Abstract
To assess the contribution of central and peripheral factors to changes in maximum voluntary force and its length dependence after eccentric muscle damage, voluntary and twitch torque were measured across a wide angular range, along with voluntary activation using twitch interpolation. Isometric torque from both maximum voluntary contractions (MVCs) and paired twitches to motor nerve stimulation were measured from 60 to 150 deg elbow flexion in 10 deg increments in eight subjects. Optimal angles were determined by curve fitting. Each subject then performed eccentric contractions until voluntary torque had decreased by ∼40%. Measurements were repeated at 2 h, 1 day and 8 days post-exercise to follow acute and longer-term changes. Before exercise, the optimal angle was in the mid-range (93 ± 10 deg; mean ±s.d.) for MVCs, and at a more extended elbow angle for the twitch (106 ± 6 deg, P < 0.05). Voluntary activation was generally high (> 94%) but depended on elbow angle, with activation being ∼4% lower at the most flexed compared to the most extended angle. Two hours after exercise, MVCs decreased 40%, while twitch torque declined 70%. All subjects showed a shift in optimal angle to longer muscle lengths for MVCs (17 ± 16 deg at 2 h, 14 ± 7 deg at day 1, P < 0.05). This shift contributed minimally (∼3%) to the reduction in torque at 90 deg, as the torque–angle relation was relatively flat around the optimum. The twitch showed a smaller shift (∼4 deg) to longer lengths which was not statistically significant. Voluntary activation was significantly impaired in the early stages after exercise (2 h and day 1, P < 0.05), particularly at short muscle lengths. By 8 days after exercise, the optimal angle had returned to pre-exercise values, but MVC, twitch torque and voluntary activation had not fully recovered. Eccentric exercise causes a short-term shift in the optimal angle for MVCs and produces a length-dependent impairment in voluntary activation. Therefore, it appears that both central and peripheral factors limit muscle performance following eccentric damage, with limits to voluntary drive being especially important at short lengths.
Unaccustomed eccentric exercise commonly results in muscle damage, characterized by a prolonged reduction in voluntary force and delayed muscle soreness (Newham et al. 1983; Newham et al. 1987; Clarkson et al. 1992; Howell et al. 1993; Prasartwuth et al. 2005). Our recent study using twitch interpolation revealed that impaired voluntary activation of the muscle contributes to the reduction in maximal voluntary force after eccentric exercise. Calculation of voluntary activation (using twitch interpolation) depends on the size of both the superimposed twitch and the resting muscle twitch (e.g. Bellemare & Bigland-Ritchie, 1984; Herbert & Gandevia, 1999; Gandevia, 2001) and previous studies have shown that the resting twitch decreases more than maximal voluntary force (MVC) after eccentric exercise (Sayers et al. 2003; Prasartwuth et al. 2005). Peripheral mechanisms also contribute to the reduced voluntary force and these include disturbances to excitation–contraction coupling (Warren et al. 1993, 1999, 2001; Balnave et al. 1997) and myofibril disruption (Friden & Lieber, 2001; Proske & Morgan, 2001). One possibility that has not been fully considered is that the prolonged reduction in voluntary force and decrease in the resting twitch may be due to changes in the optimal muscle length for force production.
In animal studies an increase in optimal length has been observed following eccentric damage. This increase has been proposed to be due to an increase in series compliance from myofibril disruption involving sarcomere disorganization (Morgan, 1990; Morgan & Allen, 1999). This acute change in length has been shown in frog (Katz, 1939; Morgan et al. 1996), toad (Wood et al. 1993; Talbot & Morgan, 1996) and rat muscles (Lynn et al. 1998). A week after eccentric exercise, some reports indicate that the increase in optimal muscle length persists and this long-term shift has been attributed to an increase in the number of in-series sarcomeres (Lynn & Morgan, 1994; cf. Koh & Herzog, 1998; McHugh et al. 1999).
As it is difficult to measure in vivo sarcomere length in humans, a functional measure such as the joint angle at which peak torque occurs (termed optimal joint angle) is used. Any change in optimal angle should reflect a change in the length–tension relation, assuming there is no change in the relationship between moment-arm and joint angle. Previous studies have demonstrated shifts in the optimal angle which are consistent with longer muscle lengths after eccentric exercise. There was a shift of ∼7 deg in the optimal angle in maximal voluntary eccentric torque in hamstrings for 10 days after eccentric exercise, although voluntary torque had recovered by day 3 (Brockett et al. 2001). Maximal voluntary torque measured in elbow flexors showed a shift of ∼15 deg in the optimal angle at day 4 after eccentric exercise (see also Saxton & Donnelly, 1996; Philippou et al. 2004). The extent to which a shift in optimal angle contributes to a reduction of voluntary torque after exercise will depend on the size of the shift, the chosen test angle, and the shape of the torque–angle relation for that muscle group.
As indicated above, both peripheral and central mechanisms could contribute to any shift in the optimal length for voluntary torque. Therefore, the present study sought to clarify both mechanisms. To assess the central contribution, voluntary activation was quantified over a wide angular range before and after eccentric exercise of elbow flexors. Because twitch dynamics are faster and the force–frequency curve is shifted such that a higher frequency is required to achieve maximum force, it has been hypothesized that voluntary activation would be reduced at short length (Gandevia & McKenzie, 1988). Available evidence suggests that this effect is minimal in fresh muscle (Gandevia & McKenzie, 1988; Babault et al. 2003; Newman et al. 2003; Del Valle & Thomas, 2004; Jaskolska et al. 2005) but there are no data on changes after exercise or eccentric muscle damage. To determine the peripheral contribution, the resting twitch was measured at each angle using supramaximal stimulation of the motor nerve. Jones et al. (1997) reported that there was a transient shift of ∼4 deg in the optimal angle to a longer length for a resting twitch in human triceps surae immediately after eccentric exercise, and this disappeared by day 2.
The aims of the present study were (i) to investigate whether there is a shift in the optimal muscle length (angle) after eccentric exercise in both the maximal voluntary torque and the resting twitch, (ii) to determine how these shifts progress compared with the changes in voluntary torque over a week of recovery, (iii) to measure how much these shifts contribute to changes in torque at the original and the new optimal length, and (iv) to assess the contributions of changes in voluntary activation to a shift of the optimal muscle length for voluntary torque after eccentric exercise.
Methods
Eight healthy subjects (three female and five male) took part. Each gave written informed consent prior to the study which was approved by the institutional ethics committee and conducted according to the Declaration of Helsinki. Subjects had a mean age of 36 ± 9 years, height of 172 ± 5 cm and weight of 69 ± 11 kg. No subjects were involved in training involving eccentric exercise with their elbow flexor muscles for at least 6 months before the study.
A series of measurements were performed before (pre-exercise), 2 h after, day 1 and day 8 after eccentric exercise. The main assessments were made at 10 angles of elbow flexion between 60 deg and 150 deg, and included maximal voluntary isometric torque (MVC), the superimposed twitch evoked by motor nerve stimulation during MVCs, and the resting twitch (Fig. 1C and D). While we have not measured moment arm formally here, it is unlikely to show a major change following eccentric muscle damage and hence we regard more extended elbow angles as indicative of lengthening of the major elbow flexor muscles (see Chang et al. 1999). Here we use the term muscle damage to cover a prolonged reduction in muscle force-generating capability associated with delayed muscle pain and tenderness following unaccustomed eccentric exercise.
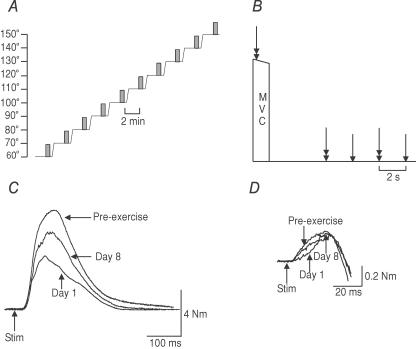
Figure 1. Measurement protocols and twitch responses of elbow flexors in one subject.
A, measurements of MVC, voluntary activation and twitch forces of elbow flexors were made at 60 deg elbow flexion and then the arm was passively extended in 10 deg steps in a custom-built myograph. Measurements were continued up to 150 deg and the sequence repeated. These sequences were performed pre-exercise, 2 h after eccentric exercise reduced the MVC by 40%, and 1 and 8 days later. Each shaded bar indicates the timing of voluntary and stimulated contractions and is expanded in panel B. B, during a MVC, paired stimuli (10 ms interval) were delivered to the motor nerve of biceps and brachialis. Paired and single stimuli were delivered after the MVC. C, resting twitches evoked by paired stimuli to the motor nerve for biceps and brachialis decreased markedly and gradually recovered by day 8. D, in this subject superimposed twitches evoked by the same stimuli during MVCs were similar in size before exercise, at day 1 and day 8.
Eccentric exercise
Controlled eccentric exercise with the elbow flexors of the non-dominant arm was used to reduce maximal voluntary isometric torque by 40%. Subjects sat with the elbow joint aligned with the axis of rotation of a pulley wheel. Subjects lowered a weight attached to the wheel (209 ± 5.2 N) from ∼60 deg flexion to full extension by eccentric contraction of the elbow flexors. The experimenter lifted the load while subjects relaxed during the shortening phase. The load was set at ∼40% of MVC at 90 deg. The exercise consisted of a series of five sets of 10 repetitions. After each five sets, subjects performed brief MVCs to monitor the reduction in maximal voluntary torque with the elbow at 90 deg flexion. Each eccentric contraction lasted 2 s with 6 s rest between repetitions and 30 s rest between sets. Contractions were performed in time with a metronome. Sets continued until the MVC fell by ∼40%. Before the exercise, subjects practiced the timing of contractions with the dominant arm.
Motor nerve stimulation
For stimulation of the motor nerve, electrical stimuli (100 µs duration, constant current, DS7, Digitimer) were delivered to intramuscular nerve fibres innervating biceps and brachialis via a surface cathode located midway between the anterior edge of the deltoid and the elbow crease and a surface anode positioned over the distal biceps tendon. Two patterns of stimulation were used: single pulses or paired pulses with a 10 ms interstimulus interval. Stimulation intensity was set 10% above the level required to produce a resting twitch of maximal amplitude. At the start of each measurement session, the stimulus intensity was set separately for each of the angles.
Maximal isometric voluntary torque, the resting twitch and voluntary activation
Subjects sat with the arm in an isometric myograph which could be set at a number of angles of flexion of the elbow. These angles ranged from 60 deg to 150 deg included angle (with 180 deg representing a straight arm). To determine the optimal angle for voluntary and twitch torque, measurements started at 60 deg and progressed in 10 deg steps to 150 deg (Fig. 1A). Subjects performed a brief MVC during which paired-pulse stimulation was delivered to evoke a superimposed twitch. When force is reduced by exercise, paired-pulse stimulation is an appropriate stimulus for estimations of voluntary activation (Edwards et al. 1977). After the MVC, a series of stimuli which comprised paired, single, paired and single pulses at 2-s intervals were delivered to the relaxed muscle (Fig. 1). The subject then remained relaxed while the arm was passively extended by 10 deg and the myograph fixed at the new angle. After 2 min, testing recommenced at the new angle. Testing through the range of angles was performed twice, separated by a 10-min rest interval. Values were averaged for the two runs.
Data analysis
Mean values were derived for all measurements within each session. Maximal voluntary torque at each angle was measured as the difference between torque at rest and the peak of the torque record. The amplitude of twitches evoked in the relaxed muscle by single or paired pulse stimulation and superimposed twitches during MVCs were also measured for each angle. Voluntary activation was quantified (Herbert & Gandevia, 1999) by comparing the amplitude of the superimposed twitch with the resting twitch evoked by paired pulse stimulation using the formula:
To determine the optimal angle for the MVC and resting twitch responses within each session, a computer-generated curve was fitted using a 4th order polynomial (mean r2= 0.94 and 0.97, respectively). Throughout the text values are given as the mean ±s.d. Statistical analysis involved one-way repeated-measures analysis of variance (ANOVA). Where significance was found, Bonferroni's post hoc comparison was used to test for significance between specific time points. The relation between voluntary activation and joint angles was assessed with Spearman's rank correlation. Statistical significance was set at the 5% level.
Results
Before eccentric exercise, the voluntary torque of elbow flexors and the twitch torque evoked by paired and single electrical stimuli depended on the length of the muscles (i.e. elbow angle). Eccentric exercise was designed to reduce the isometric maximal voluntary contraction (MVC) of the elbow flexors at 90 deg by ∼40%. Subjects required between 40 and 160 eccentric contractions to cause this reduction. At 90 deg, MVCs decreased from 53.8 ± 13.2 to 30.8 ± 4.6 N m 2 h after exercise and remained similarly depressed at day 1. The MVCs at 90 deg recovered to ∼75% of their pre-exercise value at day 8. The torque–angle curves before and after exercise are shown for a single subject (Fig. 2) and for the group of subjects (Fig. 3). Paired twitches at 90 deg decreased by ∼70% (from 12.1 ± 4.7 to 3.5 ± 1.4 N m) at 2 h and remained depressed at day 1. Twitches recovered to only ∼60% of their pre-exercise value at day 8 (Fig. 1C). Single twitches decreased markedly by 77% (from 4.4 ± 1.6 to 1.1 ± 0.3 N m) at 2 h and recovered to ∼50% of their pre-exercise value at day 8.
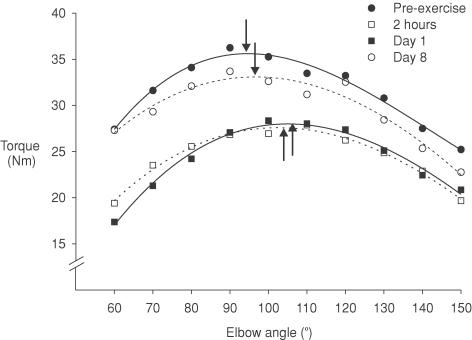
Figure 2. Maximal voluntary isometric torque–angle curves from one subject.
Data from one subject for MVCs at different elbow angles ranging from 60 deg to 150 deg before eccentric exercise (•), 2 h after (□), day 1 after (▪) and day 8 after (○). Data are the mean of two values obtained at each angle. Across the angular range, MVC was reduced at day 1 and recovered by day 8. The optimal muscle length at which the peak MVC occurred (vertical arrows) shifted acutely to a longer muscle length and returned close to the pre-exercise value at day 8.
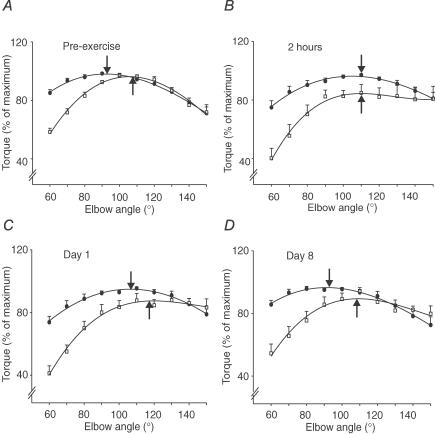
Figure 3. Group data for maximal voluntary torques and twitches from paired stimuli before eccentric exercise, 2 h, 1 and 8 days following exercise.
A, the optimal angles for MVC (•) and twitch from paired stimuli (□) before performing eccentric exercise were at 93 deg and 106 deg (arrows). Torques were normalized to their maximal values for each subject. Note that the ascending portion of the curves was steeper for the twitch than for the MVC at all time points. B, at 2 h after exercise, the optimal angles for the MVC and the twitch were at 110 deg. C, at day 1 after exercise, the optimal angle for the MVC and the twitch remained at the longer muscle length. D, at day 8 after exercise, the optimal angles for MVC and twitch were close to pre-exercise values. All data are shown as mean ±s.e.m. (n = 8).
Optimal muscle length for voluntary torques
Peak torque and optimal angle for the MVC and the twitch from paired stimuli are given in Table 1. Before eccentric exercise, the optimal angle for MVCs was 93 ± 10 deg of elbow angle (Fig. 3A). After exercise, the optimal angle was shifted to a significantly longer muscle length of 110 ± 21 deg at 2 h after exercise and 107 ± 13 deg at day 1 (Fig. 3B and C). This shift to a longer optimal length occurred in all subjects. For the group the shift was 17 ± 16 deg and 14 ± 7 deg at 2 h and day 1, respectively (Fig. 4A). By 8 days after exercise, the optimal angle had returned to pre-exercise values (Fig. 3D, Table 1). The MVC produced at 90 deg decreased by 41 ± 10% at 2 h and 44 ± 4% at day 1. These values were only ∼3% lower than the torque at the new optimal angle, which decreased by 38 ± 3% at 2 h and 42 ± 4% at day 1. Values at the two angles were not significantly different (Fig. 4B).
Table 1.
Changes in peak torque for maximal voluntary contractions (MVCs) and electrically evoked contractions (PS = paired stimuli, SS = single stimuli) before and after eccentric exercise (2 h, day 1, day 8)
| Pre-ex | 2 h | Day 1 | Day 8 | |
|---|---|---|---|---|
| Peak torque (Nm) | ||||
| MVC | 54.2 ± 13.1 | 32.8 ± 6.3** | 31.0 ± 7.3** | 42.1 ± 6.3** |
| PS | 12.9 ± 5.1 | 4.1 ± 1.8** | 4.3 ± 2.0** | 7.7 ± 4.2** |
| SS | 5.1 ± 2.0 | — | — | — |
| Optimal angle (deg) | ||||
| MVC | 93.5 ± 10.2 | 110.2 ± 21.0* | 107. ± 13.4* | 92.7 ± 14.8 |
| PS | 106.4 ± 6.2 | 110.1 ± 17.1 | 114.5 ± 14.3 | 108.5 ± 13.2 |
| SS | 119.2 ± 9.4 | — | — | — |
Values shown are the mean ±s.d. for the group of 8 subjects.
P < 0.05, comparison with pre-exercise value
P < 0.01, comparison with pre-exercise value.
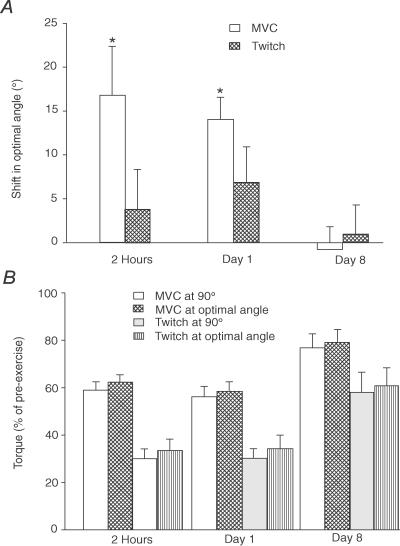
Figure 4. Shift in the optimal angle for maximal voluntary torque and the twitch torque evoked by paired stimuli.
A, over the first 24 h after eccentric exercise, the optimal angle shifted to a significantly longer length for MVC (open bars). The trend for a shift in the twitch was not significant (cross-hatched bars). Optimal angles were the same as pre-exercise values at day 8. B, voluntary torque and twitch evoked by paired stimuli at 90 deg and at the optimal angle. For the MVCs and twitches, there was no significant difference between torque at 90 deg and at the optimal angle at 2 h, day 1 and day 8. All data are shown as the mean ±s.e.m. (n = 8). Asterisks indicate a significant change from pre-exercise values.
Optimal muscle length for the resting twitches
Before eccentric exercise, the optimal angle for paired and single twitches was 106 ± 6 deg and 119 ± 9 deg of elbow angle, respectively (Fig. 3A, Table 1). For the paired twitch (interstimulus interval 10 ms), this shifted to longer lengths by 4 deg to 110 ± 17 deg at 2 h after exercise and by 8 deg to 114 ± 14 deg at day 1 (Fig. 3B and C). These shifts were not significantly different for the group from pre-exercise values. However, there was a tendency for the twitch torques to plateau (not decrease) at lengths beyond the optimal angle. The optimal angle had returned to the pre-exercise value after 8 days (Fig. 3D). For single twitches, the optimal angle was 119 ± 9 deg before exercise, but the optimum could not be determined reliably after exercise as the responses tended to increase in amplitude at the most extended angles. Comparison of the size of the paired twitches at 90 deg (decreased by 70 ± 4% at 2 h and 69 ± 4% at day 1) and at the new optimal angles (decreased by 67 ± 5% at 2 h and 66 ± 6% at day 1) showed no significant differences (Fig. 4B).
Voluntary activation
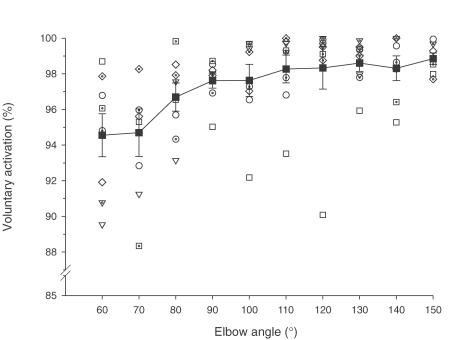
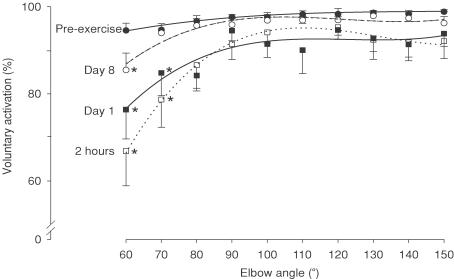
Prior to eccentric exercise, voluntary activation tested with motor nerve stimulation during brief MVCs was high across the range of test angles (> 94%). For the group, voluntary activation tended to be slightly lower at the shortest test length (60 deg). Although this was not statistically significant in a repeated measures ANOVA, a Spearman rank correlation on the means showed an association (P < 0.001). Furthermore, 5 of the 8 subjects had lower activation at short lengths before exercise when assessed individually (Rs; P < 0.05, Fig. 5). In the early stages (2 h and day 1) after exercise voluntary activation was markedly impaired over the angular range (P < 0.001). This was most evident at short lengths, being significantly lower (by 15–20%) at 60 deg and 70 deg compared to pre-exercise (P < 0.001, Fig. 6). Thus, there was an initial length dependence of voluntary activation and this increased after exercise. By day 8, voluntary activation had almost returned to pre-exercise levels although significant depression remained at 60 deg.
Figure 5. Voluntary activation of elbow flexors before exercise for individual subjects and the group.
Data for voluntary activation in MVCs for individual subjects obtained under control conditions are plotted across the angular range. Each open or dotted-open symbol shows data from an individual subject. Five of eight subjects had significantly lower voluntary activation at short lengths (see text). The mean for the group is also shown (filled squares with lines). At short lengths, voluntary activation was reduced for the group (P < 0.05).
Figure 6. Voluntary activation of elbow flexors across the angular range before and after eccentric exercise.
Voluntary activation during MVCs before exercise (•) was high across the angular range (see Fig. 5). However, after exercise voluntary activation was impaired (P < 0.001), especially at short muscle lengths (60 deg and 70 deg) at 2 h (□) and at day 1 (▪), and only at 60 deg at day 8 (○). Data are shown as the mean ±s.e.m. (n = 8). Asterisks indicate a significant change from pre-exercise values at specific angles.
Discussion
When maximal voluntary torque is reduced by unaccustomed eccentric exercise of the elbow flexor muscles, both voluntary torque and twitch torque remain low for several days if measurements are made at a fixed joint angle (90 deg of elbow flexion). The present study investigated whether changes in length–tension properties were important in this loss of torque, and assessed the contribution of length-dependent changes in voluntary activation.
Shifts in the optimal muscle length were found for the MVCs after exercise up to day 1, and returned to pre-exercise values at day 8. However, due to the relatively flat angle–torque relation over the mid-range of angles, these shifts contributed minimally (3%) to the drop in torque at the original test angle (90 deg). Voluntary activation has been found for the first time to be impaired at short muscle lengths and it was further reduced after exercise. The following discussion will focus on these length-dependent changes in voluntary torque, twitch-evoked torque, and voluntary activation after eccentric exercise.
Length dependence of voluntary and twitch torque
Before exercise, maximal voluntary torque and twitch torque evoked by paired- and single-pulse electrical stimulation all showed the classical dependence on muscle length. Similar findings have been reported previously for human elbow flexors (e.g. Hansen et al. 2003). Factors that contribute to the torque–angle relationship of a muscle group include sarcomere length (Gordon et al. 1966; Herzog et al. 1992), the length–tension properties of the series elastic component, muscle architecture, and the relationship between moment arm and joint angle (An et al. 1981, 1989).
For MVCs, the optimal elbow angle was 93 ± 10 deg with similar slopes for the ascending and descending limbs. The optimal angle for the paired-pulse twitches was at a more extended elbow angle (106 ± 7 deg), with a further extension of optimal angle for single twitches (119 ± 9 deg). Similar findings have been reported previously in animal muscles (Rack & Westbury, 1969; Roszek et al. 1994) and in human elbow flexors (Hansen et al. 2003). Comparison of curves for MVCs and resting twitches showed that at short muscle lengths, the twitches produced a lower fraction of the torque generated at the optimal joint angle compared to the voluntary torque. This difference may be related to the reduced ability of the twitch contractions to take up the compliance from the non-contractile elements of the muscle and tendon. A difference in the length dependence of calcium sensitivity between twitches and high-frequency contractions might also contribute (Roszek et al. 1994). In addition, voluntary torques reflect the sum of torques produced by all elbow flexors (An et al. 1989), whereas that of evoked twitches includes torque produced only by biceps and brachialis.
As in our previous study (Prasartwuth et al. 2005), eccentric exercise of the elbow flexors was set individually to reduce each subject's isometric MVC by 40% with the elbow flexed to 90 deg. At this angle, the single twitches decreased by 77 ± 10% and the paired-pulse twitches by 70 ± 12%. As there was no recovery of voluntary or twitch torque between 2 and 24 h after the exercise, the drops in torque could not be explained by metabolic fatigue but were primarily due to muscle damage. The prolonged force deficit is likely to be the direct or indirect result of mechanical (sarcomere) disruption (Morgan, 1990; Jones et al. 1997; Morgan & Allen, 1999), including an impairment in the excitation–contraction coupling process (Warren et al. 2001).
The present study found that the optimal angle shifted acutely to longer muscle lengths (by 17 ± 16 deg) for the MVC 2 h after exercise and remained longer at day 1. Similarly shifts have been reported previously (Saxton & Donnelly, 1996; Jones et al. 1997; Brockett et al. 2001; Philippou et al. 2004). It has been proposed that eccentric contractions disrupt some sarcomeres and increase their compliance so that longer muscle lengths are needed for active sarcomeres to reach their optimal lengths. Alternatively, calcium sensitivity is known to be muscle length dependent (Balnave & Allen, 1996), and calcium release has been shown to be reduced following eccentric contractions (Balnave & Allen, 1995) so this could conceivably shift optimal length. Such calcium-related effects following eccentric contractions may or may not be a direct reflection of the proposed sarcomere disruption. Other less plausible possibilities include a sustained increase in the compliance of the series elastic component, or a change in the relationship between moment-arm and joint angle.
For paired stimuli, shifts were not significant being smaller and more variable, though similar in direction and magnitude to reports for human ankle extensors (Jones et al. 1997). Twitch torques tended to plateau at longer muscle lengths after exercise, which may explain the greater variability in shifts compared to voluntary contractions. The altered shape of the twitch torque–angle curves after eccentric exercise means that comparison of shifts for voluntary and twitch curves should be made with caution. One factor that might contribute to the failure to find a descending limb for the single twitch is spread of the stimulus to additional muscles at extended joint angles. Local oedema may exacerbate this problem after eccentric exercise (Shellock et al. 1991; Nurenberg et al. 1992). A second factor is that eccentric exercise has different effects on the length dependence of calcium sensitivity for twitch or low-frequency stimuli compared to high-frequency stimuli (Balnave & Allen, 1996). Finally the increase in passive tension after eccentric exercise which has been attributed to ‘contraction clots’ (Whitehead et al. 2001, 2003) may also contribute.
Although the optimal angles for torque shifted, the changes contributed little to the prolonged reduction of voluntary torque and marked drop in the resting twitch seen in the mid-range after eccentric exercise. When the voluntary and twitch torques produced at the new optimal angles were compared to those produced at 90 deg, the difference was minimal (only 2–3%). The torque–angle relationship is relatively flat around 90 deg (mid-range) for the elbow flexors. As a consequence, a shift does not produce much force drop, so underestimation of the force deficit is small if measurements are made only at 90 deg elbow flexion. Such a shift in optimal angle would be more important if initial measurements had been made at shorter muscle length, or if the muscle group being tested had a narrower torque–angle relationship.
Length dependence of voluntary activation
As the MVC depends on voluntary activation as well as the force-generating capacity of the muscle, voluntary activation must also be considered. Prior to eccentric exercise, voluntary activation tended to be lower at flexed elbow angles, with a difference of about 4% between the most flexed and most extended angles. Length dependence of voluntary activation has not been reported previously. The reason for poor voluntary activation at flexed angles is not clear. However, twitches are smaller and shorter in duration at shorter muscle lengths and require higher frequencies to produce tetanic forces in stimulated contractions (Rack & Westbury, 1969; Gandevia & McKenzie, 1988). This suggests that more neural drive could be required to engage the muscle maximally when it is short. As motor unit firing rates are not higher during voluntary isometric contractions at short muscle lengths (Bigland-Ritchie et al. 1992; Del Valle & Thomas, 2004), lower voluntary activation would be predicted. Surprisingly, previous studies of voluntary activation in limb muscles show little change in activation for triceps brachii, adductor digiti minimi, tibialis anterior and quadriceps at short muscle length (Gandevia & McKenzie, 1988; Newman et al. 2003; Del Valle & Thomas, 2004; Kooistra et al. 2005). This may be due to the relatively small changes over the range of angles studied and the variability of voluntary activation within and between subjects. In contrast to limb muscles, voluntary activation of the diaphragm declines at long muscle lengths (McKenzie et al. 1996).
After exercise, voluntary activation was significantly impaired, being worse at the most flexed angles (60 deg and 70 deg). Thus, force production may be more sensitive to decreases in neural drive when the muscle is short. Increased length dependence in the ability to drive the muscle maximally might contribute to the shift of optimal angle for voluntary torque. To assess this, voluntary torque–angle curves were re-fitted to exclude the short lengths (60 deg and 70 deg). Presumably due to the steepness and consistency of the ascending limb of the curve, elimination of these values for short lengths did not affect the optimal angles or the size of the shift in optimal angle after eccentric exercise. Thus the shift in optimal length for maximal voluntary torque after eccentric muscle damage represents more than a decrease in voluntary activation at short lengths.
In the present study, values for voluntary activation after eccentric exercise were higher (90% at 90 deg) than for our previous study using the same exercise protocol (75% at 90 deg (Prasartwuth et al. 2005). The difference in the current study was the use of paired pulses for estimation of voluntary activation, instead of single pulses. While the use of single or paired stimuli should not be a factor in estimation of voluntary activation for undamaged muscles (Herbert & Gandevia, 1999), it does seem to lead to differences after eccentric damage. The disproportionate loss of twitch force compared to voluntary force was less marked for paired pulses. Thus paired pulses may be better suited to estimates of voluntary activation following eccentric contractions, since they are a closer approximation to a tetanic contraction.
Longer-term recovery after eccentric exercise
It has been proposed that during recovery from eccentric exercise, extra sarcomeres are added to the myofibrils to increase optimal muscle length and protect against further eccentric damage (Lynn & Morgan, 1994; Lynn et al. 1998). Two shifts in optimal length have been proposed: an acute shift due to disrupted sarcomeres, and a longer-term shift reflecting longitudinal addition of sarcomeres. In humans, one previous study has reported indirect evidence of this in hamstrings, measured as a sustained increase in optimal muscle length when voluntary force had recovered (Brockett et al. 2001). As judged by shifts in the optimal length for MVCs, we found no evidence that sarcomeres were added by day 8. At this time, peak torque occurred at its original optimal length, although MVC and twitch torques were still significantly decreased. Thus muscle recovery was not complete, so that later addition of sarcomeres cannot be ruled out.
The twitch torque was still reduced by 50% at day 8, compared to a 20% reduction for the MVC. Thus, in terms of assessment, twitch force may be a more sensitive indicator of complete recovery following eccentric exercise than the MVC. Furthermore, this disproportionate loss of force for single and paired twitches may represent greater deficits in force for submaximal contractions than would be predicted from an MVC. In functional terms, MVCs may underestimate the true deficit in muscle performance for everyday tasks after muscle damage, and these tasks may be especially difficult to perform at short muscle lengths.
In summary, both maximal voluntary torque and voluntary activation change with muscle length (joint angle) for elbow flexors after damaging eccentric exercise: the optimal angle shifts to longer lengths for MVCs and voluntary activation is impaired, particularly at short muscle lengths. By day 8, optimal angle recovers to pre-exercise values, while both maximal voluntary and twitch evoked torque are not fully recovered and voluntary activation at the shortest test length is still impaired. Both central and peripheral factors contribute to the loss of muscle force after eccentric damage, with a clear muscle length dependence in the impairment of voluntary drive.
Acknowledgments
This work was supported by the National Health and Medical Research Council (of Australia). We are grateful to Professor Uwe Proske for helpful comments on the draft manuscript.
References
- An KN, Hui FC, Morrey BF, Linschied RL, Chao EY. Muscles across the elbow joint: a biomechanical analysis. J Biomech. 1981;14:659–669. doi: 10.1016/0021-9290(81)90048-8. [DOI] [PubMed] [Google Scholar]
- An KN, Kaufman KR, Chao EY. Physiological considerations of muscle force through the elbow joint. J Biomech. 1989;22:1249–1256. doi: 10.1016/0021-9290(89)90227-3. [DOI] [PubMed] [Google Scholar]
- Babault N, Pousson M, Michaut A, Van Hoecke J. Effect of quadriceps femoris muscle length on neural activation during isometric and concentric contractions. J Appl Physiol. 2003;94:983–990. doi: 10.1152/japplphysiol.00717.2002. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol. 1995;488:25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal. J Physiol. 1996;492:705–713. doi: 10.1113/jphysiol.1996.sp021339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave CD, Davey DF, Allen DG. Distribution of sarcomere length and intracellular calcium in mouse skeletal muscle following stretch-induced injury. J Physiol. 1997;502:649–659. doi: 10.1111/j.1469-7793.1997.649bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare F, Bigland-Ritchie B. Assessment of human diaphragm strength and activation using phrenic nerve stimulation. Respir Physiol. 1984;58:263–277. doi: 10.1016/0034-5687(84)90003-3. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Furbush FH, Gandevia SC, Thomas CK. Voluntary discharge frequencies of human motoneurons at different muscle lengths. Muscle Nerve. 1992;15:130–137. doi: 10.1002/mus.880150203. [DOI] [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Med Sci Sports Exerc. 2001;33:783–790. doi: 10.1097/00005768-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Chang YW, Su FC, Wu FW, An KW. Optimum length of muscle contraction. Clin Biomech. 1999;14:537–542. doi: 10.1016/s0268-0033(99)00014-5. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24:512–520. [PubMed] [Google Scholar]
- Del Valle A, Thomas CK. Motor unit firing rates during isometric voluntary contractions performed at different muscle lengths. Can J Physiol Pharmacol. 2004;82:769–776. doi: 10.1139/y04-084. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand. 2001;171:321–326. doi: 10.1046/j.1365-201x.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK. Activation of human muscles at short muscle lengths during maximal static efforts. J Physiol. 1988;407:599–613. doi: 10.1113/jphysiol.1988.sp017434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EA, Lee HD, Barrett K, Herzog W. The shape of the force-elbow angle relationship for maximal voluntary contractions and sub-maximal electrically induced contractions in human elbow flexors. J Biomech. 2003;36:1713–1718. doi: 10.1016/s0021-9290(03)00167-2. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol. 1999;82:2271–2283. doi: 10.1152/jn.1999.82.5.2271. [DOI] [PubMed] [Google Scholar]
- Herzog W, Kamal S, Clarke HD. Myofilament lengths of cat skeletal muscle: theoretical considerations and functional implications. J Biomech. 1992;25:945–948. doi: 10.1016/0021-9290(92)90235-s. [DOI] [PubMed] [Google Scholar]
- Howell JN, Chleboun G, Conatser R. Muscle stiffness, strength loss, swelling and soreness following exercise-induced injury in humans. J Physiol. 1993;464:183–196. doi: 10.1113/jphysiol.1993.sp019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskolska A, Kisiel K, Adach Z, Jaskolski A. The influence of elbow joint angle on different phases of relaxation from maximal voluntary contraction. Biol Sport. 2005;22:89–104. doi: 10.1139/h00-030. [DOI] [PubMed] [Google Scholar]
- Jones C, Allen T, Talbot J, Morgan DL, Proske U. Changes in the mechanical properties of human and amphibian muscle after eccentric exercise. Eur J Appl Physiol Occup Physiol. 1997;76:21–31. doi: 10.1007/s004210050208. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, Herzog W. Eccentric training does not increase sarcomere number in rabbit dorsiflexor muscles. J Biomech. 1998;31:499–501. doi: 10.1016/s0021-9290(98)00032-3. [DOI] [PubMed] [Google Scholar]
- Kooistra RD, de Ruiter CJ, de Haan A. Muscle activation and blood flow do not explain the muscle length-dependent variation in quadriceps isometric endurance. J Appl Physiol. 2005;98:810–816. doi: 10.1152/japplphysiol.00712.2004. [DOI] [PubMed] [Google Scholar]
- Lynn R, Morgan DL. Decline running produces more sarcomeres in rat vastus intermedius muscle fibers than does incline running. J Appl Physiol. 1994;77:1439–1444. doi: 10.1152/jappl.1994.77.3.1439. [DOI] [PubMed] [Google Scholar]
- Lynn R, Talbot JA, Morgan DL. Differences in rat skeletal muscles after incline and decline running. J Appl Physiol. 1998;85:98–104. doi: 10.1152/jappl.1998.85.1.98. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Connolly DA, Eston RG, Gleim GW. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 1999;27:157–170. doi: 10.2165/00007256-199927030-00002. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Allen GM, Gandevia SC. Reduced voluntary drive to the human diaphragm at low lung volumes. Respir Physiol. 1996;105:69–76. doi: 10.1016/0034-5687(96)00021-7. [DOI] [PubMed] [Google Scholar]
- Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Allen DG. Early events in stretch-induced muscle damage. J Appl Physiol. 1999;87:2007–2015. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Claflin DR, Julian FJ. The effects of repeated active stretches on tension generation and myoplasmic calcium in frog single muscle fibres. J Physiol. 1996;497:665–674. doi: 10.1113/jphysiol.1996.sp021798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham DJ, Jones DA, Clarkson PM. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol. 1987;63:1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Newham DJ, McPhail G, Mills KR, Edwards RH. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Newman SA, Jones G, Newham DJ. Quadriceps voluntary activation at different joint angles measured by two stimulation techniques. Eur J Appl Physiol. 2003;89:496–499. doi: 10.1007/s00421-003-0836-0. [DOI] [PubMed] [Google Scholar]
- Nurenberg P, Giddings CJ, Stray-Gundersen J, Fleckenstein JL, Gonyea WJ, Peshock RM. MR imaging-guided muscle biopsy for correlation of increased signal intensity with ultrastructural change and delayed-onset muscle soreness after exercise. Radiology. 1992;184:865–869. doi: 10.1148/radiology.184.3.1509081. [DOI] [PubMed] [Google Scholar]
- Philippou A, Bogdanis GC, Nevill AM, Maridaki M. Changes in the angle-force curve of human elbow flexors following eccentric and isometric exercise. Eur J Appl Physiol. 2004;93:237–244. doi: 10.1007/s00421-004-1209-z. [DOI] [PubMed] [Google Scholar]
- Prasartwuth O, Taylor JL, Gandevia SC. Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J Physiol. 2005;567:337–348. doi: 10.1113/jphysiol.2005.087767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack PM, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969;204:443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszek B, Guus CB, Huijing PA. Decreasing stimulation frequency-dependent length-force characteristics of rat muscle. J Appl Physiol. 1994;77:2115–2124. doi: 10.1152/jappl.1994.77.5.2115. [DOI] [PubMed] [Google Scholar]
- Saxton JM, Donnelly AE. Length-specific impairment of skeletal muscle contractile function after eccentric muscle actions in man. Clin Sci. 1996;90:119–125. doi: 10.1042/cs0900119. [DOI] [PubMed] [Google Scholar]
- Sayers SP, Peters BT, Knight CA, Urso ML, Parkington J, Clarkson PM. Short-term immobilization after eccentric exercise. Part I: contractile properties. Med Sci Sports Exerc. 2003;35:753–761. doi: 10.1249/01.MSS.0000064932.55998.CC. [DOI] [PubMed] [Google Scholar]
- Shellock FG, Fukunaga T, Mink JH, Edgerton VR. Exertional muscle injury: evaluation of concentric versus eccentric actions with serial MR imaging. Radiology. 1991;179:659–664. doi: 10.1148/radiology.179.3.2027970. [DOI] [PubMed] [Google Scholar]
- Talbot JA, Morgan DL. Quantitative analysis of sarcomere non-uniformities in active muscle following a stretch. J Muscle Res Cell Motil. 1996;17:261–268. doi: 10.1007/BF00124247. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exerc Sport Sci Rev. 2001;29:82–87. doi: 10.1097/00003677-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Shah SJ, Armstrong RB. Uncoupling of in vivo torque production from EMG in mouse muscles injured by eccentric contractions. J Physiol. 1999;515:609–619. doi: 10.1111/j.1469-7793.1999.609ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Hayes DA, Karwoski CJ, Prior BM, Armstrong RB. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol. 1993;468:487–499. doi: 10.1113/jphysiol.1993.sp019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead NP, Morgan DL, Gregory JE, Proske U. Rises in whole muscle passive tension of mammalian muscle after eccentric contractions at different lengths. J Appl Physiol. 2003;95:1224–1234. doi: 10.1152/japplphysiol.00163.2003. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Weerakkody NS, Gregory JE, Morgan DL, Proske U. Changes in passive tension of muscle in humans and animals after eccentric exercise. J Physiol. 2001;533:593–604. doi: 10.1111/j.1469-7793.2001.0593a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Morgan DL, Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. Am J Physiol. 1993;265:792–800. doi: 10.1152/ajpcell.1993.265.3.C792. [DOI] [PubMed] [Google Scholar]