Abstract
Cross-bridge kinetics underlying stretch-induced force transients was studied in fibres with different myosin light chain (MLC) isoforms from skeletal muscles of rabbit and rat. The force transients were induced by stepwise stretches (< 0.3% of fibre length) applied on maximally Ca2+-activated skinned fibres. Fast fibre types IIB, IID (or IIX) and IIA and the slow fibre type I containing the myosin heavy chain isoforms MHC-IIb, MHC-IId (or MHC-IIx), MHC-IIa and MHC-I, respectively, were investigated. The MLC isoform content varied within fibre types. Fast fibre types contained the fast regulatory MLC isoform MLC2f and different proportions of the fast alkali MLC isoforms MLC1f and MLC3f. Type I fibres contained the slow regulatory MLC isoform MLC2s and the slow alkali MLC isoform MLC1s. Slow MLC isoforms were also present in several type IIA fibres. The kinetics of force transients differed by a factor of about 30 between fibre types (order from fastest to slowest kinetics: IIB > IID > IIA ≫ I). The kinetics of the force transients was not dependent on the relative content of MLC1f and MLC3f. Type IIA fibres containing fast and slow MLC isoforms were about 1.2 times slower than type IIA fibres containing only fast MLC isoforms. We conclude that while the cross-bridge kinetics is mainly determined by the MHC isoforms present, it is affected by fast and slow MLC isoforms but not by the relative content of MLC1f and MLC3f. Thus, the physiological role of fast and slow MLC isoforms in type IIA fibres is a fine-tuning of the cross-bridge kinetics.
Muscle contraction is due to cyclic interactions of myosin and actin (cross-bridge cycles). Myosin is a hexameric protein consisting of two heavy chains (MHCs) and two pairs of light chains (MLCs) (Lowey & Risby, 1971). The rod parts of the MHCs form the backbone of the myosin filament. The globular ends (myosin heads, cross-bridges) of the MHCs project from the myosin filament. An 8.5-nm long α-helix connects the globular end with the rod part of each MHC. One so called ‘alkali’ or ‘essential’ MLC and one ‘regulatory’ MLC wrap around this helix, which is also known as the ‘myosin neck’ (Rayment et al. 1993). The globular ends of the MHCs contain the binding site for actin and the binding site for ATP, which is hydrolysed during contractile activity. The myosin neck is thought to act as a lever arm, which amplifies small conformational changes occurring in the catalytic domain of the MHC during ATP hydrolysis cycles into larger movement (Rayment et al. 1993; Uyeda et al. 1996). As reviewed by Lowey & Trybus (1995), the MLCs seem to be necessary for increasing the rigidity of the lever arm while transmitting force during the myosin head power stroke.
In striated muscle a large variety of isoforms of MHC and MLC exist (for review, see Pette & Staron, 1990; Schiaffino & Reggiani, 1996). Fast fibres of adult mouse (rat, rabbit) skeletal muscles are divided into three types based on their content of the MHC isoforms MHC-IIa (type IIA), MHC-IId (or MHC-IIx) (type IID or IIX) or MHC-IIb (type IIB). All these fibre types usually contain the fast regulatory MLC isoform (MLC2f) and different ratios of the fast alkali MLC isoforms MLC1f and MLC3f. Slow fibres (type I) contain the MHC isoform MHC-I, which is usually associated with the slow regulatory MLC isoform MLC2s and the slow alkali MLC isoform MLC1s. In several cases, type IIA fibres, in addition to fast MLC isoforms contain also slow MLC isoforms (rat: Mizusawa et al. 1982; Danieli-Betto et al. 1990; Bortolotto et al. 2000; rabbit: Salviati et al. 1982; Staron & Pette, 1987; for review see Stephenson, 2001; Bicer & Reiser, 2004).
As reviewed by Bottinelli (2001) the contribution of MHC isoforms to the performance of muscle fibre types is well established whereas the role of MLC isoforms is less known. In type IIB and type IID fibres of rat and rabbit, a higher proportion of MLC1f is associated with a smaller unloaded shortening velocity (Greaser et al. 1988; Sweeney et al. 1988; Bottinelli et al. 1994a; Sweeney, 1995). In contrast, different ratios of MLC1f and MLC3f do not seem to influence the ATPase activity of type IIB fibres of rat (Reggiani et al. 1997). In human type IIA fibres the presence of the slow regulatory MLC isoform MLC2s was shown to be associated with lower unloaded shortening velocities (D'Antona et al. 2002).
The measurement of both unloaded shortening velocity and ATPase activity involves many cross-bridge cycles, and therefore they may provide information only about the rate of the whole cross-bridge cycle. In contrast, force transients following stepwise length changes applied on maximally activated fibres reveal more detailed information about cross-bridge kinetics (Huxley & Simmons, 1971; Heinl et al. 1974; Ford et al. 1977). The force transients seem to be due to a synchronization of a fraction of myosin heads by the rapid change in the fibre length. This assumption is supported by the finding that the kinetics of the force transients shows a conspicuous correlation with the MHC isoforms present in fibres (rabbit: Kawai & Schachat, 1984; Andruchov et al. 2004b; rat: Galler et al. 1994, 1996, 1997; human: Hilber et al. 1999; mouse: Andruchov et al. 2004a). Furthermore, a variation in the kinetics of force transients was found in flight muscles of Drosophila where the head part of MHC was genetically modified (Swank et al. 2002). Stepwise stretches result in a simultaneous force increase followed by force decay and a delayed force increase (Heinl et al. 1974; Galler et al. 1994). Although there is some diversity in the interpretation, it is widely accepted that these transients are determined by specific steps, and not the whole cross-bridge cycle (Kawai & Brandt, 1980; Kawai & Zhao, 1993; Piazzesi et al. 1997; Ranatunga et al. 2002; Davis et al. 2002).
The role of MLC isoforms in determining the cross-bridge kinetics underlying stretch-induced force transients has not yet been investigated. In the present study we examined if different MLC isoforms alter the time course of stretch-induced force transients in specific muscle fibre types. The study was undertaken on maximally Ca2+-activated single fibres of various skeletal muscles of rabbit and rat. The isoforms of MLC and MHC were analysed after completion of the mechanical experiments. Our data suggest that while the cross-bridge kinetics underlying the force transients is mainly determined by the MHC isoforms present, it is affected by fast and slow MLC isoforms but not by the relative content of MLC1f and MLC3f. Thus, the physiological role of fast and slow MLC isoforms in type IIA fibres is a fine-tuning of the cross-bridge kinetics.
Methods
Muscle preparation
Adult rabbits (New Zealand White, 10–18 months) and rats (Fisher 344 strain, 4–12 months) were used in this study. The animals were anaesthetized with sodium pentobarbitone (55 mg kg−1 injected i.p.) and killed by bleeding, which is in accordance with the ethical rule of the country (protocol approved by the Ethics Committee of Salzburg University). The following muscles were quickly excised: masseter, diaphragm, soleus, gastrocnemius and adductor magnus from rabbits; soleus, gastrocnemius, vastus lateralis, tibialis anterior and plantaris from rats.
For obtaining single skinned muscle fibres, a procedure similar to that described by Wang & Kawai (1996) was used. The procedure was carried out at 0–4°C. After excision, muscles were immediately put into Sylgard-coated plates containing the following skinning solution (mm): 132 sodium propionate, 5 EGTA, 7 Na2H2ATP, 2 MgCl2, 1 dithiothreitol (DTT), 10 Mops (pH adjusted to 6.9 at 22°C with KOH). Muscles were cut into several strips of about 1–2 mm in diameter. Each strip was slightly stretched and pinned to the bottom of the plate. After several hours in this solution muscles were transferred into another skinning solution in which sodium propionate was replaced by potassium propionate. After periods of 1–2 h 10, 25 and finally 50% (v/v) glycerol were added in succession. Muscle preparations were kept for up to two months in cold storage solution (−25°C), which was a 1: 1 volumetric mixture of glycerol and relaxation solution (composition see below, pH adjusted to 6.9 at 22°C).
Mechanical measurements
The experimental apparatus and the method for mechanical measurements have been previously described (Galler & Hilber, 1994). The attachment points for the muscle fibres on the mechanical apparatus were two vertical epoxy carbon fibre needles of ∼100 μm tip diameter. The needles were attached to the silicon beams of two force transducer elements (AE 801, SensoNor, Norway). One element, acting as the force sensor (resonance frequency about 7.5 kHz) was connected mechanically to a micrometer screw and electrically to a force bridge amplifier, while the other element was a dummy, glued on the lever arm of a stepping motor. Rapid changes of the fibre length (≤ 1 ms) were achieved by a feedback-controlled stepping motor based on a Ling vibrator. The ability to make rapid solution changes was provided by a cuvette transporting system. Laser diffractometry (He–Ne laser, 632.8 nm, 4 mW) was used for measuring the sarcomere length.
The solutions used during the mechanical measurements were based on those described by Stephenson & Williams (1982) and contained (mm): 60 Hepes, 8 Na2H2ATP, 10 sodium creatine phosphate, 10 NaN3, 1 DTT, 40 g l−1 dextran T-500, 30 units ml−1 creatine phosphokinase and 1 mm free Mg2+. In addition, the relaxation solution (pCa > 8, pCa = −log [Ca2+]free) contained 50 mm EGTA. The maximal activation solution (pCa 4.3) contained 50 mm Ca-EGTA and the preactivation solution (low Ca2+-buffering capacity, pCa about 7) 50 mm hexamethylenediamine-N,N,N,N-tetraacetic acid (HDTA). The pH was adjusted to 7.10 with KOH at 22°C. The concentrations of free Ca2+ and free Mg2+ of the solutions were determined with ion-selective electrodes (Galler, 1999).
Single fibres were dissected at around −5°C in storage solution and glued to the tips of the two needles of the apparatus with the tissue glue Vetseal (Braun Melsungen, Germany). After gluing, the fibre ends were fixed by superfusion with a fine rapidly flowing downwards-directed stream of stained glutaraldehyde solution (8% glutaraldehyde, 5% Toluidine Blue, fixative) for 2–3 s. This procedure was fulfilled in a rigor solution (10 mm imidazole, 2.5 mm EGTA, 7.5 mm EDTA, 134 mm potassium propionate, pH 6.9) with a lower specific mass than the fixative. In this way a sharp boundary between the functional part of the fibre and the fixed ends was created. This method improves considerably the maintenance of the sarcomere homogeneity and the constancy of the mechanical properties during prolonged activations (Hilber & Galler, 1998).
Prior to the experiment, the length of the fibres was adjusted to exactly the slack position (resting length) in relaxation solution, and both fibre dimensions and sarcomere length were recorded. Fibre cross-sectional area was calculated using the largest and the smallest diameters measured along the fibre. The active length of the fibres ranged between 1.2 and 2.7 mm. The mean diameter of fibres was 53.4 ± 1.0 μm (range 35–80 μm) for rabbit and 43.4 ± 0.5 μm (range 25–60 μm) for rat. After transferring the fibre to the preactivating solution (∼1 min) and subsequently to the maximal activating solution a steady force was reached. The maximal isometric tension was determined by dividing this force by the cross-sectional area. Quick (≤ 1 ms) stretches, < 0.3% of fibre length in amplitude and 1–4 s in duration, were applied to induce force transients. The mechanical experiments were carried out at 22°C.
Gel electrophoresis
After completion of the mechanical measurements, the muscle preparations were removed from the apparatus for biochemical analysis of MHC and MLC isoforms. The muscle fragments were dissolved in SDS lysis buffer (62 mm Tris-HCl, pH 6.8, 10% (v/v) glycerol, 5% (w/v) SDS, 5% (v/v) 2-mercaptoethanol). The samples were heated at 65°C for 15 min. The MHC isoforms were analysed using a SDS–polyacrylamide (7.5%) gel system (separation gel: 7.428% (w/v) acrylamide (AA) and 0.072% (w/v) N,N′-methylene-bis-acrylamide (BAA); stacking gel: 3.96% (w/v) AA, 0.04% (w/v) (BAA). The MLC isoforms were analysed using a gradient (10–20%) SDS–polyacrylamide gel system. The separation gel contained 19.467% (w/v) AA and 0.533% (w/v) BAA on the bottom and 9.733% (w/v) AA and 0.267% (w/v) BAA on the top. The stacking gel contained 3.861% (w/v) AA and 0.106% (w/v) BAA. Ten microlitres of muscle extract containing about 0.7 nl of fibre was dispensed into each well. SDS-PAGE was carried out on 0.75-mm-thick slab gels with 16-cm-long glasses of an SE 600 system (Hoefer, San Francisco, USA). A constant voltage of 180 V was applied for 24 h in the case of MLC analysis and 28 h in the case of MHC analysis. After electrophoresis, gels were silver stained.
Western blots
Western blot analysis was performed for identifying the migration of MLC isoforms and for distinguishing them from other myofibrillar proteins. In this case, two gradient SDS-PAGE gels were run in parallel with the same set of samples; one was silver stained, the other was used for transferring the proteins to a Hybond-P membrane (Amersham Biosciences, QC, Canada). The transfer was performed in the semi-dry condition (semi-dry transfer unit TE 70, Amersham Biosciences), and a current of about 1 mA cm−2 was applied for 1.5 h. Two different monoclonal antibodies were necessary to detect the regulatory and alkali myosin light chains. One of these antibodies was specific for alkali MLC, the other for regulatory MLC. These antibodies did not distinguish between isoforms of respective proteins. The antibody for detecting the alkali myosin light chain isoforms MLC1f, MLC3f and MLC1s was purchased from Alexis (Grünberg, Germany; the monoclonal antibody to human ventricular light chain 1). The specific reactivity of this antibody with skeletal muscle alkali light chain isoforms was shown in a previous study (Cornillon et al. 1992). The antibody for detecting the regulatory myosin light chain isoforms MLC2f and MLC2s was purchased by Sigma (St Louis, USA; myosin light chain 20 K). After treating with primary antibodies, membranes were treated with anti-mouse antibody labelled with horseradish peroxidase (Amersham Biosciences). The protein bands were visualized with a chemiluminescence method (ECL plus detection reagents, Amersham Biosciences). This method is based on oxidation of the reagent luminal, a reaction which is catalysed by peroxidase in the presence of peroxide. This reaction enhances the luminescence of luminal, which was detected with photosensitive paper.
Densitometric analysis of MLC isoforms
For quantitative analysis of MLC isoforms, densitometry of protein bands was applied. The gels were scanned (scanner, HP ScanJet 6100C) and the densities of the silver-stained protein bands were determined using ImageJ software (http://www.nih.gov). Assuming 200 μm myosin heads in a fibre (e.g. Goldman et al. 1982) the protein concentration of regulatory MLC was calculated to be maximally 0.6 ng per mm2 of the stained protein band. This is in the linear range of silver-stain density and protein concentration, which is saturated at about 2 ng mm−2 in gels of the same thickness (Merril, 1990). The regulatory MLC isoform (MLC2f or MLC2f + MLC2s) was taken as a reference for MLC quantification because it is a measure for the number of myosin heads. Each myosin head is associated with one regulatory MHC (Lowey & Risby, 1971).
The density of protein bands of silver-stained SDS-PAGE is usually proportional to both the number and the size of protein molecules. Therefore, for calculating the molar ratio between proteins their densities were corrected by their molecular weight. Thus, in fast twitch fibres (containing only fast MLC isoforms) the molar ratio MLC3f/MLC2f was calculated using the following equation:
The terms on the left of the equation refer to the concentrations of corresponding proteins, d2f and d3f are the densities of MLC2f and MLC3f bands, and Mr,2f and Mr,3f are the relative molecular weights of MLC2f (18.9 kDa) and MLC3f (16.6 kDa), respectively.
The MLC3f/MLC2f ratio is an indicator for the fraction of MLC3f per myosin head, and ‘1 – MLC3f/MLC2f’ is an indicator for the fraction of MLC1f per myosin head. This is because each myosin head is associated with one MLC2f and, in addition, with either one MLC1f or one MLC3f. Thus, the MLC3f/MLC2f ratio is a measure of the relative distribution of MLC1f and MLC3f.
In type IIA fibres containing MLC1s in addition to the fast alkali MLC isoforms, but only fast regulatory MLC (MLC2f), the quantification of the MLC1s content was performed by the following equation:
The terms on the left of the equation refer to the concentrations of corresponding proteins, d1s and Mr,1s are the density of protein band and the molecular weight (22.5 kDa) of MLC1s, respectively.
In type IIA fibres, containing MLC1s and MLC2s in addition to the fast MLC isoforms, the quantification of MLC1s and MLC2s content was performed by the following equations:
and
The terms on the left of the equations refer to the concentrations of the corresponding proteins, d2s and Mr,2s are the density of protein band and the molecular weight (19 kDa) of MLC2s, respectively.
Statistics
Results are expressed as means ±s.e.m. Analysis was done using ANOVA statistics. Where applicable, linear regression analysis and Kolmogorov-Smirnov tests were performed.
Results
Myosin light chain isoforms of different muscle fibre types
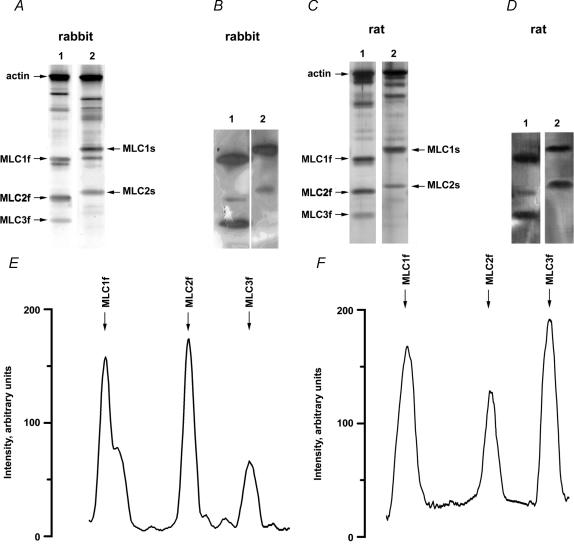
Representative gradient (10–20%) electrophoretograms of rabbit and rat skeletal muscle fibres are shown in Fig. 1A and C, respectively. In both figures type IIB fibres are shown in lanes 1 and type I fibres in lanes 2. The regulatory (MLC2f, MLC2s) and alkali (MLC1f, MLC3f, MLC1s) MLC were identified by Western blots, which are shown in Fig. 1B for rabbit fibres and in Fig. 1D for rat fibres. In more detailed studies (Weeds, 1976; Schachat et al. 1980; Julian et al. 1981; Salviati et al. 1982; Biral et al. 1999) MLC1s was shown to consist of two isoforms (MLC1sa and MLC1sb). Our electrophoresis technique did not separate these two isoforms.
Figure 1. SDS-PAGE (A and C), Western blots (B and D) and the densitometry (E and F) of myosin light chain isoforms of rabbit and rat skeletal muscle.
The figure shows silver-stained 10–20% gradient SDS-PAGE (A and C) and Western blots (B and D) of single fibres. Lanes 1 in A and C are electrophoretograms of type IIB fibres containing exclusively fast MLC isoforms (MLC1f, MLC2f, MLC3f). Likewise, lanes 2 in A and C are electrophoretograms of type I fibres containing exclusively slow MLC isoforms (MLC1s, MLC2s). Based on the reactivity with a monoclonal antibody against alkali MLCs and a monoclonal antibody against regulatory MLCs (Western blots B and D) the myosin light chain isoforms were identified as labelled in A and C. The traces in E and F are the densitometric profiles of lanes 1 in C (SDS-PAGE) and D (Western blot), respectively.
Figure 1E displays the densitometry of the electrophoretogram of the rat type IIB fibre shown in lane 1 of Fig. 1C. Figure 1F displays the densitometry of the corresponding Western blot (lane 1 of Fig. 1D). In the electrophoretogram, the densitometric profiles of MLC2f and MLC3f indicate the presence of only one band, but the densitometric profile of MLC1f contains two peaks indicating the presence of two adjacent bands. The second peak is not present in the Western blot (Fig. 1F) indicating that this second peak on the electrophoretogram must represent another protein, probably the fast isoform of troponin I, which has similar electrophoretic mobility (Bottinelli et al. 1994a; O'Connell et al. 2004).
The densitometric profiles of MLC2f and MLC3f bands in the electrophoretograms and Western blots indicate that these bands represent single proteins. Therefore, the densities of these bands can be used for measuring the relative concentrations of MLC2f and MLC3f, with the ratio MLC3f/MLC2f indicating the fraction of MLC3f per myosin head (see Methods). For numerous fibres (31 rabbit fibres, 40 rat fibres) the MLC electrophoresis was performed two times. The mean difference of the MLC3f/MLC2f ratio determined in the two gels was 0.038 ± 0.003 (s.e.m.) suggesting a good reproducibility of the method.
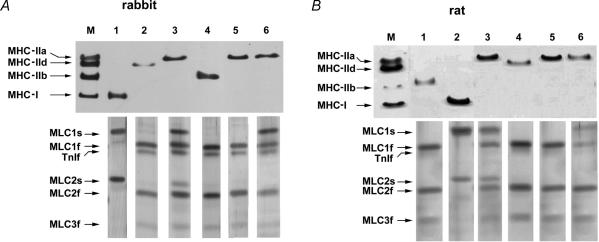
Figure 2 shows electrophoretograms of MHC and MLC isoforms in single skeletal muscle fibres for which mechanical properties were previously measured. Figure 2A shows results for rabbit fibres and Fig. 2B shows results for rat fibres. The same solubilized fibre sample was used for both MHC analysis (7.5% SDS-PAGE, upper panels) and MLC analysis (10–20% gradient SDS-PAGE, lower panels). In both rabbit and rat preparations four distinct MHC isoforms were separated, namely MHC-IIa, MHC-IId(x), MHC-IIb and MHC-I (in order of increasing electrophoretic mobility). Fibres containing only one of these isoforms were designated as fibres of type IIA, type IID(X), type IIB and type I, respectively. In contrast to these fibre types containing only one MHC isoform (pure fibres), several fibres (37% of total in rabbit and 25% in rat) contained two different MHC isoforms (hybrid fibres). In hybrid fibres the contribution of MLC isoforms to the kinetics of force transients can only be investigated at comparable ratios of MHC isoforms. The present set of data did not allow this evaluation. Therefore, only pure fibre types were considered for further analysis and hybrid fibres were discarded.
Figure 2. Silver-stained SDS-PAGE electrophoretograms of single rabbit (A) and rat (B) muscle fibres used for MHC (upper panels) and MLC (lower panels) isoform analysis.
For each fibre, the lanes of MLC electrophoretograms are displayed below the lanes of MHC electrophoretograms. A, M represents a mixture of extracts of rabbit gastrocnemius and diaphragm muscles serving as a marker for rabbit MHC isoforms. Lanes 1–6 represent electrophoretograms of single fibres for which mechanical properties were previously measured: lane 1, type I fibre containing MLC1s and MLC2s; lane 2, type IID fibre containing MLC1f, MLC2f and MLC3f; lane 3, type IIA fibre containing MLC1f, MLC2f and MLC3f as well as MLC1s and MLC2s; lane 4, type IIB fibre containing MLC1f, MLC2f and MLC3f; lane 5, type IIA fibre containing MLC1f, MLC2f and MLC3f; lane 6, type IIA fibre containing MLC1f, MLC2f and MLC3f as well as MLC1s. B, M represents an extract of diaphragm muscle of rat and serves as a marker for rat MHC isoforms. Lanes 1–6 represent electrophoretograms of single fibres for which mechanical properties were previously measured: lane 1, type IIB fibre containing MLC1f, MLC2f and MLC3f; lane 2, type I fibre containing MLC1s and MLC2s; lane 3, type IIA fibre containing MLC1f, MLC2f and MLC3f as well as MLC1s and MLC2s; lane 4, type IID fibre containing MLC1f, MLC2f and MLC3f; lane 5, type IIA fibre containing MLC1f, MLC2f and MLC3f; lane 6, type IIA fibre containing MLC1f, MLC2f and MLC3f as well as MLC1s.
Rabbit types IIB (lane 4 of Fig. 2A) and IID (lane 3 of Fig. 2A) fibres and rat types IIB (lane 1 of Fig. 2B) and IID (lane 4 of Fig. 2B) fibres exclusively contained fast MLC isoforms (MLC1f, MLC2f, MLC3f). Type I fibres of both rabbit (lane 1 of Fig. 2A) and rat (lane 2 of Fig. 2B) exclusively contained slow MLC isoforms (MLC1s, MLC2s). Type IIA fibres of rabbit and rat contained either only fast MLC isoforms or fast and slow MLC isoforms. Type IIA fibres were further classified into subtypes according to their MLC isoform complement. Type IIA(1f,2f,3f) are fibres which only contain the fast MLC isoforms MLC1f, MLC2f and MLC3f (lanes 5 of Fig. 2A and B). Type IIA(1f,2f,3f,1s) are fibres which contain the fast MLC isoforms MLC1f, MLC2f and MLC3f and the slow MLC isoform MLC1s (lanes 6 of Fig. 2A and B). Type IIA(1f,2f,3f,1s,2s) are fibres which contain the fast MLC isoforms MLC1f, MLC2f and MLC3f and the slow MLC isoforms MLC1s and MLC2s (lanes 3 of Fig. 2A and B). The same combinations of fast and slow MLC isoforms were already described for type IIA fibres of rabbit (Salviati et al. 1982; Staron & Pette, 1987) and rat (Mizusawa et al. 1982; Danieli-Betto et al. 1990; Bortolotto et al. 2000). In type IIA(1f,2f,3f,1s) fibres the fraction of MLC1s per myosin head was 0.38 ± 0.02 in rabbit (n = 4) and 0.32 ± 0.05 in rat (n = 4). In type IIA(1f,2f,3f,1s,2s) fibres the fraction of MLC1s per myosin head was 0.43 ± 0.06 for rabbit (n = 4) and 0.36 ± 0.05 for rat (n = 5); the fraction of MLC2s per myosin head was 0.31 ± 0.02 for rabbit (n = 4) and 0.33 ± 0.03 for rat (n = 5). Table 1 gives information about muscle fibre origin and frequency of type IIA fibres with various combinations of fast and slow MLC isoforms.
Table 1.
Distribution of type IIA fibres with different MLC isoform complements between different skeletal muscles of rabbit and rat
| Rabbit | Rat | |||||
|---|---|---|---|---|---|---|
| Mas | Dia | Gas | Sol | Vas | Gas | |
| Type IIA(1f,2f,3f) | 16 | 5 | 3 | 0 | 5 | 3 |
| Type IIA(1f,2f,3f,1 s) | 4 | 0 | 0 | 1 | 1 | 2 |
| Type IIA(1f,2f,3f,1 s,2 s) | 3 | 1 | 0 | 1 | 2 | 2 |
The type IIA fibres are classified according to their MLC isoform complement given in brackets. The characters 1f, 2f, 3f, 1s and 2s refer to MLC1f, MLC2f, MLC3f, MLC1s and MLC2s, respectively. The number of fibres originating from soleus (Sol), vastus lateralis (Vas), gastrocnemius (Gas), masseter (Mas) and diaphragm (Dia) is given.
Force transients of fibre types with different fast myosin alkali light chain isoforms
The resting sarcomere length was 2.20 ± 0.01 μm in rabbit muscle fibres and 2.34 ± 0.01 μm in rat muscle fibres. No significant differences (P > 0.6) between different fibre types were found within both species. Maximally Ca2+-activated isometric tension of fast fibres (rabbit: 293 ± 14 mN mm−2; rat: 278 ± 16 mN mm−2) was significantly larger (P < 0.01) than that of slow fibres (rabbit: 211 ± 11 mN mm−2; rat: 189 ± 14 mN mm−2), which is in agreement with other studies (Julian et al. 1981; Bottinelli et al. 1994a; Andruchov et al. 2004a). Within fast fibre types there was no significant difference in maximal tension (P > 0.2). Maximal tension was also not different in type IIA fibres containing different MLC isoform complement (P > 0.2).
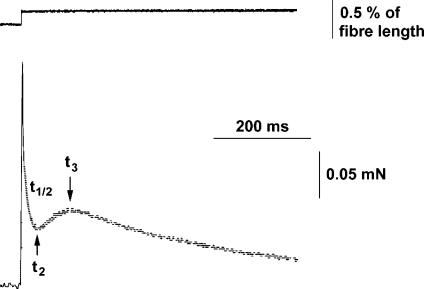
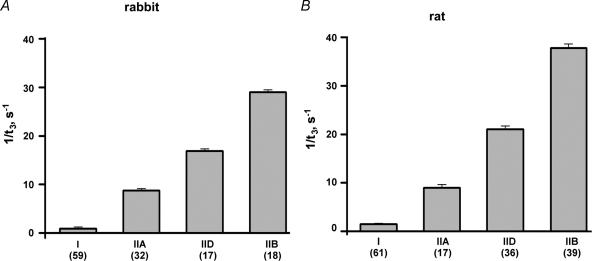
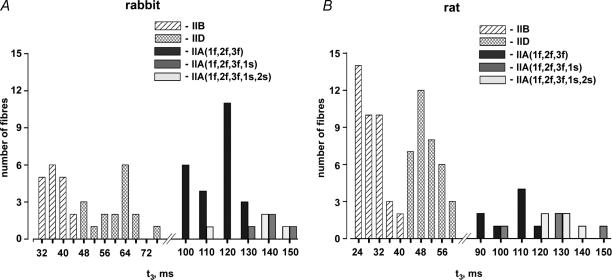
Maximally Ca2+-activated fibres were stretched by < 0.3% in a stepwise (< 1 ms) manner. The stretches caused an immediate rise in force, followed by a decrease and a delayed force increase (stretch activation, Fig. 3). The following kinetic parameters were evaluated: t2, which is the time from the beginning of the stretch to the lowest force value before the onset of delayed force increase, and t3, which is the time from the beginning of the stretch to the peak value of the delayed force increase. In addition, the half-time of force decay (t½) was evaluated within the time interval starting 3 ms after beginning of the stretch and ending at t2. The time parameters t2, t½ and t3 of all fibre types are listed in Table 2. As already reported for different mammalian species (rat: Galler et al. 1994, 1996, 1997; rabbit: Andruchov et al. 2004b; human: Hilber et al. 1999; mouse: Andruchov et al. 2004a) the kinetics of stretch-induced force transients was strikingly different in different fibre types (Fig. 4A and B). In both rabbit and rat the order from fastest to slowest kinetics was: IIB > IID > IIA ≫ I. As shown in the histogram of Fig. 5, the t3 values of different fibre types (but not of IIA subtypes, see later) never did overlap.
Figure 3. Typical original recordings of length (upper panel) and force (lower panel) in a stretch experiment on a maximally Ca2+-activated rabbit type IIA fibre.
The following kinetic parameters were determined in order to characterize the time course of stretch induced force transient: t2, which is the time from beginning of stretch to the lowest force before the onset of delayed force increase; t½, which is the half-time of the force decay starting 3 ms after beginning of the stretch and ending at t2; and t3, which is the time from the beginning of the stretch to the peak of the delayed force increase.
Table 2.
Time parameters t½, t2 and t3 of stretch-induced force transients in fibre types and IIA subtypes of rabbit and rat skeletal muscle
| Rabbit | Rat | |||||||
|---|---|---|---|---|---|---|---|---|
| Fibre types | n | t2 (ms) | t½ (ms) | t3 (ms) | n | t2 (ms) | t½ (ms) | t3 (ms) |
| IIB(1f,2f,3f) | 18 | 10.6 ± 0.4 | 2.4 ± 0.1 | 34.9 ± 0.9 | 39 | 8.7 ± 0.3 | 2.1 ± 0.1 | 27.3 ± 0.8 |
| IID(1f,2f,3f) | 17 | 21.6 ± 0.8 | 4.2 ± 0.1 | 60.3 ± 1.7 | 36 | 16.9 ± 0.4 | 3.7 ± 0.1 | 48.4 ± 1.0 |
| IIA(1f,2f,3f) | 24 | 39.0 ± 1.1 | 7.3 ± 0.3 | 110.1 ± 2.0 | 8 | 34.9 ± 1.7 | 6.9 ± 0.2 | 101.1 ± 3.9 |
| IIA(1f,2f,3f,1s) | 4 | 49.3 ± 3.2* | 8.5 ± 0.8* | 136.8 ± 5.8† | 4 | 40.3 ± 2.9* | 8.6 ± 0.7* | 121.8 ± 8.9† |
| IIA(1f,2f,3f,1s,2s) | 4 | 47.8 ± 5.5* | 8.4 ± 0.6* | 132.0 ± 2.0† | 5 | 43.4 ± 2.5* | 8.4 ± 0.3* | 127.2 ± 5.6† |
| I(1s,2s) | 59 | 286.3 ± 12.1 | 43.5 ± 1.2 | 1216.0 ± 57.4 | 61 | 203.7 ± 5.3 | 34.8 ± 0.6 | 704.0 ± 14.5 |
The data are means ±s.e.m. The MLC isoform complement of different fibre types is given in brackets. The characters 1f, 2f, 3f, 1s, 2s refer to MLC1f, MLC2f, MLC3f, MLC1s and MLC2s, respectively. t2 is the time from the beginning of the stretch to the lowest force value before the onset of delayed force increase; t½ is the half-time of the force decay starting 3 ms after beginning of the stretch and ending at t2; t3 is the time from the beginning of the stretch to the peak of the delayed force increase. All values are significantly different between fibres with different MHC content (P < 0.001). The symbols * and # characterize significant differences of IIA fibre subtypes from type IIA(1f,2f,3f)
P < 0.05 for
P < 0.01 for.
Figure 4. Kinetics of stretch-induced delayed force increase in different fibre types of rabbit (A) and rat (B) expressed as 1/t3.
The datea are means ±s.e.m. and the number of the fibres tested is given in parentheses for each type.
Figure 5. Histogram showing the distribution of the kinetics parameter t3 in fast fibre types of rabbit (A) and rat (B).
The type IIA fibres are classified according to their MLC isoform complement given in brackets. The characters 1f, 2f, 3f, 1s and 2s refer to MLC1f, MLC2f, MLC3f, MLC1s and MLC2s, respectively. Kolmogorov-Smirnov tests (P > 0.1) suggest that the data are normally distributed.
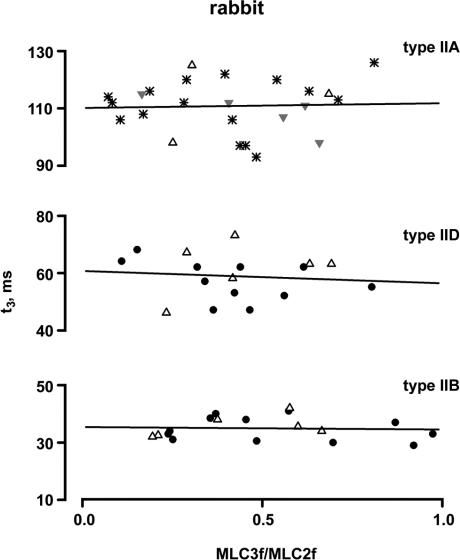
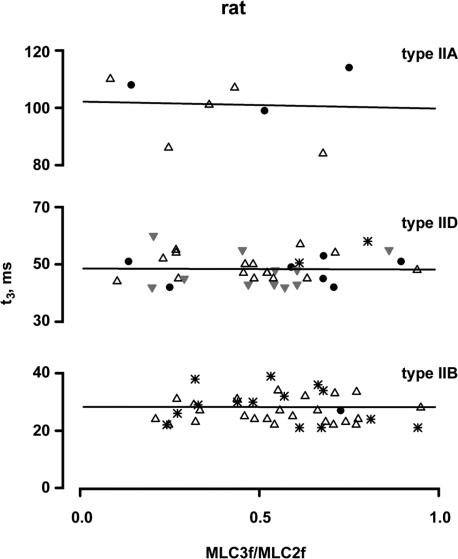
The relationship between t3 and the MLC3f/MLC2f ratio of the fibre types IIB, IID and IIA is shown in Fig. 6 for rabbit and in Fig. 7 for rat. Fibres from different muscles are indicated by different symbols. It can be seen that t3 is not dependent on the MLC3f/MLC2f ratio in all three fast fibre types. The slope of the regression lines did not deviate from zero in both rabbit and rat (P > 0.26, r2 < 0.14). This was true for fibres of individual muscles as well as for fibres of different muscles (for details, see legends of Figs 6 and 7). Similarly to t3, t2 and t½ also were not dependent on the MLC3f/MLC2f ratio in all three fast fibre types. The slopes of the linear regression lines did not deviate from zero (P > 0.36 and r2 < 0.09 for fibres of individual muscles; P > 0.46 and r2 < 0.03 for fibres of different muscles).
Figure 6. Relationships between t3 and the MLC3f/MLC2f ratio in rabbit fast fibre types.
All fibres contained only fast MLC isoforms. The molar ratio of MLC3f/MLC2f serves as a measure for the relative concentrations of MLC1f and MLC3f. Each point represents the t3 value of an individual fibre. Different symbols indicate the muscle origin (•, adductor magnus; ▵, gastrocnemius;  , diaphragm; *, masseter). The straight lines represent the best fits by linear regression analysis to the data points for all fibres of the same type; the slopes of all fitted lines did not significantly deviate from zero (type IIB: P = 0.81, r2 = 0.004; type IID: P = 0.69, r2 = 0.01; type IIA: P = 0.67, r2 = 0.009). The same was true for the regression lines of individual muscles (adductor magnus type IIB fibres, P = 0.52, r2 = 0.04; adductor magnus type IID fibres, P = 0.26, r2 = 0.14, masseter type IIA fibres, P = 0.56, r2 = 0.06.
, diaphragm; *, masseter). The straight lines represent the best fits by linear regression analysis to the data points for all fibres of the same type; the slopes of all fitted lines did not significantly deviate from zero (type IIB: P = 0.81, r2 = 0.004; type IID: P = 0.69, r2 = 0.01; type IIA: P = 0.67, r2 = 0.009). The same was true for the regression lines of individual muscles (adductor magnus type IIB fibres, P = 0.52, r2 = 0.04; adductor magnus type IID fibres, P = 0.26, r2 = 0.14, masseter type IIA fibres, P = 0.56, r2 = 0.06.
Figure 7. Relationships between t3 and the MLC3f/MLC2f ratio in rat fast fibre types.
All fibres contained only the fast MLC isoforms. The molar ratio of MLC3f/MLC2f serves as a measure for the relative concentrations of MLC1f and MLC3f. Each point represents the mean t3 value of an individual fibre. Different symbols indicate the muscle origin (•, gastrocnemius; ▵, vastus lateralis,  , plantaris; *, tibialis anterior). The straight lines represent the best fits by linear regression analysis to the data points of all fibres of the same type; the slopes of all fitted lines did not significantly deviate from zero (type IIB: P = 0.86, r2 = 0.06; type IID P = 0.93, r2 = 0.0002; type IIA: P = 0.88, r2 = 0.004). The same was true for the regression lines of individual muscles (vastus lateralis type IIB fibres, P = 0.76, r2 = 0.004; tibialis anterior type IIB fibres, P = 0.44, r2 = 0.05; vastus lateralis type IID fibres, P = 0.91, r2 = 0.001; gastrocnemius type IID fibres, P = 0.78, r2 = 0.02; plantaris type IID fibres, P = 0.99, r2 = 0.000002).
, plantaris; *, tibialis anterior). The straight lines represent the best fits by linear regression analysis to the data points of all fibres of the same type; the slopes of all fitted lines did not significantly deviate from zero (type IIB: P = 0.86, r2 = 0.06; type IID P = 0.93, r2 = 0.0002; type IIA: P = 0.88, r2 = 0.004). The same was true for the regression lines of individual muscles (vastus lateralis type IIB fibres, P = 0.76, r2 = 0.004; tibialis anterior type IIB fibres, P = 0.44, r2 = 0.05; vastus lateralis type IID fibres, P = 0.91, r2 = 0.001; gastrocnemius type IID fibres, P = 0.78, r2 = 0.02; plantaris type IID fibres, P = 0.99, r2 = 0.000002).
Force transients of type IIA fibres with fast and slow myosin light chain isoforms
The kinetics of force transients of type IIA fibres differed depending on the presence or absence of about 30–40% slow MLC isoforms. In Table 2, the t2, t½ and t3 values of different IIA fibre subtypes originating from different muscles are pooled. Both type IIA(1f,2f,3f,1s) fibres and type IIA(1f,2f,3f,1s,2s) fibres exhibited significantly (t2, t½: P < 0.05; t3: P < 0.01) slower kinetics of force transients than type IIA(1f,2f,3f) fibres (Fig. 5). On the other hand, no differences in the kinetics of force transients were found between type IIA(1f,2f,3f,1s) and type IIA(1f,2f,3f,1s,2s) fibres (P > 0.3, for t2, t½ and t3, Table 2). Since only in the rabbit masseter muscle were a sufficient number of type IIA fibre subtypes found, only this muscle was analysed individually. Within this muscle, both type IIA(1f,2f,3f,1s) fibres (t2 = 43.7 ± 5.3 ms, t½ = 8.6 ± 0.7 ms, t3 = 131.2 ± 10.9 ms) and type IIA-(1f,2f,3f,1s,2s) fibres (t2 = 49.2 ± 3.2 ms, t½ = 8.3 ± 0.5 ms, t3 = 136.8 ± 5.8 ms) exhibited significantly (t2, t½: P < 0.05; t3: P < 0.01) slower kinetics than type IIA(1f,2f,3f) fibres (t2 = 36.8 ± 1.2 ms, t½ = 7.2 ± 0.4 ms, t3 = 111.1 ± 2.4 ms).
Discussion
This is the first study of the role of MLC isoforms in the cross-bridge kinetics underlying stretch-induced force transients of skeletal muscle fibres. The study was undertaken on different muscle fibre types with a varying content of MLC isoforms. In both rabbit and rat, type IIB, type IID and many type IIA fibres contained only fast MLC isoforms (MLC1f, MLC2f, MLC3f). The proportion of MLC1f and MLC3f varied within each of these fibre types. Other type IIA fibres contained both fast and slow MLC isoforms. Our study helps to elucidate the physiological role of different MLC isoforms in the cross-bridge kinetics of different fibre types.
Kinetics of force transients and isoforms of myosin light chains
Although there is some diversity in the interpretation of stretch-induced force transients, there is agreement on the assumption that the late phase of the force decay (> ∼3 ms) and the delayed force increase are determined by specific steps of the cross-bridge cycle (Kawai & Brandt, 1980; Kawai & Zhao, 1993; Piazzesi et al. 1997; Ranatunga et al. 2002; Davis et al. 2002). The initial fast force decay (< ∼3 ms) was not evaluated in the present study. In studies of sinusoidal analysis where the concentrations of ATP, phosphate and ADP were changed (Kawai & Brandt, 1980; Kawai & Zhao, 1993; Galler et al. 2005), it was suggested that the late phase of force decay (process C of sinusoidal analysis) represents the detachment and the delayed force increase (process B of sinusoidal analysis) represents the attachment of myosin heads. Other investigators do not relate attachment and detachment so strictly to the various phases of stretch-induced force transients (Piazzesi et al. 1997; Ranatunga et al. 2002; Davis et al. 2002).
As observed in previous studies (rat: Galler et al. 1994, 1996, 1997; rabbit: Andruchov et al. 2004b; human: Hilber et al. 1999; mouse: Andruchov et al. 2004a) the rates of stretch-induced force transients of single fibres were found to be conspicuously correlated with their MHC isoform content. The time parameters t2, t½ and t3 differed up to about 30 times and increased in the order IIB, IID, IIA and I. In all fast fibre types t2, t½ and t3 did not depend on the MLC3f/MLC2f ratio. Therefore, it can be concluded that the cross-bridge kinetics underlying stretch-induced force transients does not depend on the relative distribution of the alkali MLC isoforms MLC1f and MLC3f.
In type IIA fibres the kinetics of force transients varied depending on their content of fast and slow MLC isoforms (IIA fibre subtypes). If fast MLC isoforms are partially (about 30–40%) replaced by slow MLC isoforms, the time parameters t2, t½ and t3 are about 1.2 times larger. Type I fibres contain exclusively slow MLC isoforms and exhibit strikingly slow kinetics. One could argue that the slow kinetics is due to the slow MLC isoforms and not to MHC-I. However, this is unlikely as type I fibres are up to 10 times slower than type IIA fibres, whereas the presence of about 30–40% slow MLC isoforms reduces the kinetics of type IIA fibres only by a factor of 1.2. The possibility that fibre kinetics would be reduced further by a factor of 8 if the content of slow MLC isoforms would increase from 30–40% to 100% is highly unlikely. Therefore, it appears that the very slow kinetics of type I fibres is mainly due to the MHC-I isoform and not due to the coexpressed slow MLC isoforms. Fast and slow MLC isoforms obviously can only fine-tune the cross-bridge kinetics underlying stretch-induced force transients; its main time course is determined by the MHC isoform.
It seems that the presence of MLC1s in type IIA fibres is sufficient to reduce the cross-bridge kinetics. The additional presence of MLC2s does not seem to be required. This is suggested by the observation that type IIA (1f,2f,3f,1s) fibres and type IIA(1f,2f,3f,1s,2s) fibres exhibited similar kinetics of force transients. They both were 1.2 times slower than type IIA(1f,2f,3f) fibres. The contribution of MLC2s to the kinetics must be investigated in fibres which contain MLC2s but not MLC1s. Such type IIA fibres were not found in the present study on rabbit and rat, but were observed in larger mammals (human: Larsson & Moss, 1993; D'Antona et al. 2002). Here the presence of MLC2s was associated with a slower unloaded shortening velocity of fibres.
Molecular aspects
The amino acid sequence of alkali MLC isoforms differs in specific regions (for review see Timson, 2003). The most striking difference is an N-terminal extension, which is present in MLC1f and MLC1s but not in MLC3f. This extension is rich in proline and has four lysines at the distal end. The size and the positively charged residues of lysine enable this extension to interact with the negatively charged C-terminus of actin (Trayer et al. 1987; Andreev et al. 1999). A further difference between the alkali MLC isoforms exists in the region where this subunit binds to the α-helix of the myosin neck (MHC-binding region). This region is identical in MLC1f and MLC3f, but differs in MLC1s.
Neither the force transients nor the ATPase activity (isometric conditions: Bottinelli et al. 1994b; Reggiani et al. 1997; actomyosin in solution: Pastra-Landis et al. 1983) depend on the fraction of MLC1f and MLC3f per myosin head. On the other hand, the speed of filament sliding is slowed by MLC1f (Greaser et al. 1988; Lowey et al. 1993; Bottinelli et al. 1994a) which is probably due to interaction of its N-terminal extension with actin (Sweeney, 1995; Morano et al. 1995; Andreev et al. 1999). Thus it appears that the binding of the N-terminal extension of MLC1f to actin slows the speed of filament sliding but not the cross-bridge kinetics underlying the force transients and the rate of the whole cross-bridge cycle (ATPase activity). These circumstances can be explained by assuming that MLC1f binding to actin reduces the distance of filament displacement during one cross-bridge cycle, but does not affect the kinetics. In contrast to skeletal muscle, in cardiac muscle the interaction of the N-terminal extension of alkali MLC with actin seems to slow down the cross-bridge kinetics (sinusoidal analysis, Miller et al. 2005).
The observation that MLC1s is associated with slower kinetics than MLC1f and MLC3f suggests that differences in the MHC-binding region of slow and fast MLC isoforms affect crossbidge kinetics. This is supported by a study of mouse heart (Vemuri et al. 1999). Here a single point mutation in the MHC-binding region of alkali MLC resulted in a significant alteration of the kinetics of stretch-induced force. The effects of the MHC binding region of MLC isoforms cannot be explained straightforwardly. However, it is possible that different MHC-binding regions provide different rigidities to the myosin neck, which functions as a lever arm. Different rigidities of the myosin necks would result in a different mechanical strain. A high strain is thought to decelerate the kinetics of certain steps of the cross-bridge cycle (Huxley & Simmons, 1971; Reconditi et al. 2004). Possibly, the binding of MLC1s, in comparison with MLC1f and MLC3f, reveals a larger rigidity of the myosin neck, which would results in a larger strain and a slower kinetics of the cross-bridge.
Acknowledgments
The authors are thankful to Professor D. G. Stephenson (Melbourne) and H. Grassberger (Salzburg) for valuable comments and critical readings of the manuscript. The work was supported by a grant of the Austrian Science Foundation (FWF-P16709-B09).
References
- Andreev OA, Saraswat LD, Lowey S, Slaughter C, Borejdo J. Interaction of the N-terminus of chicken skeletal essential light chain 1 with F-actin. Biochemistry. 1999;38:2480–2485. doi: 10.1021/bi981706x. [DOI] [PubMed] [Google Scholar]
- Andruchov O, Andruchova O, Wang Y, Galler S. Kinetic properties of myosin heavy chain isoforms in mouse skeletal muscle: Comparison with rat, rabbit and human and correlation with amino acid sequence. Am J Physiol Cell Physiol. 2004a;287:C1725–C1732. doi: 10.1152/ajpcell.00255.2004. [DOI] [PubMed] [Google Scholar]
- Andruchov O, Wang Y, Andruchova O, Galler S. Functional properties of skinned rabbit skeletal and cardiac muscle preparations containing α-cardiac myosin heavy chain. Pflugers Arch. 2004b;448:44–53. doi: 10.1007/s00424-003-1229-2. [DOI] [PubMed] [Google Scholar]
- Bicer S, Reiser PJ. Myosin light chain isoform expression among single mammalian skeletal muscle fibers: specie variation. J Muscle Res Cell Motil. 2004;25:622–633. doi: 10.1007/s10974-004-5070-9. [DOI] [PubMed] [Google Scholar]
- Biral D, Ballarin F, Toscano I, Salviati G, Yu F, Larsson L, Betto R. Gender- and thyroid hormone-related transitions of essential myosin light chain isoform expression in rat soleus muscle during ageing. Acta Physiol Scand. 1999;167:317–323. doi: 10.1046/j.1365-201x.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bortolotto SK, Stephenson DG, Stephenson GMM. MHC isoforms composition and Ca2+- or Sr2+- activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;279:C1564–C1577. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch. 2001;443:6–17. doi: 10.1007/s004240100700. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994a;478:341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Reggiani C, Stienen GJM. Myofibrillar ATPase activity durino isometric contraction and isomyosin composition in rat single skinned muscle fibres. J Physiol. 1994b;481:663–675. doi: 10.1113/jphysiol.1994.sp020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornillon B, Cathiard AM, Eldin P, Anoal M, Cardinaud R, Liautard JP, et al. Probing myosin light chain 1 structure with monoclonal antibodies. J Muscle Res Cell Motil. 1992;13:329–340. doi: 10.1007/BF01766461. [DOI] [PubMed] [Google Scholar]
- D'Antona G, Megighian A, Bortolotto S, Pellegrino MA, Ragona RM, Staffieri A, et al. Contractile properties and myosin heavy chain isoform composition in single fibre of human laryngeal muscles. J Muscle Res Cell Motil. 2002;23:187–195. doi: 10.1023/a:1020963021105. [DOI] [PubMed] [Google Scholar]
- Danieli-Betto D, Betto R, Midrio M. Calcium sensitivity and myofibrillar protein isoforms of rat skinned skeletal muscle fibres. Pflugers Arch. 1990;417:303–308. doi: 10.1007/BF00370996. [DOI] [PubMed] [Google Scholar]
- Davis JS, Satorius CL, Epstein ND. Kinetic effects of myosin regulatory light chain phosphorylation on skeletal muscle contraction. Biophys J. 2002;83:359–370. doi: 10.1016/S0006-3495(02)75175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford LE, Huxley AF, Simmons RM. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977;269:441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S. Ca2+ Sr2+ force relationships and kinetic properties of fast-twitch rat leg muscle fibre subtypes. Acta Physiol Scand. 1999;167:131–141. doi: 10.1046/j.1365-201x.1999.0600x.x. [DOI] [PubMed] [Google Scholar]
- Galler S, Hilber K. Unloaded shortening of skinned mammalian skeletal muscle fibres. Effects of the experimental approach and passive force. J Muscle Res Cell Motil. 1994;15:400–412. doi: 10.1007/BF00122114. [DOI] [PubMed] [Google Scholar]
- Galler S, Hilber K, Pette D. Force responses following stepwise length changes of rat skeletal muscle fibre types. J Physiol. 1996;493:219–227. doi: 10.1113/jphysiol.1996.sp021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S, Hilber K, Pette D. Stretch activation and myosin heavy chain isoforms of rat, rabbit and human skeletal muscle fibres. J Muscle Res Cell Motil. 1997;18:441–448. doi: 10.1023/a:1018646814843. [DOI] [PubMed] [Google Scholar]
- Galler S, Schmitt T, Pette D. Stretch activation, unloaded shortening velocity, and myosin heavy chain isoforms of rat skeletal muscle fibres. J Physiol. 1994;478:513–521. doi: 10.1113/jphysiol.1994.sp020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S, Wang G, Kawai M. Elementary steps of the cross-bridge cycle in fast-twitch fiber types from rabbit skeletal muscles. Biophys J. 2005;89:3248–3260. doi: 10.1529/biophysj.104.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman YE, Hibberd MG, McCray JA, Trentham DR. Relaxation of muscle fibes by photolysis of caged ATP. Nature. 1982;300:701–705. doi: 10.1038/300701a0. [DOI] [PubMed] [Google Scholar]
- Greaser ML, Moss RL, Reiser PJ. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chain. J Physiol. 1988;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinl P, Kuhn HJ, Rüegg JC. Tension responses to quick length changes of glycerinated skeletal muscle fibers from frog and tortoise. J Physiol. 1974;237:243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilber K, Galler S. Improvement of the measurements on skinned muscle fibres by fixation of the fibre ends with glutaraldehyde. J Muscle Res Cell Motil. 1998;19:365–372. doi: 10.1023/a:1005393519811. [DOI] [PubMed] [Google Scholar]
- Hilber K, Galler S, Gohlsch B, Pette D. Kinetic properties of myosin heavy chain isoforms in single fibers from human skeletal muscle. FEBS Lett. 1999;455:267–270. doi: 10.1016/s0014-5793(99)00903-5. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Julian FJ, Moss RL, Waller GS. Mechanical properties and myosin light chain composition of skinned muscle fibres from adult and new-born rabbits. J Physiol. 1981;311:201–218. doi: 10.1113/jphysiol.1981.sp013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Brandt PW. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980;1:279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kawai M, Schachat FH. Differences in the transient response of fast and slow skeletal muscle fibres. Correlations between complex modulus and myosin light chains. Biophys J. 1984;45:1145–1151. doi: 10.1016/S0006-3495(84)84262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Zhao Y. Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibres. Biophys J. 1993;65:638–651. doi: 10.1016/S0006-3495(93)81109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscle. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S, Risby D. Light chains from fast and slow muscle myosins. Nature. 1971;234:81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Lowey S, Trybus KM. Role of skeletal and smooth muscle myosin light chain. Biophys J. 1995;68:120s–128s. [PMC free article] [PubMed] [Google Scholar]
- Lowey S, Waller GS, Trybus KM. Function of skeletal muscle myosin heavy and light chain isoforms by an in vitro motility assay. J Biol Chem. 1993;268:20414–20418. [PubMed] [Google Scholar]
- Merril CR. Gel-staining techniques. Meth Enzymol. 1990;182:477–488. doi: 10.1016/0076-6879(90)82038-4. [DOI] [PubMed] [Google Scholar]
- Miller MS, Palmer BM, Ruch S, Martin LA, Farman GP, Wang Y, et al. The essential light chain N-terminal extension alters force and fiber kinetics in mouse cardiac muscle. J Biol Chem. 2005;280:34427–34434. doi: 10.1074/jbc.M508430200. [DOI] [PubMed] [Google Scholar]
- Mizusawa H, Takagi A, Sugita H, Toyokura Y. Coexistence of fast and slow types of myosin light chains in single fiber of rat soleus muscle. J Biochem. 1982;91:423–425. doi: 10.1093/oxfordjournals.jbchem.a133706. [DOI] [PubMed] [Google Scholar]
- Morano I, Ritter O, Bonz A, Timek T, Vahl CF, Michel G. Myosin light chain–actin interaction regulates cardiac contractility. Circ Res. 1995;76:720–725. doi: 10.1161/01.res.76.5.720. [DOI] [PubMed] [Google Scholar]
- O'Connell B, Nguyen LT, Stephenson GM. A single-fibre study of the relationship between MHC and TnC isoform composition in rat skeletal muscle. Biochem J. 2004;378:269–274. doi: 10.1042/BJ20031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastra-Landis SC, Huiatt T, Lowey S. Assembly and kinetic properties of myosin light chain isozymes from fast skeletal muscle. J Mol Biol. 1983;170:403–422. doi: 10.1016/s0022-2836(83)80155-7. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V. Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres. J Physiol. 1997;498:3–15. doi: 10.1113/jphysiol.1997.sp021837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW, Coupland ME, Mutungi G. An asymmetry in the phosphate dependence of the transients induced by length perturbation in mammalian (rabbit psoas) muscle fibres. J Physiol. 2002;542:899–910. doi: 10.1113/jphysiol.2002.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, et al. Three-dimensional structure of a myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reconditi M, Linari M, Lucii L, Stewart A, Sun YB, Boesecke P, et al. The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature. 2004;428:578–581. doi: 10.1038/nature02380. [DOI] [PubMed] [Google Scholar]
- Reggiani C, Potma EJ, Bottinelli R, Canepari M, Pellegrino MA, Stienen GJM. Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat. J Physiol. 1997;502:449–460. doi: 10.1111/j.1469-7793.1997.449bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G, Betto R, Danieli-Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal muscle fibres. Biochem J. 1982;207:261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachat FH, Bronson DD, McDonald OB. Two kinds of slow skeletal muscle fibers which differ in their myosin light chain complement. FEBS Lett. 1980;122:80–82. doi: 10.1016/0014-5793(80)80406-6. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Staron RS, Pette D. The multiplicity of myosin light chain and heavy chain combinations in histochemically typed single fibres. Rabbit soleus muscle. Biochem J. 1987;243:687–693. doi: 10.1042/bj2430687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson GMM. Hybrid skeletal muscle fibres: a rare or common phenomenon? Clin Exp Pharmacol Physiol. 2001;28:692–702. doi: 10.1046/j.1440-1681.2001.03505.x. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Effect of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank DM, Knowles AF, Suggs JA, Sarsoza F, Lee A, Maughan DW, Bernstein SI. The myosin converter domain modulates muscle performance. Nat Cell Biol. 2002;4:312–316. doi: 10.1038/ncb776. [DOI] [PubMed] [Google Scholar]
- Sweeney HL. Function of N-terminus of the myosin essential light chain of vertebrate striated muscle. Biophys J. 1995;68:112s–119s. [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Kushmerick MJ, Mabuchi K, Sreter FA, Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988;263:9034–9039. [PubMed] [Google Scholar]
- Timson DJ. Fine tuning of myosin motor: the role of essential light chain in striated muscle myosin. Biochimie. 2003;85:639–645. doi: 10.1016/s0300-9084(03)00131-7. [DOI] [PubMed] [Google Scholar]
- Trayer IP, Trayer HR, Levine BA. Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study. Eur J Biochem. 1987;164:259–266. doi: 10.1111/j.1432-1033.1987.tb11019.x. [DOI] [PubMed] [Google Scholar]
- Uyeda TQP, Abramson PD, Spudich JA. The neck region of the myosin motor domain acts as a lever arm to generate movement. Proc Natl Acad Sci U S A. 1996;93:4459–4464. doi: 10.1073/pnas.93.9.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri R, Lankford EB, Poetter K, Hassanzadeh S, Takeda K, Yu Z-X, et al. The stretch-activation response may be critical to the proper functioning of the mammalian heart. Proc Natl Acad Sci U S A. 1999;96:1048–1053. doi: 10.1073/pnas.96.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Kawai M. Effects of MgATP and MgADP on the cross-bridge kinetics of rabbit soleus slow-twitch muscle fibers. Biophys J. 1996;71:1450–1461. doi: 10.1016/S0006-3495(96)79346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds AG. Light chains from slow-twitch muscle myosin. Eur J Biochem. 1976;66:157–173. doi: 10.1111/j.1432-1033.1976.tb10436.x. [DOI] [PubMed] [Google Scholar]