Abstract
Mitochondrial fatty acid transport is a rate-limiting step in long chain fatty acid (LCFA) oxidation. In rat skeletal muscle, the transport of LCFA at the level of mitochondria is regulated by carnitine palmitoyltransferase I (CPTI) activity and the content of malonyl-CoA (M-CoA); however, this relationship is not consistently observed in humans. Recently, fatty acid translocase (FAT)/CD36 was identified on mitochondria isolated from rat and human skeletal muscle and found to be involved in LCFA oxidation. The present study investigated the effects of exercise (120 min of cycling at ∼60% V̇O2peak) on CPTI palmitoyl-CoA and M-CoA kinetics, and on the presence and functional significance of FAT/CD36 on skeletal muscle mitochondria. Whole body fat oxidation rates progressively increased during exercise (P < 0.05), and concomitantly M-CoA inhibition of CPTI was progressively attenuated. Compared to rest, 120 min of cycling reduced (P < 0.05) the inhibition of 0.7, 2, 5 and 10 μm M-CoA by 16%, 21%, 30% and 34%, respectively. Whole body fat oxidation and palmitate oxidation rates in isolated mitochondria progressively increased (P < 0.05) during exercise, and were positively correlated (r = 0.78). Mitochondrial FAT/CD36 protein increased by 63% (P < 0.05) during exercise and was significantly (P < 0.05) correlated with mitochondrial palmitate oxidation rates at all time points (r= 0.41). However, the strongest (P < 0.05) correlation was observed following 120 min of cycling (r= 0.63). Importantly, the addition of sulfo-N-succimidyloleate, a specific inhibitor of FAT/CD36, reduced mitochondrial palmitate oxidation to ∼20%, indicating FAT/CD36 is functionally significant with respect to LCFA oxidation. We hypothesize that exercise-induced increases in fatty acid oxidation occur as a result of an increased ability to transport LCFA into mitochondria. We further suggest that decreased CPTI M-CoA sensitivity and increased mitochondrial FAT/CD36 protein are both important for increasing whole body fatty acid oxidation during prolonged exercise.
As exercise duration increases there is a greater reliance on fatty acids to provide the reducing equivalents necessary for aerobic oxidation in the electron transport chain (Watt et al. 2003). Prior to oxidation, LCFAs are activated by long-chain acyl-CoA synthetase. They cannot passively cross into the mitochondrial inner matrix, where oxidation occurs, and therefore carnitine-dependent transport must precede oxidation. It is currently believed that the activity of carnitine palmitoyltransferase I (CPTI), which catalyses the transesterification of LCFA-CoA to LCFA-carnitine, is the rate-limiting enzyme in the carnitine-dependent transport of LCFA (see McGarry & Brown, 1997 for review). In rat skeletal muscle, CPTI activity is allosterically regulated by malonyl-CoA (M-CoA), and M-CoA levels have been shown to decrease during exercise (Winder et al. 1989, 1990) permitting an increased rate of fatty acid oxidation. However, studies in humans suggest that M-CoA levels may not decrease sufficiently during moderate intensity exercise to explain the increases in fatty acid oxidation (Odland et al. 1996, 1998; Dean et al. 2000). Further studies have been conducted in search of other regulators of CPTI activity during exercise, but without success (Starritt et al. 2000; Bezaire et al. 2004). Although M-CoA levels may not change in human skeletal tissue during exercise, alterations in compartmentalization/cellular distribution or the sensitivity of CPTI to M-CoA may explain the changes in LCFA oxidation that have been observed.
Recently, it has been proposed that mitochondrial LCFA transport may involve other proteins in addition to CPTI (Campbell et al. 2004). Fatty acid translocase (FAT/CD36) is a multiligand scavenger receptor with several functions, one of which is to interact with, and transport LCFAs across phospholipid bilayers (Abumrad et al. 1993; Matsuno et al. 1996; Bonen et al. 2004; Coort et al. 2004). A role for FAT/CD36 in LCFA transport has been identified in rat adipocytes (Abumrad et al. 1993) and skeletal muscle (Bonen et al. 2000). More recently, FAT/CD36 has been identified on the mitochondrial membrane of resting rat (Campbell et al. 2004) and human (Bezaire et al. 2005) skeletal muscle and was shown to be required for LCFA oxidation. It has been shown that FAT/CD36 protein content correlates with oxidative capacity, and electrically stimulated muscle contraction increased FAT/CD36 in rat mitochondria without alterations in total muscle content (Campbell et al. 2004). Given the short duration of the exercise protocol used (30 min), and the unaltered total protein level of FAT/CD36, it has been proposed that FAT/CD36 translocates to the mitochondria in response to exercise (Campbell et al. 2004). This suggests that there is a level of regulation of fatty acid oxidation that has not previously been recognized. Whether a similar mechanism occurs in human muscle remains to be established.
The purpose of this study was to compare exercise-induced increases in whole body fatty acid oxidation and isolated mitochondrial palmitate oxidation rates to measurements associated with mitochondrial transport. We examined two regulators of mitochondrial LCFA transport, CPTI M-CoA sensitivity and the involvement of FAT/CD36 protein in fatty acid oxidation. We hypothesized that 120 min of exercise would reduce M-CoA inhibition of CPTI activity. In addition, we hypothesized that 120 min of exercise would increase the capacity of isolated mitochondria to oxidize palmitate in association with an increase in mitochondrial FAT/CD36 content.
Methods
Subjects
Fifteen healthy, recreationally active, individuals volunteered for this study (n = 10 males, 5 females; age: 22 ± 1 years; weight: 76 ± 3 kg; BMI: 24 ± 1 kg m−2; and V̇O2peak: 48 ± 2 ml min−1 (kg body mass (bm)−1); (means ±s.e.m.). All subjects completed the same experimental protocol. Volunteers were randomly divided into two groups for the purposes of in vitro analysis as a result of the limitations in the amount of skeletal muscle tissue that can be obtained from each subject. There were no significant differences in the characteristics of the two groups so data were pooled for the analysis of whole body metabolic and blood measures, as well as for Western blot protein determination. Group A consisted of eight subjects (6 males, 2 females), from whom muscle samples were used for mitochondrial palmitate oxidation studies (age: 22 ± 1 years; weight: 72 ± 5 kg; BMI: 24 ± 1 kg m−2; and V̇O2peak: 48 ± 3 ml min−1(kg bm)−1). Group B consisted of seven subjects (4 males, 3 females) from whom muscle samples were used for CPTI analysis (21 ± 1 years; weight: 80 ± 4 kg; BMI: 25 ± 1 kg m−2; and V̇O2peak: 49 ± 4 ml min−1 (kg bm)−1). Female subjects were in the early follicular phase of their menstrual cycle at the time of the experiments involving muscle biopsies. Subjects were fully informed of the purpose of the experiments and of any possible risk before giving written consent to participate. The study was approved by the University of Guelph Ethics Committee and conformed to the Declaration of Helsinki.
Pre-experimental protocol
Subjects visited the laboratory on three occasions and were asked to refrain from exercise in the 48 h prior to each visit. On the first visit, peak pulmonary O2 uptake (V̇O2peak) was measured with a metabolic cart (SensorMedics Vmax model, CA, USA) during an incremental exercise test on a cycle ergometer (LODE Instrument, Groningen, the Netherlands). On the following two visits participants cycled for 2 h at ∼60% V̇O2peak on the Lode cycle ergometer following a 12 h overnight fast. The first visit was used to familiarize the subjects with the 2 h procedure and to confirm the power output required to attain ∼60% V̇O2peak.
Experimental protocol
On the third visit, subjects arrived at the laboratory following a 12 h overnight fast. A catheter was inserted into a peripheral arm vein and both legs were prepared for muscle biopsies of the vastus lateralis muscle. Resting ventilatory and blood samples were obtained and a muscle sample was obtained under local anaesthesia (2% lidocaine without adrenaline) using the percutaneous needle biopsy technique described by Bergstrom (1975). Ventilatory and blood samples were obtained during exercise at 15, 30, 60, 90 and 120 min and additional biopsies were sampled at 30 and 120 min. In Group A, two muscle biopsies (total ∼400 mg) were sampled at each time point for the determination of mitochondrial palmitate oxidation. In Group B, one muscle biopsy (∼200 mg) was sampled at each time point and for the determination of CPTI activity.
Immediately following tissue sampling visible fat and connective tissue were dissected free from the muscle and the samples were blotted to remove excess blood. The majority of the tissue was used for the immediate isolation of mitochondria for the determination of CPTI activity and palmitate oxidation rates and for the analysis of selected proteins with Western blotting. A second small section of the muscle biopsy sample (∼10 mg) was homogenized and frozen in liquid N2 for the subsequent analysis of citrate synthase (CS) activity.
Determination of blood metabolites
Venous blood was sampled directly into vacutainers containing heparin, and partitioned into two fractions. An aliquot of 200 μl of whole blood was added to 1 ml of 0.6 m perchloric acid (PCA) and centrifuged. The deproteinized supernatant was stored at −80°C and later analysed for glucose, lactate and glycerol (Bergmeyer, 1974). A second aliquot of whole blood was immediately centrifuged; the plasma was removed and stored at −80°C. The plasma was later analysed for free fatty acids (FFAs) (Wako NEFA C-test kit, Wako Chemicals, Richmond, VA, USA).
Isolation of mitochondria from skeletal muscle
Differential centrifugation was used to obtain pure and intact mitochondria containing both intermyofibrillar (IMF) and subsarcelommal (SS) fractions (Jimenez et al. 2002). The IMF and SS mitochondria were pooled due to the limited amount of muscle tissue and the requirements for in vitro analysis. The mitochondria were resuspended in 1 μl of medium III per initial milligram of tissue and used for CPTI activity measurements, or mitochondria were further diluted to a final volume of 500 μl for palmitate oxidation measurements.
For Western blotting analysis, mitochondria were further purified using a Percoll gradient (Sigma-Aldrich). Samples were centrifuged at 20 000 g for 1 h and the mitochondrial layer was removed. The Percoll was removed from the sample by further centrifuging at 20 000 g for 5 h. At this point the mitochondria are no longer metabolically viable, but are suitable for Western blotting.
Mitochondrial lipid oxidation measurements
Labeled CO2 production and acid soluble trapped 14C from palmitate oxidation were measured following a 30 min incubation of viable mitochondria in a sealed system as previously described (Campbell et al. 2004; Bezaire et al. 2005). Briefly, viable mitochondria (100 μl) were added to a 900 μl aliquot of pregassed (5% CO2 and 95% O2, constantly shaking at 37°C for 15 min) modified Krebs–Ringer buffer, which was then sealed. The 20 ml glass scintillation vial contained a microcentrifuge tube with 500 μl of 1 m benzethonium hydroxide inserted into a 1.5 ml centrifuge tube to capture 14CO2 produced during the oxidation reaction. The reaction was initiated by the addition of a 6: 1 palmitate: BSA complex (containing 10 μCi of [1-14C]palmitate, for a final palmitate concentration of 1.8 mm) administered by syringe through the rubber cap. The reaction continued for 30 min at 37°C and was terminated with the addition of ice-cold 12 n PCA by syringe through the rubber cap.
A fraction of the reaction medium was removed through the cap and quantified by liquid scintillation to determine the isotopic fixation as previously described (Campbell et al. 2004; Bezaire et al. 2005). Gaseous CO2 produced from oxidation of [1-14C]palmitate was measured by acidifying the remaining reaction mixture in the 20 ml glass scintillation vial with 1.0 ml of 1 m H2SO4. Liberated 14CO2 was trapped by benzethonium hydroxide over a 90 min incubation period at room temperature. The microcentrifuge tube containing the 14CO2 was put in a scintillation vial, and radioactivity was counted.
Inhibition studies with sulfo-N-succimidyloleate (SSO) were performed by preincubating mitochondria with SSO dissolved in dimethylsulfoxide (DMSO) for 30 min as described by Bezaire et al. (2005). Based on dose–response experiments the final SSO concentration was set at 200 μm (Bezaire et al. 2005). For control purposes, the same volume (1 μl) of DMSO was added to vials that were not supplemented with SSO.
Carnitine palmitoyltransferase-I activity
The forward radioisotope assay was used for the determination of CPTI activity as described by McGarry et al. (1983) with minor modifications. Briefly, the assay was conducted at 37°C and initiated by the addition of 10 μl of mitochondrial suspension (1: 3 dilution) to 10 μl of varying palmitoyl-CoA concentrations (18.75, 37.5, 75, 150 and 300 μm) and 80 μl of a standard reaction medium (l-[3H]carnitine; GE Healthcare (Amersham Biosciences), UK). The reaction containing 300 μm palmitoyl-CoA was also carried out in the presence of various M-CoA concentrations (0.2, 0.7, 2, 5 and 10 μm). The reaction was stopped after 6 min with the addition of ice-cold HCl. Palmitoyl-[3H]carnitine was extracted in water-saturated butanol in a process involving three washes with distilled water and subsequent re-centrifugation steps to separate the butanol phase, in which the radioactivity was counted.
Control studies were performed by the addition of SSO to the reaction mixture prior to initiation of the reaction. The final concentration of SSO was set at 200 μm. As a control, the same volume of DMSO was added to the control tubes. Maximal CPTI activity was expressed in terms of the whole muscle (nmol min−1 (g wet muscle)−1), and was normalized to the ratio of CS activity in intact mitochondrial suspensions to total muscle CS activity to account for the quality of the mitochondrial preparation (see below). CPTI M-CoA and palmitoyl-CoA kinetic data were expressed as a percentage of maximal CPTI activity.
Citrate synthase activity
Citrate synthase (CS) activity was determined in isolated mitochondria as well as in aliquots of homogenized whole muscle as previously described (Ramsay & Naismith, 2003; Bezaire et al. 2005). The net difference between CS activity in intact mitochondria and mitochondria subjected to repeat freeze thawing, provided a measure of the viability of the mitochondria, as well as, when compared to the total muscle CS activity, provided a measure of the amount of mitochondria recovered during our isolation procedure (Bezaire et al. 2005). The CS activity was assayed spectrophotometrically at 37°C by measuring the disappearance of NADH (Srere, 1969).
Western blotting
Purified isolated mitochondrial fractions were analysed for total protein (BCA protein assay). Twenty-five micrograms of denatured proteins from each sample were separated by electrophoresis on 8% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. The monoclonal antibody MO25 was used to detect FAT/CD36 (Matsuno et al. 1996). Commercially available antibodies were used to detect cytochrome c oxidase IV (COXIV; Santa Cruz Biotechnology, Santa Cruz, CA, USA), Na+,K+-ATPase α1 subunit (Upstate Biotechnology, Charlottesville, VA, USA), and sarcoplasmic reticulum calcium ATPase (SERCA1) (Affinity BioReagents Inc., Golden, CO, USA). An internal control of previously extracted human muscle crude membrane was used in each gel. Blots were quantified using chemiluminescence and the ChemiGenius 2 Bioimaging system (SynGene, Cambridge, UK).
Calculation of whole body oxidation rates
Whole body CHO and fat oxidation rates (g min−1) were calculated according to the following equations (Peronnet & Massicotte, 1991): CHO oxidation = 4.585 V̇CO2 production (l min−1) − 3.226 O2 production (l min−1); fat oxidation = 1.695 O2 production (l min−1) − 1.701 CO2 production (l min−1). To convert CHO and fat oxidation rates to kilojoules per minute, values were multiplied by 16.19 and 40.80, respectively.
Statistics
All data are presented as the mean ±s.e.m. Differences between control and SSO treatments were analysed with Student's paired t test. One-way analysis of variance was used to determine significance between all other treatments. When significance was obtained, Fisher's LSD post hoc analysis was completed. Associations between variables were investigated using Pearson's correlation analyses, as appropriate. Statistical significance was accepted at P < 0.05.
Results
Whole body metabolic measurements
V̇O2 was greater (P < 0.05) than rest at all time points during exercise, and progressively increased from 55% V̇O2peak at 15 min to 63% V̇O2peak at 120 min (Table 1). The V̇CO2peak and total oxidation rates increased with exercise by ∼8% from 30 min to 120 min of cycling (Table 1). The respiratory exchange ratio (RER) was greater than rest at all time points during exercise, and progressively decreased from 0.90 at 30 min to 0.87 at 120 min (Fig. 1A). Whole body fat oxidation rates increased by 54% from 30 to 120 min of exercise (Fig. 1B). Whole body CHO oxidation rates decreased by 12% from 30 to 120 min of exercise (Fig. 1B).
Table 1.
Whole body metabolic measurements during 120 min of cycling at ∼60% V̇O2peak
| 0 | 15 | 30 | 60 | 90 | 120 min | |
|---|---|---|---|---|---|---|
| V̇O2 (l min−1) | 0.40 ± 0.03 | 1.88 ± 0.10 | 1.98 ± 0.11 | 2.06 ± 0.11# | 2.13 ± 0.12#† | 2.17 ± 0.14#† |
| % V̇O2max | 11.6 ± 0.6 | 55.2 ± 3.0 | 57.7 ± 2.8 | 60.0 ± 2.9# | 61.8 ± 3.0#† | 62.7 ± 2.8#† |
| V̇CO2 (l min−1) | 0.33 ± 0.02 | 1.74 ± 0.09 | 1.79 ± 0.10 | 1.84 ± 0.10 | 1.87 ± 0.10# | 1.88 ± 0.11#† |
| Calculated total oxidation (kJ min−1) | 9.4 ± 0.4 | 40.2 ± 2.1 | 42.1 ± 2.3# | 43.7 ± 2.4# | 45.0 ± 2.6#† | 45.8 ± 2.9#†‡ |
Values are means ±s.e.m. (n = 15). All time points are significantly (P < 0.05) different from rest
significantly different from 15 min
significantly different from 30 min
significantly different from 60 min.
Figure 1. Whole body metabolic measures during 120 min of cycling at ∼60% V̇O2peak.
Values are means ±s.e.m. (n = 15). A, respiratory exchange ratio (RER). B, calculated fat and carbohydrate (CHO) rates expressed in kJ min−1. All time points are significantly (P < 0.05) different from rest; *significantly different from 15 min, †significantly different from 30 min, and ‡significantly different from 60 min.
Blood measurements
Blood glucose remained constant for the initial 30 min of exercise and progressively decreased (P < 0.05) following 60 min (Table 2). Blood lactate was greater than rest at all time points, and remained constant throughout exercise (Table 2). Plasma FFA and glycerol levels remained stable for the initial 30 min of exercise, and then significantly increased during the final 90 min of exercise (Table 2).
Table 2.
Whole blood metabolites and plasma FFA concentrations during 120 min of cycling at ∼60% V̇O2peak
| 0 | 15 | 30 | 60 | 90 | 120 | |
|---|---|---|---|---|---|---|
| Glucose (mm) | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.1 ± 0.1 | 4.0 ± 0.1* | 3.8 ± 0.1*# | 3.5 ± 0.1*#†‡§ |
| Lactate (mm) | 0.5 ± 0.1 | 2.0 ± 0.3* | 1.8 ± 0.3* | 1.5 ± 0.3* | 1.5 ± 0.3* | 1.5 ± 0.2* |
| FFA (mm) | 0.32 ± 0.03 | 0.31 ± 0.03 | 0.36 ± 0.05 | 0.59 ± 0.10*#† | 0.71 ± 0.10*#† | 0.89 ± 0.10*#†‡§ |
| Glycerol (μm) | 36 ± 7 | 47 ± 8 | 77 ± 26 | 145 ± 36*#† | 180 ± 29*#† | 234 ± 34*#†‡§ |
Values are means ±s.e.m. (n = 15).
Significantly (P < 0.05) different from rest
significantly different from 15 min
significantly different from 30 min
significantly different from 60 min
significantly different from 90 min.
Mitochondrial purification
Across all experiments, mitochondrial recovery from skeletal muscle was 26 ± 2%, while the quality of the preparation was 88 ± 2% (see Methods). Western blotting demonstrated that the isolation procedure successfully yielded highly purified mitochondria without contamination from other sources. Specifically, Western blotting demonstrated the absence of SERCA (110 kDa), and Na+,K+-ATPase (112 kDa) proteins, and the presence of COXIV (22 kDa) on purified mitochondrial extracts from human skeletal muscle (data not shown).
Mitochondrial palmitate oxidation
Isolated mitochondrial palmitate oxidation rates were higher (P < 0.05) following 120 min of exercise compared to rest (Fig. 2). In the presence of SSO, a specific inhibitor of FAT/CD36 (Coort et al. 2002), palmitate oxidation was dramatically reduced at all time points (Fig. 2). Isolated mitochondrial FAT/CD36-dependent palmitate oxidation rates (subtraction of the 200 μm SSO oxidation rates from control values) were also higher following 120 min of exercise compared to rest and 30 min (Fig. 2).
Figure 2. In vitro palmitate oxidation rates during 120 min of cycling at ∼60% V̇O2peak.
Values are means ±s.e.m. expressed in nmol mg−1 h−1 (n = 8). FAT/CD36-dependent palmitate oxidation was individually calculated as the difference between oxidation rates with and without 200 μm SSO. *Significantly (P < 0.05) different from rest, †significantly different from 30 min, and ‡significantly different from control.
CPTI activity
Maximal CPTI activity (at 300 μm palmitoyl-CoA) was not altered during 120 min of exercise (Fig. 3), nor was it inhibited by the addition of 200 μm SSO (Fig. 3). CPTI activity at various concentrations of palmitoyl-CoA was also not altered during exercise, nor was the palmitoyl-CoA Michaelis constant (KM) (Fig. 4A). However, the sensitivity of CPTI to the inhibitor M-CoA was progressively reduced following 120 min of exercise without altering the concentration required for 50% inhibition (IC50) (Fig. 4B). Compared to rest, CPTI activity following 30 and 120 min was higher in the presence of 5.0 and 10.0 μm M-CoA (Fig. 4B). Also, following 120 min of exercise, CPTI activity was elevated in the presence of 0.7 and 2.0 μm M-CoA.
Figure 3. Maximal CPTI activity during 120 min of cycling at ∼60% V̇O2peak, and CPTI activity in the presence of 200 μmol SSO.
Values are means ±s.e.m. expressed in nmol min−1 (g wet wt)−1 (n = 7).
Figure 4. Carnitine palmitoyltransferase I (CPTI) kinetics during 120 min of cycling at ∼60% V̇O2peak.
Values are means ±s.e.m., as a percentage of maximal activity (n = 7). A, palmitoyl-CoA kinetics. B, malonyl-CoA kinetics at 300 μm palmitoyl-CoA. *Significantly (P < 0.05) different from rest, and †significantly different from 30 min.
Mitochondrial FAT/CD36 protein content
Western blots performed on all subjects (n = 15) demonstrated a progressive increase (P < 0.05) in mitochondrial FAT/CD36 protein. Following 120 min of cycling the content of FAT/CD36 on the mitochondrial membrane increased by 59% (Fig. 5).
Figure 5. Representative FAT/CD36 Western blot performed on isolated mitochondria following 120 min of cycling at ∼60% V̇O2peak.
Values are means ±s.e.m., in arbitrary units (n = 15). *Significantly (P < 0.05) different from rest, and †significantly different from 30 min.
Correlation analysis
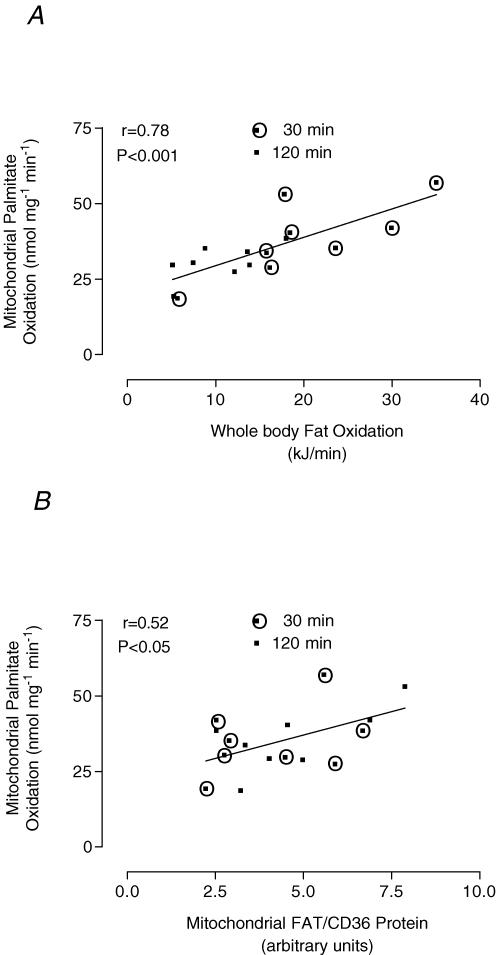
A significant correlation (P < 0.001) (r = 0.78) existed between estimated whole body fat oxidation rates and mitochondrial palmitate oxidation rates during exercise (30 and 120 min) (Fig. 6A). Mitochondrial FAT/CD36 protein content and mitochondrial palmitate oxidation rates observed at all time points significantly correlated (P = 0.05, r = 0.41; data not shown). Mitochondrial FAT/CD36 protein content and palmitate oxidation rates analysed in isolation at rest and 30 min, when fatty acid oxidation was the lowest, did not correlate (P = 0.17, r = 0.39 and P = 0.15, r = 0.43, respectively; data not shown). However, a significant correlation was observed during exercise (30 and 120 min) (P < 0.05, r = 0.52) and following 120 min when fatty acid oxidation was greatest (P < 0.05, r = 0.63) (Fig. 6B).
Figure 6. Pearson correlations following120 min of cycling at ∼60% V̇O2peak.
A, calculated correlation between estimated whole body fat oxidation rates and isolated mitochondrial palmitate oxidation rates at 30 and 120 min. B, Pearson correlation calculated between mitochondrial FAT/CD36 protein and isolated mitochondrial palmitate oxidation rates at 30 and 120 min.
Discussion
In the present study we examined the relationship between increases in whole body fat oxidation and two putative mitochondrial LCFA transport regulators in response to prolonged exercise. Specifically, we investigated palmitoyl-CoA and M-CoA CPTI sensitivity, and the presence and functional significance of FAT/CD36 on skeletal muscle mitochondria, during 120 min of cycling at ∼60% V̇O2peak. In accordance with our hypothesis, 120 min of cycling decreased the sensitivity of CPTI to M-CoA and increased FAT/CD36 mitochondrial protein content. Interestingly, the increased mitochondrial palmitate oxidation rates during exercise correlated with both whole body fat oxidation rates and FAT/CD36 mitochondrial protein content, suggesting an important role for mitochondrial FAT/CD36 in LCFA transport during exercise.
Generally, absolute fatty acid oxidation reaches maximal rates at ∼64% V̇O2peak, and about 50–70% of the total fat oxidized at this intensity is derived from plasma LCFA (see van Loon for review, 2004). Although plasma LCFAs have been reported to represent up to 70% of the fat oxidized during moderate intensity exercise, the mobilization of LCFAs cannot entirely explain alterations in LCFA oxidation, as increases in whole body fat oxidation have been observed at the start of exercise without elevations in plasma FFA (O'Neill et al. 2004; Watt et al. 2004). Regardless of the source, LCFA transport into mitochondria must precede its oxidation. In rat muscle it appears that CPTI activation is critical to increasing LCFA oxidation (Chien et al. 2000), and recently involvement of FAT/CD36 in this process has also been implicated (Campbell et al. 2004). Thus we examined whether similar mechanisms contribute to increasing LCFA oxidation during exercise in isolated mitochondria.
CPTI activity
The small reductions in M-CoA levels observed during prolonged exercise cannot fully explain the increases in fatty acid transport and oxidation that have been observed in humans during exercise (Odland et al. 1996, 1998; Dean et al. 2000; Roepstorff et al. 2005). The results from this study are the first to show a decrease in the CPTI M-CoA sensitivity following exercise in human skeletal tissue. An alteration in total CPTI protein is not a likely explanation for the decreased M-CoA kinetics given the short duration of the exercise protocol, the unaltered maximal CPTI activity, and the constant palmitoyl-CoA sensitivity. Although the M-CoA sensitivity was altered following 30 min of exercise, importantly, the M-CoA sensitivity following 120 min of cycling was further attenuated. This study clearly depicts a situation where, despite constant or elevated M-CoA levels, CPTI activity can increase fatty acid transport into mitochondria as exercise duration increases beyond 30 min.
The CPTI enzyme, although anchored to the mitochondrial outer membrane, possess two hydrophilic cytosolic domains. It has also been shown that changing the electrical charge of only a single amino acid in the C-terminus can drastically alter the sensitivity of CPTI for M-CoA (Napal et al. 2003). The existence of a cytosolic N/C interaction has been demonstrated to be required for M-CoA binding in rat liver tissue (Faye et al. 2005), but remains to be demonstrated in skeletal tissue. This interaction may create a plausible mechanism for the reduced M-CoA sensitivity observed in this study. Although this mechanism appears to exist in liver tissue, it has not been shown to occur in skeletal muscle, and currently the regulation of M-CoA kinetics in skeletal muscle remains speculative.
FAT/CD36 regulation
The LCFA transport protein FAT/CD36 has been isolated in adipocytes (Abumrad et al. 1993) and on plasma membranes (Bonen et al. 2004; Bonen et al. 2004; Coort et al. 2004), and been shown to influence the transport of LCFA across plasma membrane in both rat and human skeletal tissue. Recently, it has also been demonstrated that FAT/CD36 is present on mitochondrial membranes of rat (Campbell et al. 2004) and human (Bezaire et al. 2005) skeletal muscle. Mitochondrial FAT/CD36 was shown to be required for palmitate and palmitoylcarnitine oxidation in human skeletal muscle, and as a result it has been proposed that FAT/CD36 facilitates the transport of LCFA-carnitine to CPTII (Bezaire et al. 2005).
The palmitate oxidation results of the present study confirmed the findings of Bezaire et al. (2005) by demonstrating the necessary presence of FAT/CD36 for isolated mitochondria LCFA oxidation, and the inhibition of palmitate oxidation with the addition of SSO, a blocker of FAT/CD36. Importantly, it has previously been shown that the SSO concentration used in this study had no effect on either pyruvate or octanoate oxidation (Campbell et al. 2004; Bezaire et al. 2005). These observations demonstrate the specificity of SSO to inhibit FAT/CD36 and the importance of FAT/CD36 for the proper functioning of mitochondrial LCFA transport and oxidation during exercise. The importance of mitochondrial FAT/CD36 in a resting situation is still unclear. However, the present findings demonstrated that exercise induced an increase in mitochondrial FAT/CD36 protein content which correlated with rates of palmitate oxidation in isolated mitochondria, confirming previous results from our laboratory in rat skeletal muscle (Campbell et al. 2004). Since rat mitochondrial FAT/CD36 content increased in response to acute (30 min) muscle contraction, and the observation that total muscle FAT/CD36 was unaltered, it has been proposed that FAT/CD36 translocated to the mitochondria during exercise (Campbell et al. 2004), similar to its apparent translocation to the sarcolemma (Bonen et al. 2000). While the feasibility of translocation from an intracellular pool appears plausible, the mechanisms responsible for increased mitochondrial FAT/CD36 protein during exercise remains to be identified.
A significant correlation existed between FAT/CD36 mitochondrial protein and mitochondrial palmitate oxidation rates following 120 min of cycling, as well as when data collected at 30 min and 120 min were combined. Although Campbell et al. (2004) previously reported that mitochondrial FAT/CD36 levels from several rat tissues followed an oxidative potential hierarchy, it has been reported that resting human mitochondrial FAT/CD36 protein does not correlate with palmitate oxidation on its own (Bezaire et al. 2005). This was also observed in the present study. Potentially this discrepancy can be explained as a result of variations in the training status, oxidative potential and fibre types between subjects. The implication is that at rest, as a result of the limited requirements for fatty acid transport and oxidation, mitochondrial FAT/CD36 may not be a limiting factor. However, mitochondrial FAT/CD36 appears to add flexibility to the regulatory system by increasing translocation to the mitochondrial membrane when LCFA transport and oxidation rates are increased. During prolonged exercise a greater reliance on FAT/CD36-mediated transport may develop; however, the mechanism responsible for this alteration remains unknown.
It still remains to be shown how FAT/CD36 participates in transport at the mitochondrial membrane. In contrast to results in rat skeletal muscle, in which SSO inhibited CPTI activity (Campbell et al. 2004), the present study and that of Bezaire et al. (2005) observed that SSO did not inhibit CPTI activity in human skeletal muscle. Moreover, since SSO inhibited palmitate oxidation in isolated mitochondria, the present study supports the interpretation of the role of mitochondrial FAT/CD36 originally proposed by Bezaire et al. (2005). They suggested that FAT/CD36 is located downstream of CPTI, facilitating the transport of LCFA-carnitine from CPTI to CPTII.
In summary, we are the first to demonstrate that the sensitivity of CPTI to M-CoA decreases, and mitochondrial FAT/CD36 content increases, in response to exercise-induced increases in whole body and isolated mitochondrial LCFA oxidation. Although decreased CPTI M-CoA sensitivity coincided with increased mitochondrial FAT/CD36 protein content, it is unclear at this time if they independently increase LCFA transport. We propose that CPTI M-CoA sensitivity and mitochondrial FAT/CD36 are not redundant but rather complementary mechanisms that influence mitochondrial LCFA transport and oxidation.
References
- Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- Bergmeyer HU. Methods in Enzymatic Analysis. New York: Academic Press; 1974. [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Laboratory Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Bezaire V, Bruce CR, Heigenhauser GJF, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: Essential roles in fatty acid transport. Am J Physiol Endocrinol Metab. 2005 doi: 10.1152/ajpendo.00312.2005. in press; DOI 10.1152/ajpendo.00312.2005. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Heigenhauser GJF, Spriet LL. Regulation of CPT I activity in intermyofibrillar and subsarcolemmal mitochondria from human and rat skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E85–E91. doi: 10.1152/ajpendo.00237.2003. [DOI] [PubMed] [Google Scholar]
- Bonen A, Campbell S, Benton C, Chabowski A, Coort S, Han Z, Koonen D, Glatz J, Luiken J. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutrition Soc. 2004;63:245–249. doi: 10.1079/PNS2004331. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275:14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- Bonen A, Parolin M, Steinberg G, Calles-escandon J, Heigenhauser G, Dyck D. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- Campbell S, Tandon N, Woldegiorgis G, Luiken J, Glatz J, Bonen A. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–36241. doi: 10.1074/jbc.M400566200. [DOI] [PubMed] [Google Scholar]
- Chien D, Dean D, Saha A, Flatt J, Ruderman N. Malonyl-CoA content and fatty acid oxidation in rat muscle and liver in vivo. Am J Physiol Endocrinal Metab. 2000;279:E259–E265. doi: 10.1152/ajpendo.2000.279.2.E259. [DOI] [PubMed] [Google Scholar]
- Coort S, Luiken J, van der Vusse G, Bonen A, Glatz J. Increased FAT (fatty acid translocase)/CD36-mediated long-chain fatty acid uptake in cardiac myocytes from obese Zucker rats. Biochem Soc Trans. 2004;32:83–85. doi: 10.1042/bst0320083. [DOI] [PubMed] [Google Scholar]
- Coort SL, Willems J, Coumans WA, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem. 2002;239:213–219. [PubMed] [Google Scholar]
- Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA, Ruderman N. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- Faye A, Borthwick K, Esnous C, Price N, Gobin S, Jackson V, Zammit V, Girard J, Prip-buus C. Demonstration of N- and C-terminal domain intramolecular interactions in rat liver carnitine palmitoyltransferase 1 that determine its degree of malonyl-CoA sensitivity. Biochem J. 2005;387:67–76. doi: 10.1042/BJ20041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Yvon C, Lehr L, Leger B, Keller P, Russell A, Kuhne F, Flandin P, Giacobino JP, Muzzin P. Expression of uncoupling protein-3 in subsarcolemmal and intermyofibrillar mitochondria of various mouse muscle types and its modulation by fasting. Eur J Biochem. 2002;269:2878–2884. doi: 10.1046/j.1432-1033.2002.02953.x. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Diaz-Ricart M, Montgomery RR, Aster RG, Jamieson GA, Tandon NN. Inhibition of platelet adhesion to collagen by monoclonal anti-CD36 antibodies. Br J Haematol. 1996;92:960–967. doi: 10.1046/j.1365-2141.1996.422962.x. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Mills SE, Long CS, Foster DW. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983;214:21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napal L, Dai J, Treber M, Haro D, Marrero PF, Woldegiorgis G. Single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of rat liver carnitine palmitoyltransferase I increases its malonyl-CoA sensitivity close to that observed with the muscle isoform of the enzyme. J Biol Chem. 2003;278:34084–34089. doi: 10.1074/jbc.M305826200. [DOI] [PubMed] [Google Scholar]
- O'Neill M, Watt MJ, Heigenhauser GJ, Spriet LL. Effects of reduced free fatty acid availability on hormone-sensitive lipase activity in human skeletal muscle during aerobic exercise. J Appl Physiol. 2004;97:1938–1945. doi: 10.1152/japplphysiol.01135.2003. [DOI] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJ, Lopaschuk GD, Spriet LL. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am J Physiol. 1996;270:541–544. doi: 10.1152/ajpendo.1996.270.3.E541. [DOI] [PubMed] [Google Scholar]
- Odland LM, Howlett RA, Heigenhauser GJ, Hultman E, Spriet LL. Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am J Physiol. 1998;274:E1080–E1085. doi: 10.1152/ajpendo.1998.274.6.E1080. [DOI] [PubMed] [Google Scholar]
- Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Ramsay RR, Naismith JH. A snapshot of carnitine acetyltransferase. Trends Inbiochem Sci. 2003;28:343–346. doi: 10.1016/S0968-0004(03)00137-3. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinalmetab. 2005;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Methods Enzymol. 1969;8:3–5. [Google Scholar]
- Starritt EC, Howlett RA, Heigenhauser GJ, Spriet LL. Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E462–E462. doi: 10.1152/ajpendo.2000.278.3.E462. [DOI] [PubMed] [Google Scholar]
- van Loon LJ. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J Appl Physiol. 2004;97:1170–1187. doi: 10.1152/japplphysiol.00368.2004. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJ, O'Neill M, Spriet LL. Hormone-sensitive lipase activity and fatty acyl-CoA content in human skeletal muscle during prolonged exercise. J Appl Physiol. 2003;95:314–321. doi: 10.1152/japplphysiol.01181.2002. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E120–E127. doi: 10.1152/ajpendo.00542.2003. [DOI] [PubMed] [Google Scholar]
- Winder WW, Arogyasami J, Elayan IM, Cartmill D. Time course of exercise-induced decline in malonyl-CoA in different muscle types. Am J Physiol. 1990;259:E266–E271. doi: 10.1152/ajpendo.1990.259.2.E266. [DOI] [PubMed] [Google Scholar]
- Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S, Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol. 1997;82:219–225. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]