Abstract
Extensive epidemiological and experimental evidence indicates that a sub-optimal environment during fetal and neonatal development in both humans and animals may programme offspring susceptibility to later development of chronic diseases including obesity and diabetes that are the result of altered carbohydrate metabolism. We determined the effects of protein restriction during pregnancy and/or lactation on growth, serum leptin, and glucose and insulin responses to a glucose tolerance test in male and female offspring at 110 days postnatal life. We fed Wistar rats a normal control 20% casein diet (C) or a restricted diet (R) of 10% casein during pregnancy. Female but not male R pups weighed less than C at birth. After delivery, mothers received the C or R diet during lactation to provide four offspring groups: CC (first letter maternal pregnancy diet and second maternal lactation diet), RR, CR and RC. All offspring were fed ad libitum with C diet after weaning. Relative food intake correlated inversely with weight. Offspring serum leptin correlated with body weight and relative, but not absolute, food intake in both male and female pups. Serum leptin was reduced in RR female pups compared with CC and increased in RC males compared with CC at 110 days of age. Offspring underwent a glucose tolerance test (GTT) at 110 days postnatal life. Female RR and CR offspring showed a lower insulin to glucose ratio than CC. At 110 days of age male RR and CR also showed some evidence of increased insulin sensitivity. Male but not female RC offspring showed evidence of insulin resistance compared with CC. Cholesterol was similar and triglycerides (TG) higher in male compared with female CC. Cholesterol and TG were higher in males than females in RR, CR and RC (P < 0.05). Cholesterol and TG did not differ between groups in females. Cholesterol and TG were elevated in RC compared with CC males. Nutrient restriction in lactation increased relative whole protein and decreased whole lipid in both males and females. RC females showed decreased relative levels of protein and increased fat. We conclude that maternal protein restriction during either pregnancy and/or lactation alters postnatal growth, appetitive behaviour, leptin physiology, TG and cholesterol concentrations and modifies glucose metabolism and insulin resistance in a sex- and time window of exposure-specific manner.
Extensive epidemiological and laboratory evidence indicates that a suboptimal environment during fetal and neonatal development in both humans and experimental animals impacts on offspring susceptibility to later development of altered carbohydrate metabolism (Dahri et al. 1991; Ravelli et al. 1999; Petry & Hales, 2000; Zambrano et al. 2005a). The concept of ‘developmental programming’ proposes that challenges during an organism's development evoke a persistent physiological response in the offspring. Epidemiological investigations such as those conducted on the children conceived during the Dutch famine of 1944–1945 have highlighted the association between poor maternal nutrition, lowered birth weight and subsequent adult disease (Ravelli et al. 1999; Roseboom, 2001). Several different experimental animal protocols have been used for the evaluation of developmental programming of metabolism: (1) fetal exposure to levels of glucocorticoids that are inappropriately high for the current stage of development (Nyirenda et al. 2001); (2) global nutrient restriction (Garofano et al. 1997, 1998); (3) maternal exposure to an isocaloric low protein diet (Stewart et al. 1975; Ozanne et al. 1996; Reusens & Remacle, 2001b); (4) uterine blood flow restriction (Simmons et al. 2001); and (5) experimental maternal diabetes (Holemans et al. 1997; Holemans et al. 2003).
In the present study we examined the offspring of female rats exposed to protein restriction during pregnancy and/or lactation to determine: (1) effects on body weight, food intake and leptin physiology; (2) glucose, insulin, triglyceride and cholesterol metabolism; (3) whether effects are dependent on the stage of development at which protein restriction occurs – pregnancy or lactation; (4) whether the exposures during fetal development and lactation interact; and (5) whether these effects show offspring sex specificity. We fed one group of virgin Wistar rats a normal control diet (C) during pregnancy and lactation (CC – first letter pregnancy diet and second letter lactation diet). Additional pregnant rats were fed a restricted 50% protein isocaloric diet (R) during pregnancy and/or lactation to provide three further groups: RR, CR and RC. All offspring ate the control diet after weaning. We present here a comparison of the metabolic phenotypes of the male and female offspring.
Methods
Care and maintenance of animals
Details of protein restriction and breeding have been published previously (Zambrano et al. 2005b). Briefly, mothers were virgin female albino Wistar rats aged between 10 and 12 weeks and weighing 220 ± 20 g (mean ±s.e.m.) obtained from the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (Mexico City, Mexico). Female rats with regular cycles were maintained on Purina Laboratory Chow 5001. Rats were maintained under controlled lighting (lights on from 07:00 to 19:00 h at 22–23°C). All procedures were approved by the Animal Experimentation Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán, Mexico City.
Female rats were mated overnight with proven male breeders and the day on which spermatozoa were present in a vaginal smear was designated as day of conception – day 0 of pregnancy. Only rats that were pregnant within 5 days of introduction of the male were retained in the study. Pregnant rats were transferred to individual metabolism cages and allocated at random to one of two groups to be fed either a 20% casein (control diet) or a 10% casein isocaloric (restricted) diet (Zambrano et al. 2005b). Food and water were available ad libitum for all animals.
Pregnant and lactating rats were weighed every day through pregnancy and until pups were removed at weaning. Food was provided in the form of large flat biscuits which were retained behind a grill through which the rats nibbled. A weighed excess of feed was provided each day. The amount remaining after 24 h was weighed. On day 20 post-conception, pregnant rats were transferred to normal rat cages to provide optimal conditions for delivery which occurred in the early daylight hours between 09:00 h and 12:00 h on post-conceptual day 22. Day of delivery was considered as day 0 of post natal life. Food intake continued to be monitored during this period.
All rats were delivered by spontaneous vaginal delivery. Timing of delivery, litter size and pup weight were recorded at birth. Ano-genital distance, anterior–posterior abdominal distance and head diameter were measured with calipers. Our published data indicate that female pups have an ano-genital distance of 1.67 ± 0.13 mm (n = 291 pups from 43 litters; mean ±s.e.m.) and males 3.26 ± 0.22 mm (n = 252 pups from 43 litters) (Zambrano et al. 2005a). Thus a value of 2.5 mm is more than 2 s.d.'s from the mean of either group and sex was judged according to whether the ano-genital distance was greater than (male) or less than (female) 2.5 mm. To ensure homogeneity of study subjects, litters of over 14 pups were not included in the study. Litters of 12–14 pups were adjusted to 12 pups for each dam while maintaining as close to a 1 : 1 sex ratio as possible. Four groups were established: CC in which dams that received the control diet during pregnancy continued to be fed the control diet during lactation; RR in which dams that had received the restricted diet during pregnancy continued to receive the restricted diet during lactation; CR in which dams that received the control diet during pregnancy received the restricted diet during lactation; and RC in which dams that received the restricted diet during pregnancy were provided with the control diet during lactation. After weaning (postnatal day 21) all pups were fed with control (20% casein) diet ad libitum. Pups continued to be weighed daily.
Measurement of food intake after weaning
Two rats of the same sex and from the same experimental group were housed per cage. Food was provided in the form of large flat biscuits as above. The amount of food provided each day was weighed as was the amount remaining after 24 h. The amount consumed was averaged between the two rats.
Glucose tolerance test at 110 days postnatal life
One or two rats of each sex from each litter were fasted overnight before an intraperitoneal glucose tolerance test in which one gram per kilogram body weight d-glucose was administered i.p. at 09:00 h (Zambrano et al. 2005a). Resting blood samples were used for the leptin, triglyceride (TG) and cholesterol analysis in addition to glucose and insulin. Where two pups from one litter were studied, their data were averaged.
Biochemical analyses
Carcass components
Rats were rapidly killed by decapitation using a rodent guillotine (Thomas Scientific, USA) and kept frozen in small plastic bags until they were analysed. Before analysis, the abdomen was opened and the stomach and caecum were excised and discarded. After weighing (wet weight), each carcass was chopped into small pieces, placed in a tared beaker and dried at 60°C to constant weight. The weight lost is considered to be body water. The dried carcasses were ground-up and aliquots were taken for fat determination by the Soxhlet method (AOAC, 2002) and total nitrogen (N) by the Kjeldahl method (AOAC, 2002).
Blood glucose measurement
Serum glucose concentrations were determined spectrophotometrically using the enzymatic hexokinase method (Beckman Coulter, Co. Fullerton, CA, USA). Intra- and interassay coefficients of variations were < 2% and < 3%, respectively.
Insulin radioimmunoassay
Serum insulin concentrations were determined by radio-immuno assay (RIA) using commercial rat kits from Linco Research, Inc. (St Charles, MO, USA), Cat. no. RI-13K. The intra- and interassay coefficients of variations were < 4% and < 6%, respectively.
Triglycerides and cholesterol measurement
Serum triglycerides and cholesterol concentrations were determined enzymatically with the Synchron CX auto analyser (Beckman Coulter, Co.). Intra- and interassay coefficients of variation were < 7% and < 6% for triglycerides and < 4% and < 3% for cholesterol, respectively.
Leptin radioimmunoassay
Serum leptin concentrations were determined by RIA using commercial rat kits from Linco Research, Inc., Cat. no. RL-83K. The intra- and interassay coefficients of variations were < 4% and < 5%, respectively.
Statistical analysis
For the glucose tolerance tests, the area under the curve (AUC) was calculated using Sigmaplot 7 (2001). Insulin resistance index (IRI) in the baseline sample was calculated from the formula IRI = glucose × insulin/22.5 (Nandhini et al. 2005). All data are presented as mean ±s.e.m. Differences between groups were compared using multiple analysis of variance (ANOVA) followed by Dunnett's test. Unpaired Student's t test was used to compare male and female data for baseline values and areas under the curve for the same variable and IRI. χ2 test was used to determine differences in the duration of pregnancy and timing of delivery. Leptin, food intake and body weight correlation were calculated using a Pearson correlation. P≤ 0.05 was considered significant.
Results
Details of delivery
Delivery occurred on day 22. There were no differences in timing of delivery, litter size and litter sex distribution between C and R. Male pup weight at delivery did not differ between groups. Birth weight of female pups of control-fed mothers (6.1 ± 0.08, n = 16) was greater than that of restricted mothers (5.7 ± 0.10; n = 22).
Offspring weight and food intake
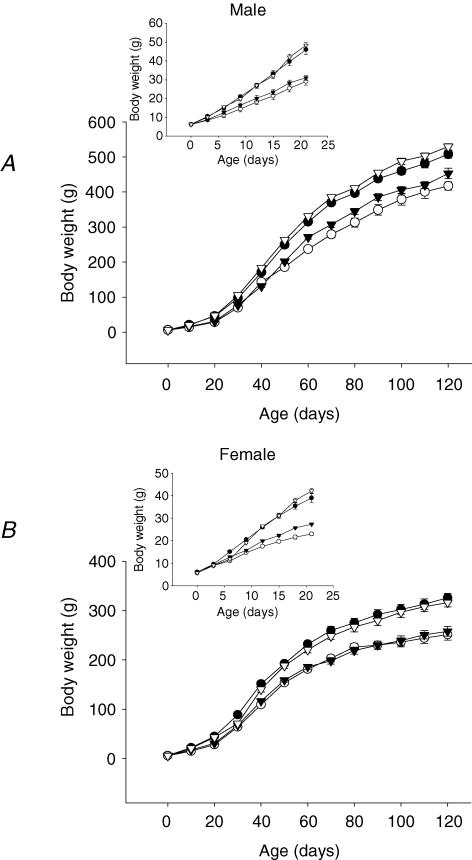
Female but not male R offspring weighed less than C at birth. Figure 1 shows daily pup postnatal weight from birth to 21 days and at 10 day intervals until 130 days. In both sexes by 21 days, both offspring groups whose mothers had a restricted diet during lactation weighed less than those whose mothers were on the control diet (Table 1). The differences between groups were similar at 100 days postnatal life with the exception that CR did not differ from RC (Table 1). Total food intake at 100 days was less in CR than CC male pups and in CR and RR in females. The comparison of greatest interest and significance in food intake expressed per unit body weight was the two groups whose mothers had a restricted diet during pregnancy. In males the difference between RR and RC was not quite significant (P < 0.07) while in females relative intake was significantly decreased in RC compared with RR. The food intake expressed per unit body weight of female pups was higher than male pups in all four groups but the difference only reached significance for the CR groups (P < 0.001; Table 1).
Figure 1. Mean body weight of (A) male and (B) female offspring rats.
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). CC (•); RR (○); CR (▾); RC (▿) inserts represents 0 to 21 days of life in large. Data represented as mean ±s.e.m. from 5 CC litters, 5 RR litters, 5 CR litters, and 4 RC litters. *P < 0.05 RR and CR versus CC and RC.
Table 1.
Body weight, absolute and relative food intake and serum leptin concentration in male and female pups
| CC | RR | CR | RC | |
|---|---|---|---|---|
| A. Body weight | ||||
| Male (21 d; g) | 46.0 ± 2.73a | 29.1 ± 2.09b | 31.0 ± 1.30b | 48.4 ± 1.30a |
| Female (21 d; g) | 38.7 ± 1.93a* | 22.9 ± 0.69b* | 27.3 ± 0.36b* | 42.9 ± 1.04a* |
| B. Serum leptin concentration in males | ||||
| Leptin (ng ml−1) | 4.7 ± 0.83a | 2.3 ± 0.26a* | 5.2 ± 0.60a* | 9.3 ± 1.56b* |
| Body weight (g) | 513.2 ± 10.5a* | 417.7 ± 10.8b* | 458.7 ± 17.2b* | 531.1 ± 8.6a* |
| Food intake (g) | 33.8 ± 1.77a* | 29.8 ± 1.03a,b* | 26.6 ± 0.76b* | 32.6 ± 0.49a* |
| Food intake (g (g body wt)−1) | 0.066 ± 0.003a,b | 0.072 ± 0.004a | 0.058 ± 0.001b* | 0.061 ± 0.002a,b |
| C. Serum leptin concentration in females | ||||
| Leptin (ng ml−1) | 3.4 ± 0.46a | 1.4 ± 0.21b* | 1.8 ± 0.30b,c* | 2.3 ± 0.28a,c* |
| Body weight (g) | 326.1 ± 10.9a* | 252.4 ± 16.4b* | 262.9 ± 10.6b,c* | 317.7 ± 17.5a,c* |
| Food intake (g) | 22.8 ± 1.03a* | 19.5 ± 0.19b* | 19.5 ± 0.40b* | 20.1 ± 2.20a,b* |
| Food intake (g (g body wt)−1) | 0.070 ± 0.003a,b | 0.079 ± 0.004a | 0.075 ± 0.003a* | 0.063 ± 0.002b |
A, male and female pup body weight at 21 days of age. Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). n = 5–6 litters. B, male offspring serum leptin at 110 days, body weight and absolute and relative food intake at 100 days of age. From 5 CC litters, 5 RR litters, 5 CR litters, and 4 RC litters. C, female offspring serum leptin at 110 days, body weight and absolute and relative food intake at 100 days of age. From 5 CC litters, 5 RR litters, 6 CR litters, and 6 RC litters. Groups not sharing a letter are statistically different
Female versus male P≤ 0.05. All values are mean ±s.e.m.
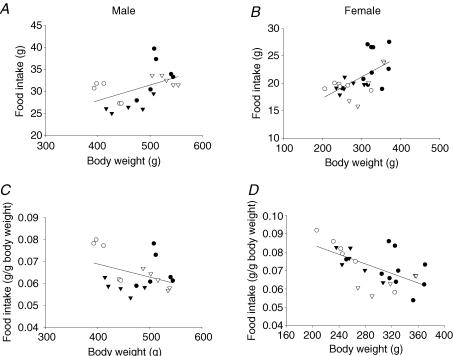
Figure 2 presents the absolute and relative food intake related to body weight for all four groups. Absolute food intake correlated positively with body weight while relative food intake correlated negatively (P = 0.05 for males and 0.001 for females).
Figure 2. Absolute and relative food intake as a function of body weight.
Absolute food intake as a function of body weight in males (A) and females (B). Males, P < 0.03 and r = 0.49; females, P < 0.001 and r = 0.59. Relative food intake per gram body weight as a function of body weight in males (C) and females (D); males, P = 0.05 and r =−0.43; females, P < 0.001 and r =−0.61. CC (•); RR (○); CR (▾); RC (▿).
Leptin concentrations at 110 days
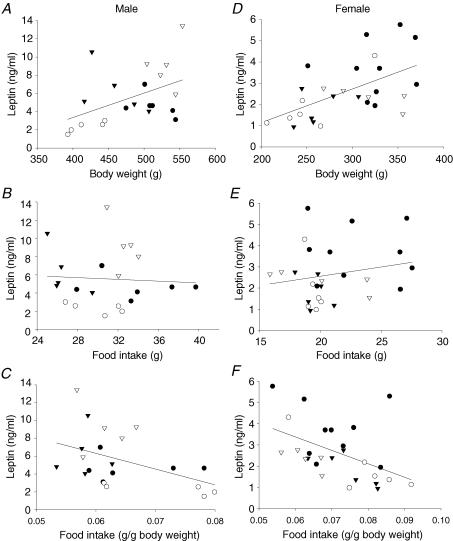
Table 1B and C shows the leptin concentrations in the four groups of male and female offspring at 110 days. Serum leptin concentration was lowest in the RR group in both males and females although the difference from CC was only significant in females. The highest leptin concentrations were in the RC male group in which leptin was more than twice the value in controls. It is of interest that similar high leptin values were not seen in the RC females. Figure 3 shows the correlation in all four groups of male and female offspring between leptin at 110 days, body weight and absolute and relative food intake at 100 days of age. Leptin correlated positively with body weight in both males and females (Fig. 3A and D). There was no correlation between leptin and food intake in either male or female offspring. However, when food intake was expressed per unit body weight, there was a strong negative correlation in the male and female offspring showing a decreased relative food intake as leptin increased.
Figure 3. Leptin as a function of body weight, and absolute and relative food uptake.
Leptin as a function of body weight in males (A) and females (D); males, P < 0.04 and r = 0.45; females, P < 0.003 and r = 0.56. Leptin as a function of absolute food intake in males (B) and females (E); males, P < 0.91 and r =−0.03; females, P < 0.38 and r = 0.18. Leptin as a function of relative food intake in males (C) and females (F); males, P < 0.04 and r =−0.46; females, P < 0.01 and r =−0.48. CC (•); RR (○); CR (▾); RC (▿).
Body fat and protein composition at 70 days of age
While there were not differences in total protein in male offspring, total protein was decreased in RC compared with female CR (Table 2). When expressed as a percentage of body weight, protein was higher in both groups restricted during lactation in males and females and lower in RC females compared with CC. Absolute lipid content was decreased in RR and CR in the male and RR in females compared with CC. Relative lipid content was decreased in RR and CR males compared with CC and RR females compared with CC. Relative lipid was increased in RC females compared with CC. Only for the control group relative protein content was higher in females than males while in the same group relative lipid content was lower in females in comparison with males. There were no sex differences for the relative protein and lipid content in the three experimental groups.
Table 2.
Offspring protein and fat absolute (g) and relative (%) body composition at 70 days of age
| CC | RR | CR | RC | |
|---|---|---|---|---|
| Males | ||||
| Protein (g) | 33.7 ± 0.6* | 32.7 ± 3.9* | 32.3 ± 0.3* | 35.8 ± 3.1* |
| Protein (%) | 44.5 ± 0.9a* | 61.0 ± 2.9b | 59.4 ± 0.4b | 49.5 ± 3.2a |
| Lipids (g) | 26.5 ± 1.2a* | 10.8 ± 1.0b* | 14.2 ± 0.2b* | 26.3 ± 1.1b* |
| Lipids (%) | 34.9 ± 1.7a | 20.9 ± 2.7b | 26.0 ± 0.3b | 36.9 ± 2.4a |
| Females | ||||
| Protein (g) | 24.0 ± 1.1a,b* | 23.8 ± 0.2a,b* | 26.0 ± 1.3a* | 20.2 ± 0.5b* |
| Protein (%) | 54.6 ± 1.4a* | 63.8 ± 0.2b | 60.2 ± 1.5b | 47.9 ± 1.1c |
| Lipids (g) | 11.6 ± 1.0a,c* | 7.3 ± 0.2b* | 10.1 ± 1.2b,c* | 14.4 ± 0.9a* |
| Lipids (%) | 26.3 ± 1.1a* | 19.6 ± 0.8b | 22.9 ± 1.5a,b | 33.8 ± 0.6c |
Mean ±s.e.m.; from 5 CC litters, 5 RR litters, 5 CR litters, and 4 RC litters; Groups not sharing a letter are statistically different: *male versus female
P≤ 0.05.
Responses to the glucose tolerance test
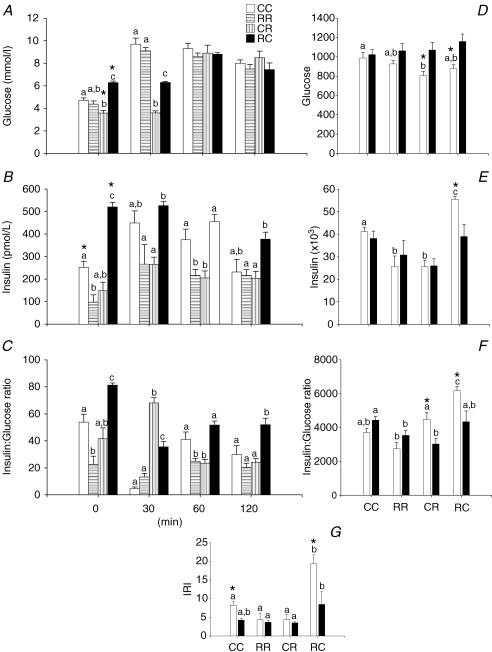
Figure 4 provides the results of the GTT conducted at 110 days postnatal life in males. The GTT data of the female offspring were presented in a previously published study (Zambrano et al. 2005a). In the male offspring evidence of both increased and decreased insulin sensitivity was presented according to the group history. Fasting glucose was lower in CR and higher in RC than CC. Fasting insulin and insulin : glucose ratio were lower in RR and higher in RC than CC. In the CR group AUC for glucose was less than CC. AUC for insulin was less in both RR and CR and increased for RC compared with CC. In the RC group AUC was higher for the insulin : glucose ratio compared with CC (P < 0.05). The RC group had a higher IRI compared with the other three groups.
Figure 4. Glucose tolerance tests.
Results from the glucose tolerance tests performed on male pups at 110 days of postnatal life. A, serum glucose (mmol l−1); B, serum insulin (pmol l−1); C, insulin to glucose ratio. Area under curve for male (□) and female (▪) during glucose tolerance test in: D, glucose; E, insulin; F, insulin : glucose ratio; and G, insulin resistance index (IRI) calculated as (glucose concentration × insulin concentration)/22.5. Data represented as mean ±s.e.m. from 4–6 litters. P < 0.05 for data not sharing at least one letter. *P < 0.05 versus female.
The only differences in fasting levels in females were higher insulin and insulin : glucose in RC compared with CC. Offspring of RR and CR mothers showed a lower insulin : glucose ratio AUC than CC (P < 0.05).
When compared with females, fasting glucose was higher in male offspring of RC mothers and lower in male offspring of CR mothers. Fasting insulin was higher in CC and RC males when compared with females. AUC for glucose was lower in CR and RC males than females while insulin was higher in RC males and the insulin : glucose AUC was higher in RC and CR males than females. Finally, the IRI was higher in CC and RC males than females.
Basal triglyceride and cholesterol concentrations
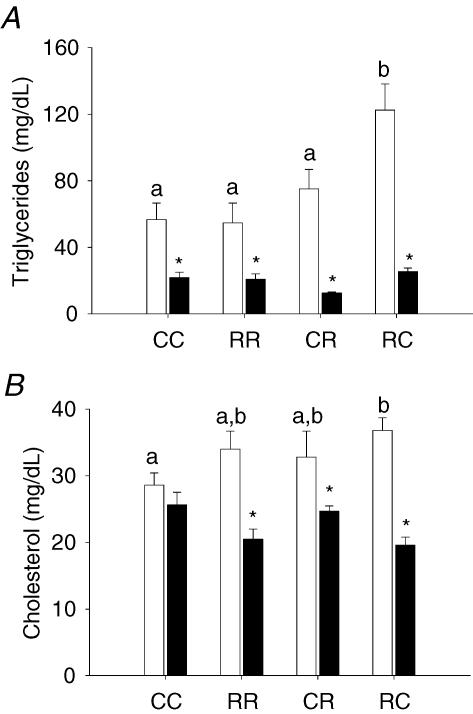
Triglyceride and cholesterol concentrations were both higher in RC male offspring than CC (Fig. 5). Concentrations of these key metabolic variables did not differ among the female groups. Triglycerides were higher in males for all four groups while cholesterol was only higher in males than females in the three experimental groups.
Figure 5. Cholesterol and triglyceride serum values.
A, male (□) and female (▪) cholesterol and B, triglyceride serum value data. Data represented as mean ±s.e.m. from 5 CC litters, 5 RR litters, 5–6 CR litters, and 4–6 RC litters. P < 0.05 for groups not sharing at least one letter. *P < 0.05 versus male.
Discussion
Previous studies in rats have shown that altered maternal carbohydrate and protein metabolism during pregnancy and/or lactation due to maternal low protein diets (Reusens & Remacle, 2001a; Sugden & Holness, 2002; Ozanne et al. 2003), global caloric restriction (Garofano et al. 1997), manipulation of the size of the rat litters in the first days of postnatal life (Engelbregt et al. 2001), maternal diabetes (Plagemann et al. 1992; Van Assche et al. 2001), maternal glucocorticoid administration (Drake & Walker, 2004; Drake et al. 2005), or bilateral uterine artery ligation in late pregnancy (Simmons et al. 2001) can result in altered carbohydrate metabolism in offspring. None of these studies separately addresses differential effects of protein restriction during gestation and lactation. This lack of data on different critical windows of exposure is also present in the only report to present data on gender differences (Sugden & Holness, 2002).
During the first 100 days, growth of the RR and CR offspring was slowed compared with CC. Our observations confirm the data provided in other studies for males that the CR group have a lower trajectory of postnatal growth over this period compared with controls and show that the same picture applies to females (Ozanne et al. 1996). Importantly we now show that changing a restricted diet for a control diet during pregancy normalizes the growth curve. However, restoration of normal weight is accompanied by a decreased percentage body protein and increased fat in female offspring. Once again it is clear that evaluation of weight alone without composition data provides an incomplete picture of the offspring physiology. Plasma cholesterol and TG were higher in males than females. There are no changes in these variables in females in any group while both were elevated in the male RC group.
None of the previous studies that attempted to dissect and evaluate effects of nutrient restriction during pregnancy and lactation contain information on food intake of the offspring. In this study, the lower food intakes in three of the four groups restricted during lactation (and a tendency in the fourth) are compatible with the lower weight. The negative correlation of food intake per unit body weight with body weight is a well known feature of mammalian metabolism (Romieu et al. 1988). In terms of overall energy balance it is interesting to note that restriction during pregnancy followed by a normal diet during lactation results in decreased relative food intake compared with restriction in both periods. Further studies need to be performed to determine the factors responsible. Alterations in uncoupling of metabolism or activity may explain these differences.
The adipose tissue-derived hormone leptin plays a key role in central nervous control of appetite and energy balance (Friedman & Halaas, 1998). As expected leptin concentrations correlated well in both sexes with body weight, and serum leptin concentrations correlated well with relative food intake per gram body weight but not total body weight. Several interesting studies have shown that there is an early peak in neonatal leptin in rodents and that this early peak may play a role in programming appetite later in life (de Oliveira Cravo et al. 2002; Vickers et al. 2005). Both males and females in our RC groups eventually become obese (author's unpublished data). We did not measure leptin in early life but the values obtained at 110 days show some interesting differences between groups. The high values in the RC males who will eventually become obese suggest that dietary influences during development may involve altered leptin sensitivity (Unger, 2002). Rats overfed during early postnatal life show a leptin-resistant state mediated by down-regulation of the hypothalamic long isoform of the leptin receptor (OB-Rb) (Lopez et al. 2005). Another observation of interest is the higher leptin concentration in males than females in the three experimental groups while the control values are the same in the two sexes. We know of few publications in which rat leptin values are given in both sexes (Leonhardt et al. 2003). In agreement with our study Engelbregt et al. (2001) reported that leptin concentrations and fat composition were higher in males than females. In contrast to our findings, Leonhardt et al. (2003) observed higher leptin values in females than males. We have no explanation for this difference.
Our observation that both males and females show evidence of increased insulin sensitivity at this stage of postnatal life is in keeping with the data presented on the male (Ozanne et al. 2003) and female offspring of protein-restricted mothers (Fernandez-Twinn et al. 2005). In the only study to date that addresses gender-related differences (Sugden & Holness, 2002), female rats were maintained on either a 20 or 8% protein diet throughout pregnancy and lactation. Then at weaning both sets of pups were placed on the 20% protein diet. Thus these two groups correspond to our CC and RR groups. These investigators also demonstrated insulin hypersensitivity in their group equivalent to our RR females at 140 days of life compared with controls while RR males showed evidence of insulin resistance by that age. At 110 days our RR males still demonstrated insulin hypersensitivity. Taken together data from rat nutrient-restriction studies in several laboratories indicate that protein restriction during development results in an initial period of insulin hypersensitivity followed in later life by insulin resistance. It appears that insulin hypersensitivity gives way to resistance earlier in male than female rats (Ozanne et al. 2003; Fernandez-Twinn et al. 2005). The reason for this difference in the time course remains to be determined. It also remains to be seen how our data relate to programming of insulin resistance in humans. Low ponderal index at birth (an indicator of IUGR) is associated with a faster postnatal growth rate and later insulin resistance. There is debate as to the relative roles of the pre- and postnatal events in human infants (Singhal et al. 2004).
Conclusions
This study used a protocol that permits identification of the window of exposure-dependent outcomes resulting from feeding pregnant rats a low protein diet during pregnancy and/or lactation. The level of nutrition available in pregnancy and lactation plays a major role in determining offspring metabolic phenotype. There are significant interactions between the effects of maternal diets in pregnancy and lactation. The greatest effects and those most likely to be harmful to long-term function (e.g. increased tissue fat and decreased protein, elevated cholesterol and TG and insulin resistance) occur when pups whose mothers were restricted during pregnancy received a normal – and hence abundant compared with restriction – diet during lactation.
Acknowledgments
This work was partially supported by the NIH (HD21350). M. D. was a recipient of a grant from PLACIRH, México.
References
- AOAC. Official Methods of Analysis of AOAC International No. 920.05, 920.39. 2002.
- Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40(Suppl. 2):115–120. doi: 10.2337/diab.40.2.s115. [DOI] [PubMed] [Google Scholar]
- de Oliveira Cravo C, Teixeira CV, Passos MC, Dutra SC, de Moura EG, Ramos C. Leptin treatment during the neonatal period is associated with higher food intake and adult body weight in rats. Horm Metab Res. 2002;34:400–405. doi: 10.1055/s-2002-33473. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Engelbregt MJ, van Weissenbruch MM, Popp-Snijders C, Lips P, Delemarre-van de Waal HA. Body mass index, body composition, and leptin at onset of puberty in male and female rats after intrauterine growth retardation and after early postnatal food restriction. Pediatr Res. 2001;50:474–478. doi: 10.1203/00006450-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373. doi: 10.1152/ajpregu.00206.2004. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat beta-cell development. Diabetologia. 1997;40:1231–1234. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B. Beta-cell mass and proliferation following late fetal and early postnatal malnutrition in the rat. Diabetologia. 1998;41:1114–1120. doi: 10.1007/s001250051038. [DOI] [PubMed] [Google Scholar]
- Holemans K, Aerts L, Van Assche FA. Lifetime consequences of abnormal fetal pancreatic development. J Physiol. 2003;547:11–20. doi: 10.1113/jphysiol.2002.036582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holemans K, Van Bree R, Verhaeghe J, Meurrens K, Van Assche FA. Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr. 1997;127:1371–1376. doi: 10.1093/jn/127.7.1371. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]
- Lopez M, Seoane LM, Tovar S, Garcia MC, Nogueiras R, Dieguez C, Senaris RM. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia. 2005;48:140–148. doi: 10.1007/s00125-004-1596-z. [DOI] [PubMed] [Google Scholar]
- Nandhini AT, Thirunavukkarasu V, Ravichandran MK, Anuradha CV. Effect of taurine on biomarkers of oxidative stress in tissues of fructose-fed insulin-resistant rats. Singapore Med J. 2005;46:82–87. [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence. J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, Hartil K, Petry CJ, Buckley AJ, Mosthaf-Seedorf L. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003;177:235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N, Smith GD. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol. 1996;271:E1128–E1134. doi: 10.1152/ajpendo.1996.271.6.E1128. [DOI] [PubMed] [Google Scholar]
- Petry CJ, Hales CN. Long-term effects on offspring of intrauterine exposure to deficits in nutrition. Hum Reprod Update. 2000;6:578–586. doi: 10.1093/humupd/6.6.578. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- Reusens B, Remacle C. Effects of maternal nutrition and metabolism on the developing endocrine pancreas. In: Barker DRP, editor. Fetal Origins of Cardiovascular and Lung Disease. Vol. 151. New York: Marcel Dekker; 2001a. pp. 339–358. [Google Scholar]
- Reusens B, Remacle C. Intergenerational effect of an adverse intrauterine environment on perturbation of glucose metabolism. Twin Res. 2001b;4:406–411. doi: 10.1375/1369052012597. [DOI] [PubMed] [Google Scholar]
- Romieu I, Willett WC, Stampfer MJ, Colditz GA, Sampson L, Rosner B, Hennekens CH, Speizer FE. Energy intake and other determinants of relative weight. Am J Clin Nutr. 1988;47:406–412. doi: 10.1093/ajcn/47.3.406. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ. The fetal origins hypothesis. Twin Res. 2001;4:iii. [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Singhal A, Cole TJ, Fewtrell M, Lucas A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet. 2004;363:1571–1578. doi: 10.1016/S0140-6736(04)16198-9. [DOI] [PubMed] [Google Scholar]
- Stewart RJ, Preece RF, Sheppard HG. Twelve generations of marginal protein deficiency. Br J Nutr. 1975;33:233–253. doi: 10.1079/bjn19750027. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol. 2002;175:757–767. doi: 10.1677/joe.0.1750757. [DOI] [PubMed] [Google Scholar]
- Unger RH. Lipotoxic diseases. Ann Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- Van Assche FA, Holemans K, Aerts L. Long-term consequences for offspring of diabetes during pregnancy. Br Med Bull. 2001;60:173–182. doi: 10.1093/bmb/60.1.173. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005a;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, Menjivar M, Larrea F, Nathanielsz PW. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005b;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]