Abstract
The Ped (preimplantation embryo development) gene, whose product is Qa-2 protein, is correlated with a faster rate of preimplantation development (Ped fast phenotype) in mice that express Qa-2 protein compared with mice with an absence of Qa-2 protein (Ped slow phenotype). In the current study, we have used two congenic mouse strains differentially expressing the Ped gene, strain B6.K1 (Ped slow; Qa-2 negative) and strain B6.K2 (Ped fast; Qa-2 positive), to investigate the effects of Ped gene expression on postnatal growth profiles, systolic blood pressure and adult organ allometry. At birth, B6.K1 mice were moderately lighter than B6.K2 mice. B6.K1 mice became heavier during postnatal life (P < 0.05) and had elevated systolic blood pressure at 21 weeks of age when compared with B6.K2 mice (P = 0.006). B6.K1 mice also demonstrated elevated serum angiotensin-converting enzyme (ACE) activity, a known regulator of blood pressure (P = 0.037). Altered organ:body weight ratios were also observed, with the B6.K1 females having a higher ratio for lungs than B6. K2 females (P = 0.014). These data provide evidence of an association between the rate of preimplantation embryo development, postnatal growth and later cardiovascular function.
During preimplantation development, the rate of embryo cleavage divisions can be influenced both by genetic determinants and environmental factors (reviewed in Warner & Brenner, 2001; Fleming et al. 2004; Warner et al. 2004). One gene that has been shown to influence the rate of mouse cleavage is the Ped (preimplantation embryo development) gene (Goldbard et al. 1982). Subsequent studies have linked Ped to the mouse major histocompatibility complex (MHC) (Warner et al. 1987b), in particular the Q7 and Q9 genes (Cai et al. 1996; Wu et al. 1999). The Q7 and Q9 genes are duplicated genes, which differ by a single nucleotide only; this leads to a glutamine in Q7 and a glutamic acid in Q9. Mouse strains express Q7 or Q9, or both, and regardless of how many genes are expressed, they show the Ped fast phenotype. Mouse strains that have a deletion of both genes do not express Qa-2 protein and therefore show the Ped slow phenotype (Cai et al. 1996). The Q7 and Q9 genes encode Qa-2 protein, a MHC class Ib molecule that is expressed throughout preimplantation development (Warner et al. 1987a). In blastocysts, Qa-2 protein is expressed on both inner cell mass and trophectoderm cells (McElhinny et al. 1998). Two phenotypes of the Ped gene, fast and slow, have been defined based upon the number of cells contained within preimplantation embryos at specific times (Goldbard et al. 1982; Brownell & Warner, 1988). The Ped fast phenotype corresponds to the presence of Qa-2 encoding genes, whilst the Ped slow phenotype corresponds to the absence of Qa-2 protein. Ped fast embryos have an increased rate of embryo cleavage and contain more cells than Ped slow embryos (Goldbard et al. 1982; Brownell & Warner, 1988). Microinjection of the Q7 and/or Q9 genes into Qa-2-negative zygotes, leading to the expression of Qa-2 on the surface of the injected embryos, increases the rate of embryo cleavage (Wu et al. 1999). Removal of the Qa-2 protein from the embryonic cell surface, through enzymatic cleavage, results in a slowing of mouse preimplantation embryo development (Tian et al. 1992). Addition of exogenous Qa-2 protein into the membrane of Ped-negative embryos increases the rate of cleavage divisions (McElhinny et al. 2000). Therefore, expression of Qa-2 protein on the surface of preimplantation embryos is required for a fast rate of preimplantation cleavage division, the Ped fast phenotype.
Embryonic and fetal development periods represent times of coordinated cell division, differentiation and growth. Perturbations in embryo environment, including reduced growth factors or other metabolites, have been shown to affect the normal patterns of embryonic cell number and rate of development (Fleming et al. 2004). Maternal undernutrition has been shown to reduce the rate of preimplantation development (Kwong et al. 2000). Offspring developing from these embryos display impaired fetal growth, altered postnatal physiology and adult hypertension. Embryos developing at a faster rate, and containing more cells in response to specific culture conditions, may have an increased chance of fetal survival (Lane & Gardner, 1997). Manipulation of preimplantation cell numbers using different experimental approaches has been shown to delay progression during later gestation after embryo transfer (reviewed in Fleming et al. 2004). Therefore, out of a normal population, those embryos that show a faster rate of preimplantation development may enjoy a selective advantage and enhanced chance of postnatal survival.
Expression of the Qa-2 protein during preimplantation development has also been shown to influence postnatal phenotype. Thus, offspring developing from Ped fast embryos have a higher chance of intrauterine survival, are heavier at birth, come from larger litters and are heavier at weaning (Warner et al. 1991, 1993; Exley & Warner, 1999). Warner & Brenner (2001) proposed that the Qa-2 protein might have a role in protecting the developing fetus from maternal natural killer cells or macrophages, which could account for the higher levels of fetal loss in embryos lacking Qa-2 protein.
Several studies have shown that altering embryonic and/or fetal development results in the subsequent onset of hypertension in the adult offspring (Langley-Evans et al. 1996, 1999; Kwong et al. 2000). Aspects of the renin–angiotensin system (RAS) in particular have been shown to be susceptible to certain challenges administered during these windows of development. The cleavage of angiotensin I to angiotensin II, a potent vasoconstrictor, by angiotensin-converting enzyme (ACE) results in an elevation of blood pressure (Skeggs et al. 1956). The angiotensin II type 1 (AT1) receptor is located in vascular smooth muscle cells, kidney, heart, adrenal glands and the brain, where its activation results in vasoconstriction, the release of aldosterone and vasopressin, renal tubular sodium reabsorption, and decreased renal blood flow (reviewed in Unger, 2002). Along with these, activation of AT1 also results in the vascular wall remodelling, typically characterized by a reduction in lumen diameter and an increase in media thickness (Unger, 2002). Angiotensin II has also been linked to endothelial dysfunction and elevated levels of superoxide anion levels (Rajagopalan et al. 1996), thus further contributing to the development of hypertension. Receptor blockade of the AT1 receptor using the agonist losartan has been shown to significantly reduce blood pressure in offspring from maternal low-protein-diet-fed rats (Sherman & Langley-Evans, 2000). As well as cleaving angiotensin I, ACE also acts to stimulate the release of aldosterone from the adrenal cortex, thus leading to the re-absorption of sodium, and it inactivates bradykinin, again facilitating in the raising and overall regulation of blood pressure (Yang & Erdos, 1967; Yang et al. 1970).
Using two congenic strains of mice, B6.K1 (Ped slow; Qa-2 negative) and B6.K2 (Ped fast; Qa-2 positive), we have investigated the influence of embryonic Ped gene expression on postnatal development, growth and physiology. Collectively, our data indicate that Ped gene expression alters postnatal growth and susceptibility to high systolic blood pressure.
Methods
Animals
B6.K1 and B6.K2 mouse colonies were established at the University of Southampton Biomedical Research Facility from the original colonies at Northeastern University, Boston Massachusetts (Warner et al. 1991); they were maintained on a 07.00–19.00 h light cycle, with controlled temperature and standard chow and water ad libitum. Animals were housed and used in accordance with Home Office regulations and approved project licence protocols. Single B6.K1 and B6.K2 females (6–8 weeks old, derived from breeding pairs that were 8–9 weeks old) were individually housed overnight without superovulation with single males of the same strain and age. Each morning, females were checked for a vaginal plug as a sign of mating. Plug-positive females were individually housed throughout gestation whilst plug negative females were replaced into original cages during the day and returned to the same male in the afternoon for up to five nights attempted mating. In total, eight females of each strain, B6.K1 (Ped slow; Qa-2 negative; experimental) and B6.K2 (Ped fast; Qa-2 positive; control), were allowed to develop their pregnancies to term. Offspring were weighed on an electronic balance on day of birth and subsequently on the same day every week for 27 weeks. On day of birth, care was taken by wearing gloves and washing hands not to transmit the smell of other mice onto the newborn pups to prevent rejection of pups by their mother. At 3 weeks of age, offspring were weaned from their mothers, and the sexes caged separately. Mice had access to standard chow and water ad libitum.
Systolic blood pressure
Systolic blood pressure was determined at 8 and 21 weeks of age by tail-cuff plethysmography using an ITC model 229 blood pressure monitor (Linton Instruments, Norfolk, UK). Mice were allowed to acclimatize to a room temperature of 27–30°C for at least 90 min before readings were taken, and to the tail-cuff apparatus for several minutes before use. Four systolic blood pressure recordings were taken per mouse at each age studied. If after 20 min all four recordings had not been taken, the mouse was released and allowed to recover for 30 min before obtaining the remaining blood pressure values. Heart rate was monitored as an indicator of stress and if it rose above 500 beats min−1, readings were stopped until the rate returned below this value (Langley-Evans et al. 1996).
Measurement of organ weight
At 27 weeks of age, following cervical dislocation, blood samples were removed via heart puncture and allowed to clot on ice. Liver, left and right kidneys, heart and lungs were dissected out, weighed, and snap frozen in liquid nitrogen and stored at −80°C. Blood samples were centrifuged at 10 000 g, and 4°C for 10 min, after which serum was aliquoted and stored at −80°C.
Serum ACE activity
The method of Raimbach & Thomas (1990) was used with modifications. A 5 μl volume of serum was mixed with 70 μl buffer (300 mm KH2PO4, 150 mm NaCl, pH 8.3) to which 50 μl hippuryl-l-histidine-l-leucine acetate salt (5 mm; Sigma, Poole, UK) in buffer was added. Immediately after mixing, samples were transferred to a shaking water bath for 45 min at 37°C. The reaction was terminated by addition of 875 μl ice-cold chloride-free buffer (100 mm KH2PO4, pH 8.3) and transferring samples to ice for 10 min. Samples containing only serum and chloride buffer were used as negative controls. Subsequently, 500 μl 0.16 m cyanuric chloride (2-4-6-trichloro-1-3-5-triazine; Sigma) in 1,4 dioxane (Sigma) was added to each sample, mixed and left at room temperature for 10 min for yellow colouration to develop. After centrifugation at 3000 g for 10 min at room temperature, the clear yellow supernatant was removed. From each sample, four 200 μl aliquots were pipetted into a 96-well plate and analysed on a plate reader (Dynatech, MR5000) at 380 nm against a blank containing 1 ml 100 mm buffer (pH 8.3) and 0.5 ml cyanuric chloride in 1,4 dioxane. For each analysis, a standard curve over the range of 1 nm to 50 μm was prepared from a 1 mm hippuric acid (Sigma) solution in 100 mm chloride buffer, pH 8.3, and treated exactly as the incubates. Each sample was analysed in duplicate, and an average activity taken. Serum ACE activity was expressed as nanomoles of hippurate formed per millilitre of serum per minute. The intra- and interassay variations were 5.75 and 8.51%, respectively.
Lung tissue ACE activity
The method of Forhead et al. (2000) was used with modifications. Samples of lung (approximately 50 mg) were homogenized in 300 μl ice-cold buffer (0.2 m H3BO3, 2 m NaCl, pH 8.3) using a PowerGen 125 homogenizer. Samples were centrifuged at 25 000 g for 10 min at 4°C, and the supernatant was removed and stored at −80°C until analysis. The pellets were homogenized in a further 300 μl buffer and the supernatants removed after centrifugation as before. Samples (10 μl) of each lung extract were mixed with 50 μl buffer and 20 μl deionized water before incubation at 37°C for 5 min. A 20 μl volume of hippuryl-l-histidine-l-leucine acetate salt (20 mm; Sigma) in the above buffer was added to each tube. Negative controls included the addition of 100 μl 1 m HCl prior to addition of the hippuryl-l-histidine-l-leucine. Samples and blanks were incubated at 37°C for 30 min. The reaction was terminated by the addition of 100 μl 1 m HCl, 100 μl 1 m NaOH, 400 μl 0.2 m KH2PO4 (pH 8.3) and 400 μl 0.16 m cyanuric chloride in 1,4 dioxane. Samples were mixed and left at room temperature for 10 min for yellow colouration to develop, before analysis as for serum ACE.
A standard curve over the range 20 μm to 4 mm comprising hippuric acid in 1 m NaOH was treated exactly as the incubates. Samples were analysed in duplicate, and an average activity was taken. Tissue protein content was measured using the Bio-Rad protein assay kit. Tissue ACE activity was expressed as nanomoles hippurate formed per milligram of protein per minute. The intra- and interassay variations were 7.31 and 8.04%, respectively.
Statistical analyses
Student's t test was used to analyse mean litter size of B6.K1 and B6.K2 mice (SigmaStat statistical software, version 2.0). All postnatal data (birth weights, postnatal weights, blood pressures, organ weights and ratios, serum and lung ACE levels and ACE correlations) were analysed using a multilevel random effects regression model (procedure XTREG in STATA 7 statistical package, StataCorp 2001) that takes into account between-litter and within-litter variation and different parameters measured from individual animals (Kwong et al. 2004). Thus, differences identified between strains throughout the study are independent of variation within strains comprising either litter size or maternal origin of litter. Results given in the text and figures are means ±s.e.m., with P < 0.05 regarded as significant in the random effects regression model.
Results
Litter size, birth weights and postnatal growth
Litter size and offspring gender ratio were not significantly different between B6.K1 and B6.K2 strains (Table 1). Although B6.K1 males and females had lower mean birth weights (1.23 ± 0.048 and 1.26 ± 0.026, n = 28–30, respectively) than B6.K2 males and females (1.34 ± 0.023 and 1.30 ± 0.022, n = 25–26, respectively), these differences were not significant. At birth B6.K1 males were 8.21% lighter, whist B6.K1 females were 2.99% lighter than B6.K2 males and females, respectively.
Table 1.
Litter and growth criteria of B6.K1 and B6.K2 matings
| P value | ||
|---|---|---|
| Number of B6.K1 litters analysed | 8 | |
| Number of B6.K2 litters analysed | 8 | |
| Mean B6.K1 litter size | 7.25 ± 0.53 | |
| Mean B6.K2 litter size | 6.38 ± 0.71 | 0.34 |
| B6.K1 male:female ratio | 1.53 | |
| B6.K2 male:female ratio | 1.8 | 0.75 |
| Combined B6.K1 birth weight (g) | 1.24 ± 0.027 (n = 58) | |
| Combined B6.K2 birth weight (g) | 1.33 ± 0.016 (n = 51) | 0.19 |
| B6.K1 males birth weight (g) | 1.23 ± 0.048 (n = 28) | |
| B6.K2 males birth weight (g) | 1.34 ± 0.023 (n = 25) | 0.19 |
| B6.K1 females birth weight (g) | 1.26 ± 0.026 (n = 30) | |
| B6.K2 females birth weight (g) | 1.30 ± 0.022 (n = 26) | 0.19 |
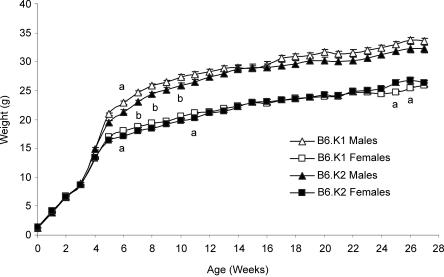
Mean weekly weights of B6.K1 and B6.K2 mice for up to 27 weeks of age are shown in Fig. 1. During weeks 4 and 5, both male and female mice exhibited accelerated growth, after which mean weight gain decreased. From week 5 onwards, B6.K1 males had an elevated mean weight compared with B6.K2 males. At week 6, both the male and female B6.K1 mice were significantly heavier (P < 0.05) than B6.K2 mice. Subsequently, during specific weeks (7, 8 and 10), B6.K1 mice were significantly heavier than B6.K2 mice when male and female data are combined (P < 0.05). At weeks 25 and 26, B6.K1 females became significantly lighter than the B6.K2 females (P < 0.05).
Figure 1. Mean body weights for B6K1 and B6.K2 mice.
Values are mean weights (±s.e.m.) for B6K1 and B6.K2 male and female offspring at birth and until 27 weeks of age; a denotes a significant difference between the B6.K1 and B6.K2 strains for either the males or the females (P < 0.05); b denotes that there is an overall significant difference between the two strains, but only when the male and female data are combined (P < 0.05, n = 23–30 per group).
Systolic blood pressure
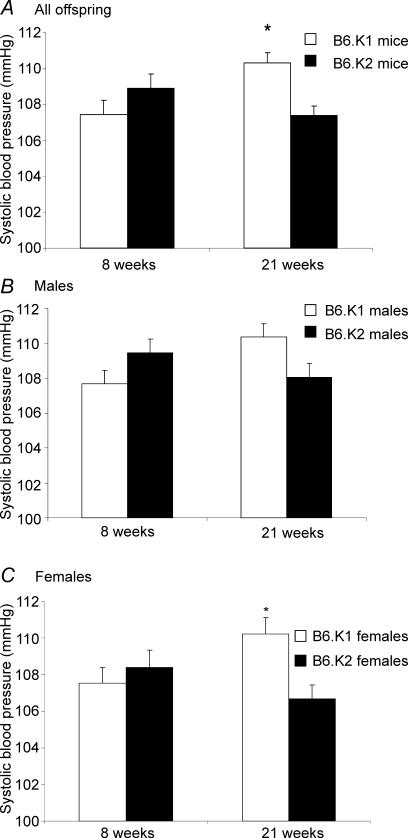
The mean systolic blood pressures of B6.K1 and B6.K2 mice at 8 and 21 weeks are shown in Fig. 2. At 8 weeks, no significant difference in blood pressure between the strains is evident. At 21 weeks, the B6.K1 mice had a significantly elevated systolic blood pressure when compared with the B6.K2 mice (110.31 ± 0.58 versus 107.38 ± 0.54 mmHg, P = 0.006) (Fig. 2A). Whilst both male and female B6.K1 mice had a higher systolic blood pressure than the B6.K2 mice when analysed separately (Fig. 2B and C), this difference was only significant in the females (110.221 ± 0.88 versus 106.69 ± 0.75 mmHg, P = 0.032) (Fig. 2C).
Figure 2. Mean systolic blood pressures for B6.K1 and B6.K2 mice.
n = 46–54 per group; A, *P = 0.006; C, *P = 0.032.
Organ allometry
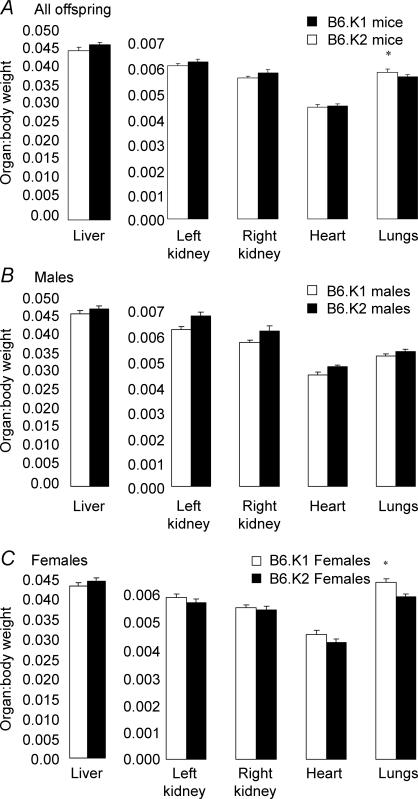
The mean organ:body weight ratios in B6.K1 and B6.K2 mice at 27 weeks are shown in Fig. 3. There was no significant difference between male B6.K1 and B6.K2 mice for any of the organs analysed. However, B6.K1 males did have a lower ratio for all of the organs when compared WITH B6.K2 males. B6.K1 females had a significantly elevated ratio for lungs when compared to B6.K2 females (P = 0.014). B6.K1 females also had an elevated ratio for their left and right kidneys and hearts, although these ratios were not significant. When data for male and female lung:body weight ratios were combined, B6.K1 mice had a significantly elevated ratio when compared with the B6.K2 mice (P = 0.013). Organ weights analysed independently of body weight showed no significant difference between male B6.K1 and B6.K2 mice for any of the organs studied; however, B6.K1 females had a significantly elevated lung weight when compared with B6.K2 females (P = 0.038, data not shown).
Figure 3. Mean organ:body weight ratios for B6.K1 and B6.K2 mice.
n = 23–27 per group; A, P = 0.013; C, *P = 0.014.
ACE activity
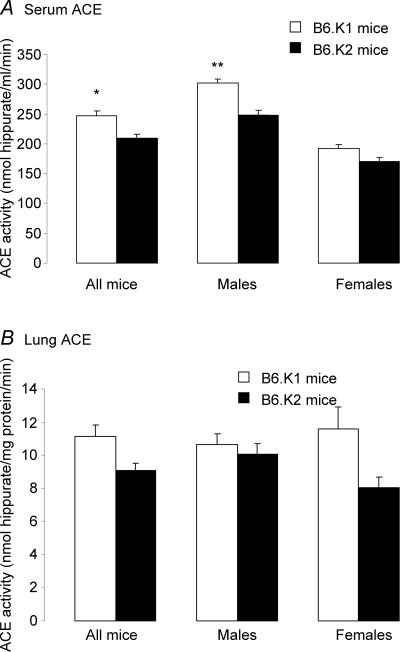
Mean ACE activity of B6.K1 and B6.K2 serum and lung tissue for individual animals is shown in Fig. 4. B6.K1 males had a significantly elevated serum ACE activity when compared with B6.K2 mice (302.1 ± 7.0 versus 247.42 ± 8.32 nmol hippurate ml−1 min−1, respectively, P = 0.02, Fig. 4A). When data for male and female mice were combined, B6.K1 mice had a significantly elevated serum ACE activity when compared with B6.K2 mice (246.87 ± 7.82 versus 209.19 ±6.74 nmol hippurate ml−1 min−1; P = 0.037). Although B6.K1 mice (both males and females) had higher lung ACE activity than B6.K2 mice (10.67 ± 0.62 versus 10.07 ± 0.62 nmol hippurate ml−1 min−1, males; 11.38 ± 1.32 versus 8.05 ± 0.64 nmol hippurate ml−1 min−1, females), this was not significant (P = 0.393 and 0.236, respectively). B6.K1 mice had an 18.01% increase in their serum ACE activity, whilst B6.K1 males had a 22.1% increase and B6.K1 females had a 12.1% increase when compared with B6.K2 offspring. B6.K1 mice had a 22.8% increase in their lung ACE activity, whilst B6.K1 males had a 6.01% increase and B6.K1 females had a 43.8% increase when compared with B6.K2 offspring.
Figure 4. Mean ACE activity of B6.K1 and B6.K2 mice.
A, serum, *P = 0.037; B, lung tissue, **P = 0.02; n = 40 per group.
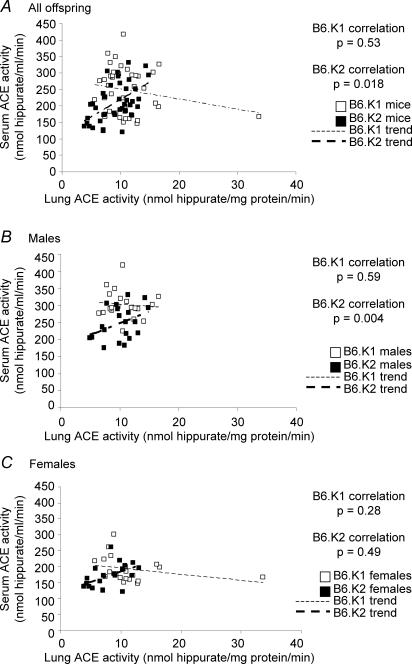
The relationship between serum and lung ACE activity is shown in Fig. 5. No correlation was evident in B6.K1 mice, whilst B6.K2 mice displayed a significant positive correlation. This correlation was primarily attributed to B6.K2 males (Fig. 5B, P = 0.004), and not B6.K2 females (Fig. 5C, P = 0.49). No significant correlation was found between serum ACE activity and body weight for B6.K1 and B6.K2 mice either as males and females analysed independently or together (data not shown). No significant correlation was found between lung ACE activity and body weight for B6.K1 and B6.K2 mice (data not shown). However, when data for males and females were analysed independently, B6.K2 males had a significant negative correlation (P < 0.001) not apparent for B6.K2 females or for B6.K1 males or females.
Figure 5. Correlation between serum and lung ACE activity in B6.K1 and B6.K2 mice at 27 weeks of age.
n = 40 per group.
Discussion
Numerous animal studies have demonstrated that alterations to the normal pattern, environment or rate of embryonic or fetal development can have long-term effects on postnatal growth, development and physiology (Thompson et al. 1995; Kwong et al. 2000; Young et al. 2001; Ecker et al. 2004). Whilst previous focus has been directed upon the environmental effects of embryo development, we investigated the postnatal development and physiology of two congenic mouse strains, B6.K1 (Ped slow; Qa-2 negative) and B6.K2 (Ped fast; Qa-2 positive), which differ only in the absence or presence of the major histocompatibility complex Q7 and Q9 genes, and thus subsequently the absence or presence of Qa-2 protein expression, respectively.
B6.K1 mice were lighter than B6.K2 mice at birth, as reported in previous studies (Warner et al. 1991, 1993), but this was not significant in the current study. Similarly, no significant differences in litter size or weight at weaning were observed, in contrast to previous studies (Warner et al. 1987a, 1991, 1993; Exley & Warner, 1999). This distinction may reflect differences in sample size or the statistical methods applied. Here, the random effects regression analysis takes into account between-litter and within-litter variation including variation in litter size. Thus, litter size between B6.K1 and B6.K2 strains did not contribute significantly to the (non-significant) birth weight relationship between the strains.
It has been proposed that as Ped fast embryos proliferate and develop faster compared with Ped slow embryos, they may reach the uterus earlier and have improved vascularized implantation sites leading to better placentation and fetal development (Brownell & Warner, 1988; McElhinny et al. 1998; Warner & Brenner, 2001; Warner et al. 2004). Studies in mice involving the asynchronous transfer of embryos have shown that more advanced embryos can implant earlier, and can give rise to offspring of increased birth weight (Aitken et al. 1977; Marsk, 1977; Ueda et al. 2003). Since the Ped gene product, i.e. Qa-2 protein, displays many functional and structural similarities to the human HLA-G protein (Geraghty et al. 1987; Jurisicova et al. 1996; Comiskey et al. 2003, 2005; Clements et al. 2005), embryos expressing the Qa-2 protein may be protected from unwanted maternal immune responses (Loke & King, 1991; Chumbley et al. 1994; Comiskey et al. 2005). This may be an additional mechanism through which Ped gene expression might enhance fetal development. Exley & Warner (1999) observed that there was a loss of Qa-2-negative embryos between day 14.5 and birth, demonstrating that the presence of Qa-2-encoding genes in the fetus confers a selective advantage later in gestation in addition to the preimplantation phenotype. However, the exact mechanism conferring any developmental advantage upon Qa-2-expressing embryos/fetuses is currently unknown.
Reduced rates of rodent embryo development have been linked with impaired fetal growth and over-compensatory ‘catch-up’ growth postnatally, resulting in adult obesity and increased risk of cardiovascular disease (Bowman & McLaren, 1970; Langley-Evans et al. 1999; Kwong et al. 2000). Over-compensatory catch-up growth may reflect the disparity between initial constrained rates of embryonic/fetal development (whether due to poor maternal nutrition or due to a genetic polymorphism; as in this study) and subsequent postnatal over abundance of nutrition (Hales & Barker, 2001; Gluckman & Hanson, 2004). The current results show a similar phenomenon between 6 and 11 weeks of age, with both B6.K1 males and females becoming significantly heavier than B6.K2 mice, despite being initially lighter at birth, with the B6.K1 males remaining heavier for the remainder of the study. Coupled with this, at 21 weeks, B6.K1 males and females have a significantly elevated systolic blood pressure when compared with the B6.K2 males and females. It would therefore appear that the reduced birth weight and subsequent postnatal catch-up growth are associated with an elevated systolic blood pressure during adult life in B6.K1 mice.
As hypertension is a multifactorial condition, its origin may lie in more than a single cause, one being altered organ allometry. It was observed that the B6.K1 females had a significantly elevated lung : body weight ratio compared with the B6.K2 females. During periods of impaired fetal development, the fetus makes compensatory adaptations to maintain the development of vital organs such as the brain. These compensations have been shown to have negative impacts on the growth and development of other organ structures (Hales et al. 1991; Kwong et al. 2000; Whorwood et al. 2001). However, overall differences in organ weight between B6.K1 and B6.K2 strains are minimal, do not include the kidney or heart, and therefore are unlikely to be major contributors to the observed difference in blood pressure.
Another factor that influences postnatal blood pressure is ACE activity. ACE cleaves angiotensin I to yield angiotensin II, a potent vasoconstrictor, thus resulting in increased blood pressure. ACE −/− mice are hypotensive, having a systolic blood pressure ∼30 mmHg below wild-type mice (Esther et al. 1997; Klein et al. 2002). Previous studies have shown that the expression profile of ACE appears to be important in the regulation of blood pressure. In humans, serum ACE levels are significantly higher in hypertensive as compared with normotensive subjects, with serum ACE activity correlating positively with plasma angiotensin II levels (Forrester et al. 1997; Nystrom et al. 1997). It may therefore be hypothesized that increased expression of ACE enzyme, or an increased activity of existing ACE, may result in an elevated blood pressure. We found that B6.K1 males had a significantly elevated serum ACE activity when compared with the B6.K2 males. When the data for the males and females were analysed independent of gender, the B6.K1 mice had a significantly elevated serum ACE activity when compared with the B6.K2 mice.
Serum ACE activity in humans has also been shown to correlate positively with body mass index (Forrester et al. 1997; Nystrom et al. 1997). Since lung ACE activity was negatively correlated to body weight in the B6.K2 males, and since serum ACE activity was positively correlated to lung ACE, then, it may be assumed that as body weight increases, overall ACE activity decreases. This reduced activity could be one factor in the lower systolic blood pressure observed for the B6.K2 mice when compared with the B6.K1 mice. In vivo, serum ACE is derived from the cleavage by secretases of endothelial-bound somatic ACE, predominantly that of the lung (Beldent et al. 1995; Sadhukhan et al. 1998; Woodman et al. 2000; Eyries et al. 2001). It may therefore be hypothesized that the more ACE that is expressed on the surface of the epithelia, then the more ACE will be available for cleavage, so raising serum ACE protein and activity levels. However, for the B6.K1 mice, as lung ACE activity increases, the serum ACE activity falls slightly. There could be several possible reasons for this, such as a decrease in the expression, availability or enzymatic activity of the secretases. However, since the B6.K1 mice had elevated serum ACE activity when compared with the B6.K2 mice, it may be that another source, such as the vasculature or the kidneys, is contributing to the elevated serum ACE activity observed.
In conclusion, it has been shown that Ped fast (Qa-2 positive) and Ped slow (Qa-2 negative) congenic mouse strains differ in their postnatal development and physiology. One explanation could be that the reduced birth weight and subsequent postnatal catch up growth have resulted in the development of aspects of the ‘metabolic syndrome’ in that the Ped slow mice display significantly elevated blood pressure and body weight. From the data presented within this study, the hypertensive condition observed appears to be attributed to altered patterns of serum and lung ACE activity, or to the differences in the postnatal growth patterns, since there are little differences in organ:body weight ratios between the two strains. The data therefore support the concept that an altered rate of preimplantation embryo development may lead to long-term consequences on postnatal growth and physiology.
Acknowledgments
This research was supported by a Medical Research Council grant (G9800761) to T.P.F. and partially supported by a National Institutes of Health grant (HD39215) to C.W.
References
- Aitken RJ, Bowman P, Gauld I. The effect of synchronous and asynchronous egg transfer on foetal weight in mice selected for large and small body size. J Embryol Exp Morphol. 1977;37:59–64. [PubMed] [Google Scholar]
- Beldent V, Michaud A, Bonnefoy C, Chauvet MT, Corvol P. Cell surface localization of proteolysis of human endothelial angiotensin I-converting enzyme. Effect of the amino-terminal domain in the solubilization process. J Biol Chem. 1995;270:28962–28969. doi: 10.1074/jbc.270.48.28962. [DOI] [PubMed] [Google Scholar]
- Bowman P, Mclaren A. Viability and growth of mouse embryos after in vitro culture and fusion. J Embryol Exp Morphol. 1970;23:693–704. [PubMed] [Google Scholar]
- Brownell MS, Warner CM. Ped gene expression by embryos cultured in vitro. Biol Reprod. 1988;39:806–811. doi: 10.1095/biolreprod39.4.806. [DOI] [PubMed] [Google Scholar]
- Cai W, Cao W, Wu L, Exley GE, Waneck GL, Karger BL, Warner CM. Sequence and transcription of Qa-2-encoding genes in mouse lymphocytes and blastocysts. Immunogenetics. 1996;45:97–107. doi: 10.1007/s002510050177. [DOI] [PubMed] [Google Scholar]
- Chumbley G, King A, Robertson K, Holmes N, Loke YW. Resistance of HLA-G and HLA-A2 transfectants to lysis by decidual NK cells. Cell Immunol. 1994;155:312–322. doi: 10.1006/cimm.1994.1125. [DOI] [PubMed] [Google Scholar]
- Clements CS, Kjer-Nielsen L, Kostenko L, Hoare HL, Dunstone MA, Moses E, Freed K, Brooks AG, Rossjohn J, Mccluskey J. Crystal structure of HLA-G: a nonclassical MHC class I molecule expressed at the fetal–maternal interface. Proc Natl Acad Sci U S A. 2005;102:3360–3365. doi: 10.1073/pnas.0409676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comiskey M, Goldstein CY, De Fazio SR, Mammolenti M, Newmark JA, Warner CM. Evidence that HLA-G is the functional homolog of mouse Qa-2, the Ped gene product. Hum Immunol. 2003;64:999–1004. doi: 10.1016/j.humimm.2003.08.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comiskey M, Warner CM, Schust DJ. MHC molecules of the preimplantation embryo and trophoblast. In: Mor G, editor. Immunology of Pregnancy. Georgetown Texas: Landes Biosciences; 2005. [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci U S A. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, Capecchi MR, Bernstein KE. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley GE, Warner CM. Selection in favor of the Ped fast haplotype occurs between mid-gestation and birth. Immunogenetics. 1999;49:653–659. doi: 10.1007/s002510050661. [DOI] [PubMed] [Google Scholar]
- Eyries M, Michaud A, Deinum J, Agrapart M, Chomilier J, Kramers C, Soubrier F. Increased shedding of angiotensin-converting enzyme by a mutation identified in the stalk region. J Biol Chem. 2001;276:5525–5532. doi: 10.1074/jbc.M007706200. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ. The embryo and its future. Biol Reprod. 2004;71:1046–1054. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Gillespie CE, Fowden AL. Role of cortisol in the ontogenic control of pulmonary and renal angiotensin-converting enzyme in fetal sheep near term. J Physiol. 2000;526:409–416. doi: 10.1111/j.1469-7793.2000.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T, Mcfarlane-Anderson N, Bennett FI, Wilks R, Cooper R, Rotimi C, Morrison L, Ward R. The angiotensin converting enzyme and blood pressure in Jamaicans. Am J Hypertens. 1997;10:519–524. doi: 10.1016/s0895-7061(97)00049-6. [DOI] [PubMed] [Google Scholar]
- Geraghty DE, Koller BH, Orr HT. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987;84:9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Goldbard SB, Verbanac KM, Warner CM. Role of the H-2 complex in preimplantation mouse embryo development. Biol Reprod. 1982;26:591–596. doi: 10.1095/biolreprod26.4.591. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisicova A, Casper RF, Maclusky NJ, Mills GB, Librach CL. HLA-G expression during preimplantation human embryo development. Proc Natl Acad Sci U S A. 1996;93:161–165. doi: 10.1073/pnas.93.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JD, Le Quach D, Cole JM, Disher K, Mongiu AK, Wang X, Bernstein KE, Sands JM. Impaired urine concentration and absence of tissue ACE: involvement of medullary transport proteins. Am J Physiol Renal Physiol. 2002;283:F517–F524. doi: 10.1152/ajprenal.00326.2001. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Osmond C, Fleming TP. Support for Barker hypothesis upheld in rat model of maternal undernutrition during the preimplantation period: application of integrated ‘random effects’ statistical model. Reprod Biomed Online. 2004;8:574–576. doi: 10.1016/s1472-6483(10)61105-4. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Nonessential amino acids and glutamine decrease the time of the first three cleavage divisions and increase compaction of mouse zygotes in vitro. J Assist Reprod Genet. 1997;14:398–403. doi: 10.1007/BF02766148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- Loke YW, King A. Recent developments in the human maternal–fetal immune interaction. Curr Opin Immunol. 1991;3:762–766. doi: 10.1016/0952-7915(91)90110-m. [DOI] [PubMed] [Google Scholar]
- McElhinny AS, Exley GE, Warner CM. Painting Qa-2 onto Ped slow preimplantation embryos increases the rate of cleavage. Am J Reprod Immunol. 2000;44:52–58. doi: 10.1111/j.8755-8920.2000.440108.x. [DOI] [PubMed] [Google Scholar]
- McElhinny AS, Kadow N, Warner CM. The expression pattern of the Qa-2 antigen in mouse preimplantation embryos and its correlation with the Ped gene phenotype. Mol Hum Reprod. 1998;4:966–971. doi: 10.1093/molehr/4.10.966. [DOI] [PubMed] [Google Scholar]
- Marsk L. Developmental precocity after asynchronous egg transfer in mice. J Embryol Exp Morph. 1977;39:127–137. [PubMed] [Google Scholar]
- Nystrom F, Karlberg BE, Ohman KP. Serum angiotensin-converting enzyme activity correlates positively with plasma angiotensin II: a population-based study of ambulatory blood pressure and the renin–angiotensin system. J Hum Hypertens. 1997;11:301–306. doi: 10.1038/sj.jhh.1000433. [DOI] [PubMed] [Google Scholar]
- Raimbach SJ, Thomas AL. Renin and angiotensin converting enzyme concentrations in the fetal and neonatal guinea-pig. J Physiol. 1990;423:441–451. doi: 10.1113/jphysiol.1990.sp018032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhukhan R, Sen GC, Ramchandran R, Sen I. The distal ectodomain of angiotensin-converting enzyme regulates its cleavage-secretion from the cell surface. Proc Natl Acad Sci U S A. 1998;95:138–143. doi: 10.1073/pnas.95.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98:269–275. [PubMed] [Google Scholar]
- Skeggs LT, Jr, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JG, Gardner DK, Pugh PA, Mcmillan WH, Tervit HR. Lamb birth weight is affected by culture system utilized during in vitro pre-elongation development of ovine embryos. Biol Reprod. 1995;53:1385–1391. doi: 10.1095/biolreprod53.6.1385. [DOI] [PubMed] [Google Scholar]
- Tian Z, Xu Y, Warner CM. Removal of Qa-2 antigen alters the Ped gene phenotype of preimplantation mouse embryos. Biol Reprod. 1992;47:271–276. doi: 10.1095/biolreprod47.2.271. [DOI] [PubMed] [Google Scholar]
- Ueda O, Yorozu K, Kamada N, Jishage K, Kawase Y, Toyoda Y, Suzuki H. Possible expansion of ‘Window of Implantation’ in pseudopregnant mice: time of implantation of embryos at different stages of development transferred into the same recipient. Biol Reprod. 2003;69:1085–1090. doi: 10.1095/biolreprod.103.017608. [DOI] [PubMed] [Google Scholar]
- Unger T. The role of the renin–angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89:3A–9A. doi: 10.1016/s0002-9149(01)02321-9. [DOI] [PubMed] [Google Scholar]
- Warner CM, Brenner CA. Genetic regulation of preimplantation embryo survival. Curr Top Dev Biol. 2001;52:151–192. doi: 10.1016/s0070-2153(01)52011-6. [DOI] [PubMed] [Google Scholar]
- Warner CM, Brownell MS, Rothschild MF. Analysis of litter size and weight in mice differing in Ped gene phenotype and the Q region of the H-2 complex. J Reprod Immunol. 1991;19:303–313. doi: 10.1016/0165-0378(91)90042-o. [DOI] [PubMed] [Google Scholar]
- Warner CM, Gollnick SO, Flaherty L, Goldbard SB. Analysis of Qa-2 antigen expression by preimplantation mouse embryos: possible relationship to the preimplantation-embryo-development (Ped) gene product. Biol Reprod. 1987a;36:611–616. doi: 10.1095/biolreprod36.3.611. [DOI] [PubMed] [Google Scholar]
- Warner CM, Gollnick SO, Goldbard SB. Linkage of the preimplantation-embryo-development (Ped) gene to the mouse major histocompatibility complex (MHC) Biol Reprod. 1987b;36:606–610. doi: 10.1095/biolreprod36.3.606. [DOI] [PubMed] [Google Scholar]
- Warner CM, Newmark JA, Comiskey M, De Fazio SR, O'Malley DM, Rajadhyaksha M, Townsend DJ, McKnight S, Roysam B, Dwyer PJ, Dimarzio CA. Genetics and imaging to assess oocyte and preimplantation embryo health. Reprod Fertil Dev. 2004;16:729–741. doi: 10.1071/rd04088. [DOI] [PubMed] [Google Scholar]
- Warner CM, Panda P, Almquist CD, Xu Y. Preferential survival of mice expressing the Qa-2 antigen. J Reprod Fertil. 1993;99:145–147. doi: 10.1530/jrf.0.0990145. [DOI] [PubMed] [Google Scholar]
- Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin II receptor in neonatal sheep. Endocrinology. 2001;142:2854–2864. doi: 10.1210/endo.142.7.8264. [DOI] [PubMed] [Google Scholar]
- Woodman ZL, Oppong SY, Cook S, Hooper NM, Schwager SL, Brandt WF, Ehlers MR, Sturrock ED. Shedding of somatic angiotensin-converting enzyme (ACE) is inefficient compared with testis ACE despite cleavage at identical stalk sites. Biochem J. 2000;347:711–718. [PMC free article] [PubMed] [Google Scholar]
- Wu L, Feng H, Warner CM. Identification of two major histocompatibility complex class Ib genes, Q7 and Q9, as the Ped gene in the mouse. Biol Reprod. 1999;60:1114–1119. doi: 10.1095/biolreprod60.5.1114. [DOI] [PubMed] [Google Scholar]
- Yang HY, Erdos EG. Second kininase in human blood plasma. Nature. 1967;215:1402–1403. doi: 10.1038/2151402a0. [DOI] [PubMed] [Google Scholar]
- Yang HY, Erdos EG, Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochlm Biophys Acta. 1970;214:374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- Young LE, Fernandes K, Mcevoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27:153–154. doi: 10.1038/84769. [DOI] [PubMed] [Google Scholar]