: CD4+ CD25+ suppresor T cells. JExpMed. 2001;193:F41–F45. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nature RevImmunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+ CD4+ regulatory T cells through GITR breaks immunological self-tolerance. NatImmunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 26.Song GY, Chung CS, Chaudry IH, Ayala A. MAPK p38 antagonism as a novel method of inhibiting lymphoid immune suppression in polymicrobial sepsis. AmJPhysiol. 2001:C662–C669. doi: 10.1152/ajpcell.2001.281.2.C662. [DOI] [PubMed] [Google Scholar]
- 27.Song GY, Chung CS, Chaudry IH, Ayala A. What is IL-10’s role in polymicrobial sepsis: anti-inflammatory agent or immune suppressant? Surgery. 1999;126:378–383. [PubMed] [Google Scholar]
- 28.Song GY, Chung CS, Cioffi WG, Ayala A. Insights into the contribution of STAT4 and STAT6 signaling to the morbidity seen in a low-mortality model of sepsis. Intensive Care Med. 2005;31:1564–1569. doi: 10.1007/s00134-005-2793-z. [DOI] [PubMed] [Google Scholar]
- 29.Sonoda K, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD-1-reactive natural killer T Cells are required for development of systemic tolerance through an immune-privileged site. JExpMed. 1999;190:1215–1225. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. JImmunol. 1999;162:392–399. [PubMed] [Google Scholar]
- 31.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25− lymphocytes. CritCare Med. 2004;32:2329–2331. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- 32.Wesche-Soldato DE, Chung CS, Lomas-Neira JL, Doughty LA, Gregory SH. In vivo delivery of caspase 8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zedler S, Biol D, Bone RC, Baue AE, Donnersmarck GHV, Faist E. T-cell reactivity and its predictive role in immunesuppression after burns. CritCare Med. 1999;27:66–72. doi: 10.1097/00003246-199901000-00028. [DOI] [PubMed] [Google Scholar]


