Abstract
PURPOSE
To use diffusion-tensor magnetic resonance (MR) imaging to measure involvement of normal-appearing white matter (WM) immediately adjacent to multiple sclerosis (MS) plaques and thus redefine actual plaque size on diffusion-tensor images through comparison with T2-weighted images of equivalent areas in healthy volunteers.
MATERIALS AND METHODS
Informed consent was not required given the retrospective nature of the study on an anonymized database. The study complied with requirements of the Health Insurance Portability and Accountability Act. Twelve patients with MS (four men, eight women; mean age, 35 years) and 14 healthy volunteers (six men, eight women; mean age, 25 years) were studied. The authors obtained fractional anisotropy (FA) values in MS plaques and in the adjacent normal-appearing WM in patients with MS and in equivalent areas in healthy volunteers. They placed regions of interest (ROIs) around the periphery of plaques and defined the total ROIs (ie, plaques plus peripheral ROIs) as abnormal if their mean FA values were at least 2 standard deviations below those of equivalent ROIs within equivalent regions in healthy volunteers. The combined area of the plaque and the peripheral ROI was compared with the area of the plaque seen on T2-weighted MR images by means of a Student paired t test (P = .05).
RESULTS
The mean plaque size on T2-weighted images was 72 mm2 ± 21 (standard deviation). The mean plaque FA value was 0.285 ± 0.088 (0.447 ± 0.069 in healthy volunteers [P < .001]; mean percentage reduction in FA in MS plaques, 37%). The mean plaque size on FA maps was 91 mm2 ± 35, a mean increase of 127% compared with the size of the original plaque on T2-weighted images (P = .03).
CONCLUSION
A significant increase in plaque size was seen when normal-appearing WM was interrogated with diffusion-tensor MR imaging. This imaging technique may represent a more sensitive method of assessing disease burden and may have a future role in determining disease burden and activity.
Magnetic resonance (MR) imaging is widely used in the evaluation of multiple sclerosis (MS). When lesion burden is assessed with standard T2-weighted techniques, findings do not correlate well with the clinical manifestations of disease activity (1-3). This suggests that there may be more widespread disease that is not appreciable with conventional MR imaging techniques and that identification of such regions on imaging studies might produce a closer correlation with clinical status. A more sensitive method of determining the true disease burden may enable more accurate evaluation of response to therapy and provide additional information to help optimize clinical management. Both diffusion-tensor imaging and MR spectroscopy have shown involvement of the normal-appearing cerebral white matter (WM) in subjects with MS, which suggests that these methods may be more sensitive than the currently available techniques and may therefore be useful additions to the imaging assessment of demyelinating conditions (1,4-8).
Diffusion-tensor imaging is a relatively recently developed MR technique that can provide in vivo data regarding the microarchitecture of the brain. Measurement of fractional anisotropy (FA), or the directional component of water diffusion in vivo, can provide information about the integrity of WM tracts. Intact WM tracts are highly anisotropic owing to the high level of organization of myelinated fibers. Myelin and cell membranes adjacent to nerve axons restrict diffusion of water molecules across the fiber pathway, thereby resulting in preferential microscopic movement of water along the long axis of myelinated fibers (9). In disease processes that disrupt the integrity of myelin sheaths and/or nerve axons, such as MS or Wallerian degeneration, the restriction of water diffusion across the fiber tract is reduced, so FA values would be expected to decrease.
Previous studies have shown that FA values are lowered in normal-appearing WM adjacent to MS plaques (4), raising the possibility that the true plaque size is underestimated with current T2-weighted techniques. Currently, many clinical trials of therapeutic agents for MS use total plaque volume as an important indicator of disease burden and response to therapy. Redefinition of plaque size with diffusion-tensor imaging may have an important effect on the conduct and interpretation of such clinical trials. We hypothesized that the area of focal WM abnormality, or plaque size (we use the histopathologic term “plaque” to refer to focal derangements of WM identified with MR imaging), would be greater on diffusion-tensor images than on conventional MR images. Thus, the purpose of our study was to use diffusion-tensor MR imaging to measure involvement of normal-appearing WM immediately adjacent to MS plaques and thus redefine the actual plaque size on diffusion-tensor MR images.
MATERIALS AND METHODS
Subjects
Our medical center's institutional review board approved this study. Informed consent was not required, given the retrospective nature of the study on an anonymized database. The study complied with requirements of the Health Insurance Portability and Accountability Act. Inclusion criteria comprised adult subjects with confirmed clinical diagnoses of MS who had undergone clinical MR imaging, including a diffusion-tensor sequence of the brain. The minimal plaque size for inclusion was 50 mm2. This size was chosen to limit any effects on FA measurements due to partial volume averaging with normal brain. A retrospective review of data from patients with the clinical diagnosis of MS who underwent clinical MR imaging with diffusion-tensor imaging at our institution during the period August 2000 to April 2003 revealed 12 patients who met our criteria (eight women, four men; mean age, 35 years; range, 25–54 years).
Fourteen volunteers who had undergone diffusion-tensor MR imaging of the brain served as our control group (eight women, six men; mean age, 25 years; range, 21–60 years). Volunteers had no pertinent medical history of neurologic or cognitive abnormality, gave informed consent before enrollment, and were imaged with institutional review board approval.
Imaging
Conventional MR imaging included transverse T2-weighted imaging with the following parameters: 2800/100 (repetition time msec/echo time msec), 22-cm2 field of view, 256 × 192 matrix, 5-mm section thickness, and 2.5-mm intersection gap. Transverse T1-weighted MR imaging was also performed before and after administration of gadopentetate dimeglu-mine (Magnevist; Berlex Laboratories, Montville, NJ). The presence of lesions representative of MS plaques on the T2-weighted images was determined by consensus between two authors (S.M.K. and Y.K.). Lesions on T2-weighted images were considered to represent MS plaques if they were oval, were oriented toward or abutting the lateral ventricles, and had no evidence of restricted diffusion. This last criterion was included to avoid erroneously including an acute lacunar infarct in the analysis. Lesions immediately adjacent to the cortical surface were excluded to avoid potential partial volume averaging effects from adjacent gray matter. All lesions on T2-weighted images that met these criteria were studied. The presence of plaque contrast enhancement and hypointense appearance on T1-weighted images were recorded. The presence or absence of enhancement in the normal-appearing WM adjacent to lesions on T2-weighted images was also recorded to evaluate for possible active demyelination without associated T2 signal change.
Diffusion-tensor MR images of the entire brain were acquired in six orthogonal directions on a 1.5-T MR imager (Signa; GE Medical Systems, Milwaukee, Wis) with a standard head coil (40 × 20-cm field of view, 128 × 64 matrix, 5-mm sections, 2.5-mm gaps, four signals acquired). The diffusion-tensor MR imaging protocol consisted of a single-shot spin-echo echo-planar sequence acquired at 12 000/107 with one signal acquired. Diffusion-sensitizing gradient encoding was applied on separate images in six directions by using a diffusion-weighted factor b of 1000 sec/mm2. The raw diffusion-tensor data were transferred to an independent workstation (Advantage Windows; GE Medical Systems) and processed with Functool software (GE Medical Systems) to generate the maps of FA.
FA Calculations and Image Analysis
We used FA as the index of anisotropy because it is generally considered to be a robust measure of anisotropy and is also the most widely used anisotropy index, permitting comparison with data from other published studies. FA also has the advantages of good contrast between white and gray matter and high contrast-to-noise ratio (10). FA represents the anisotropic portion of total diffusion, and values range from 0 to 1, where 0 represents isotropic diffusion and 1 represents extremely anisotropic diffusion. The FA value is unitless because it represents a ratio of diffusion coefficients. The calculations for FA were performed for each voxel and displayed as an anisotropy map, which was appropriately scaled for display.
Plaques were first identified on the T2-weighted image. The T2-weighted image and the corresponding diffusion-tensor (B0) image were then compared side by side, and oval regions of interest (ROIs) conforming to the size and shape of the MS plaques on T2-weighted images were placed on the diffusion-tensor image by one trained radiologist (Y.K., 6 years experience in brain MR imaging); this allowed measurement of FA changes within plaques on the corresponding FA maps. Careful attention was paid to ensure that the ROIs conformed to the shapes of the plaques as seen on T2-weighted images. The poorer resolution of diffusion-tensor images compared with T2-weighted images results in some indistinctness of the plaque margin. To avoid underestimating original plaque size, we therefore chose to include the entire area that had elevated signal intensity on the diffusion-tensor images.
The ROIs were drawn around the boundary of plaques, and, consequently, ROI size varied according to plaque size, with a range of 50–118 mm2. The FA values within regions conforming to the plaque sites were compared (by Y.K.) with FA values in identical ROIs placed at analogous regions on normal-appearing WM in the healthy volunteers. Because it is well known that FA values differ according to location within the brain, comparison ROIs were placed in brain sites of healthy volunteers in the same location relative to the index plaque in patients with MS. In each case, the analogous ROI was placed on MR images of all 14 healthy volunteers and the mean FA value for all 14 was obtained. These ROIs were placed with reference to anatomic landmarks, such as the lateral ventricles, and readily identifiable sulci, such as the calcarine sulcus or the central sulcus. There were no cases of marked cerebral atrophy in either the patient group or the healthy volunteer group, which could have represented a possible source of error in the placement of equivalent ROIs. A fellowship-trained neuroradiologist (S.M.K., 6 years experience in brain MR imaging) confirmed ROI positioning.
FA values in normal-appearing WM regions adjacent to plaques were determined as follows. After placing on FA maps an ROI around a plaque (hereafter referred to as the “central ROI”), we placed six standard oval ROIs in normal-appearing WM adjacent to the central ROI (Y.K.). The size of the adjacent ROIs (hereafter referred to as “peripheral ROIs”) was preestablished at a set size relative to the central ROI; the short-axis diameter of each peripheral ROI was half that of the central ROI. These peripheral ROIs were placed in six predefined WM periplaque locations around the margins of the central ROI to encompass most of the periplaque region (Figure). Peripheral ROIs were not placed if they were too close to gray matter, cerebrospinal fluid, or another MS plaque, to avoid potentially confounding results. Potentially confounding peripheral ROIs were not placed in eight patients. The FA value in each peripheral ROI was recorded. Then ROIs were placed in the identical locations relative to the central ROI in 14 healthy volunteers, and the mean FA value and standard deviation (SD) were recorded for each location.
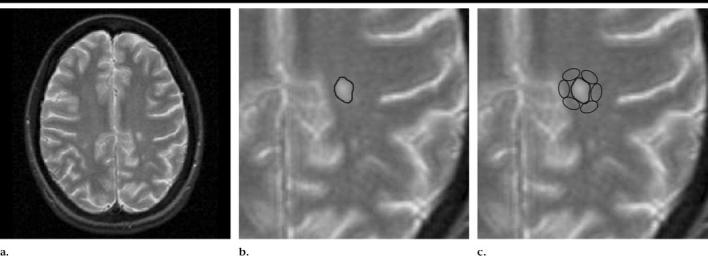
Conventional and diffusion-tensor MR images show the process used to compare regions of abnormal WM. (a) Transverse T2-weighted MR image (2800/100, 5-mm section thickness) with a focus of hyperintensity representing a plaque of demyelination in the left centrum semiovale. (b) Magnified image of a shows an ROI that has been placed to encompass the MS plaque. (c) On another magnified image of a, the area of abnormal WM, as indicated by FA values, is mapped by placing small ROIs around the MS plaque in predefined sites, as shown. The ROIs in this figure have been placed on the T2-weighted image for the purpose of clarity.
Statistical Analysis
Increase in plaque size on FA maps was determined by defining as abnormal those peripheral ROIs that were at least 2 SDs below the mean FA value for the equivalent ROIs in healthy subjects. Comparison of each ROI in the index patient to its analogous ROI in the healthy volunteers allowed us to control for healthy variability of FA in different parts of the brain. The areas of peripheral ROIs meeting this criterion were added to the area of the central plaque seen on T2-weighted images (ie, the central ROI) to calculate the new plaque size. The size of resultant regions of abnormal WM was compared with that of the corresponding plaque seen on T2-weighted images by means of a Student paired t test. Values obtained in male and female patients were also compared with a Student t test. P values of .05 or lower were considered to indicate significant differences.
We calculated the percentage decrease in FA values in plaques, compared with the FA value in identical areas in healthy volunteers. We recorded the percentage FA reduction in individual peripheral ROIs compared with identical regions in healthy volunteers, as well as the mean percentage reduction for all peripheral ROIs related to a given plaque. We also compared the FA reductions in MS plaques in female patients with those in male patients.
RESULTS
Fifteen cerebral WM plaques were studied in 12 subjects. No plaque in the study group was hypointense on T1-weighted images. One plaque showed contrast material enhancement. There was no enhancement in the normal-appearing WM adjacent to a lesion on T2-weighted images in any case.
The mean plaque size (±SD) seen on T2-weighted images was 72 mm2 ± 21 (standard error, 5.42 mm2; 95% confidence interval [CI]: 77.42, 66.58 mm2). The mean FA value for plaques was 0.285 ± 0.088 (standard error, 0.023; 95% CI: 0.262, 0.308). The mean FA value for areas of WM corresponding to plaques in healthy volunteers was 0.447 ± 0.069 (standard error, 0.018; 95% CI: 0.429, 0.465; P < .001). This corresponded to a mean percentage reduction of 37% for FA in MS plaques, compared with the FA values in healthy volunteers (range, 11%–50%). The mean FA value of MS plaques was 0.372 in men (n = 5) and 0.243 in women (n = 10) (P = .003). To control for possible confounding factors such as normal variability of FA values in different parts of the brain, we also compared the mean percentage reductions for plaque FA compared with values in healthy volunteers and found mean reductions of 41% in women and 27% in men (P = .03).
Table 1 shows the percentage changes in FA for each plaque and its peripheral ROIs compared with FAs in healthy volunteers. The mean percentage reduction in FA for all the peripheral ROIs was 16% compared with values in healthy volunteers. Mean changes in FA in the peripheral ROIs (ie, normal-appearing WM) for individual plaques ranged from a 30% reduction compared with the FA values in healthy volunteers to an increase of 4%. There was a significant reduction in the mean FA value of all peripheral ROIs compared with those in healthy volunteers (P < .001). For individual peripheral ROIs, percentage FA alterations ranged from a reduction of 41% to an increase of 23%. FA increases in the normal-appearing WM were seen in eight peripheral ROIs in four plaques; in these patients the FA value in individual ROIs was 3%–23% higher than that in healthy volunteers. In two of these four plaques, the mean FA for all peripheral ROIs was still lower than that in healthy subjects. There was no significant difference between the FAs of the plaques in these patients and those of the other plaques studied (P = .22).
TABLE 1.
Percentage Change in FA in Plaques and in Peripheral ROIs in Adjacent Normal-appearing WM
| Percentage Change in FA Compared with FA in Healthy Volunteers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peripheral ROIs |
|||||||||
| Plaque No. | Plaque FA | Plaque | ROI 1 | ROI 2 | ROI 3 | ROI 4 | ROI 5* | ROI 6* | Mean |
| 1 | 0.237 | −38 | −15 | −1 | −4 | −17 | −12 | −22 | −12 |
| 2 | 0.25 | −42 | −30 | −17 | −18 | −26 | −36 | … | −25 |
| 3 | 0.219 | −47 | −23 | +9 | 0 | −21 | −4 | −40 | −13 |
| 4 | 0.236 | −36 | −13 | −19 | −25 | 0 | … | −7 | −13 |
| 5 | 0.239 | −50 | −36 | −32 | −5 | −35 | −25 | −30 | −27 |
| 6 | 0.321 | −28 | −16 | −6 | −20 | −3 | −14 | … | −12 |
| 7 | 0.223 | −48 | −17 | −27 | −37 | −17 | −8 | −15 | −20 |
| 8 | 0.505 | −11 | +3 | −14 | −14 | +21 | +23 | +7 | +4 |
| 9 | 0.256 | −34 | −21 | −16 | −30 | −10 | −16 | … | −19 |
| 10 | 0.462 | −21 | +3 | −11 | −6 | −4 | … | −12 | −6 |
| 11 | 0.228 | −50 | −38 | −25 | −41 | −26 | −16 | … | −29 |
| 12 | 0.314 | −43 | −35 | −17 | −15 | −26 | … | −19 | −22 |
| 13 | 0.306 | −25 | −1 | +12 | −8 | 0 | +8 | −3 | +1 |
| 14 | 0.21 | −47 | −27 | −17 | −25 | −31 | −32 | −29 | −27 |
| 15 | 0.279 | −29 | −9 | −10 | −9 | −15 | −24 | −17 | −14 |
Missing values are due to cases in which a peripheral ROI was not placed, as doing so would have resulted in the inclusion of cerebrospinal fluid or gray matter.
When we applied the threshold of a 2-SD reduction in FA to peripheral ROIs, mean plaque size as determined by FA values increased from 72 mm2 ± 21 (standard error, 5.42 mm2) to 91 mm2 ± 35 (standard error, 8.95 mm2; 95% CI: 82.05, 99.95 mm2); the area range was 50–186 mm2. This represented a mean increase in plaque size to 127% of the original value (range, 100%–230%). These data are shown in Table 2. The mean increase in plaque size on FA maps was 18.6 mm2 ± 13.69 (standard error, 3.54 mm2; 95% CI: 15.06, 22.14 mm2); this difference was significant (P = .03). Nine plaques showed no plaque growth according to this threshold. Most of these plaques (seven of nine) did show a reduction in FA values in peripheral ROIs but did not reach the threshold of a reduction of 2 SD. The plaques with peripheral ROIs that did reach the 2-SD threshold are shown in Table 3. In two plaques, the mean FA value within ROIs peripheral to an MS plaque was almost the same as the mean value in healthy volunteers (Table 1, plaques 8 and 13).
TABLE 2.
Plaque Size as Determined on T2-weighted MR Images and FA Maps
| Plaque No. | Size on T2-weighted MR Images (mm2) |
Size on FA Maps (mm2)* |
Percentage Change |
|---|---|---|---|
| 1 | 53 | 53 | 100 |
| 2 | 81 | 186 | 230 |
| 3 | 58 | 58 | 100 |
| 4 | 118 | 118 | 100 |
| 5 | 53 | 89 | 168 |
| 6 | 100 | 100 | 100 |
| 7 | 60 | 89 | 148 |
| 8 | 59 | 59 | 100 |
| 9 | 106 | 106 | 100 |
| 10 | 65 | 65 | 100 |
| 11 | 73 | 105 | 144 |
| 12 | 56 | 98 | 175 |
| 13 | 74 | 74 | 100 |
| 14 | 74 | 109 | 147 |
| 15 | 50 | 50 | 100 |
Plaque size on FA maps was determined by defining as abnormal peripheral ROIs that were at least 2 SDs below the mean FA value for equivalent ROIs in healthy volunteers.
TABLE 3.
Plaques with at Least One Peripheral ROI Reaching the 2-SD Threshold for FA Reduction
| FA in Peripheral ROIs |
|||||||
|---|---|---|---|---|---|---|---|
| Plaque No. | Plaque FA | ROI 1 | ROI 2 | ROI 3 | ROI 4 | ROI 5* | ROI 6* |
| 2 | 0.25 | 0.324† | 0.323† | 0.358 | 0.330 | 0.274† | … |
| 5 | 0.239 | 0.310† | 0.295† | 0.420 | 0.289 | 0.335 | 0.340 |
| 7 | 0.223 | 0.336 | 0.292 | 0.251† | 0.358 | 0.391 | 0.349 |
| 11 | 0.228 | 0.255 | 0.387 | 0.290† | 0.318 | 0.319 | … |
| 12 | 0.314 | 0.348† | 0.492 | 0.483 | 0.397† | … | 0.349 |
| 14 | 0.21 | 0.285 | 0.288 | 0.269 | 0.262 | 0.305† | 0.314 |
Missing values are due to cases in which a peripheral ROI was not placed, as doing so would have resulted in the inclusion of cerebrospinal fluid or gray matter.
These ROIs met the 2-SD threshold (ie, were at least 2 SDs below the mean FA value for equivalent ROIs in healthy volunteers).
DISCUSSION
Although myelin loss is the primary pathologic hallmark of MS, more recent histologic assessments have indicated that both axonal injury and neuronal loss also play a role in the pathogenesis of this disease (11-15). Early features of axonal derangement have also been identified in the WM surrounding MS plaques (16). N-acetylaspartate is thought to be a nonspecific marker of axonal mass and viability; reported reductions in the levels of this metabolite throughout the brains of subjects with MS suggest that there is more widespread axonal injury than is currently identifiable with standard imaging techniques (17). Destruction of axons is usually irreversible and may therefore result in more permanent clinical disability; an imaging method that is sensitive to this process could have applications to prognostic assessment in MS. The random motion of water molecules in vivo would be expected to be accentuated in the setting of both myelin loss and axonal destruction, resulting in areas of reduced FA in regions of damaged tissue. Indeed, previous reports have indicated that FA values are globally reduced throughout the brains of subjects with MS, suggesting that diffusion-tensor imaging represents a promising method for the determination of true disease burden in such patients (4). Both FA and apparent diffusion coefficient measurements have previously shown derangements in the WM immediately adjacent to MS plaques, which indicates that these measurements may be more sensitive than conventional methods of determining the extent of WM injury (18).
In this study we found significantly reduced FA values in MS plaques compared with values for equivalent areas in healthy volunteers. In addition, the mean FA values in the WM immediately adjacent to MS plaques were also significantly reduced compared with equivalent areas in healthy volunteers. The area of focal WM abnormality, or plaque size, as determined with diffusion-tensor MR imaging was frequently markedly greater than that seen with standard clinical MR sequences. These findings suggest that the true plaque burden in cases of MS is underestimated with current standard MR imaging techniques. Many MS clinical trials use plaque volume as an important indicator of disease burden and response to therapy. Therefore, redefinition of plaque size with diffusion-tensor imaging may have an important effect on the conduct and interpretation of such trials.
The relative reductions in FA values observed in this study are less than those previously reported in an analysis comparing MS plaques with their mirror image sites in the contralateral cerebral hemisphere (19). In the earlier study, the periplaque WM was interrogated by sequentially placing small ROIs around the central ROI, eliminating regions that did not reach the threshold of a 40% reduction in FA. Given the global nature of the WM abnormality in MS, we expected that the relative reduction in plaque FA compared with the FA in healthy subjects would be even greater than that in the earlier study. In that study, mean FA reductions of 41% were seen in MS plaques compared with normal-appearing WM in the contralateral hemisphere, with similar relative FA reductions in much of the periplaque WM.
There is generally good agreement between the mean plaque FA values in our current study and the earlier study (0.285 vs 0.251, respectively) and between the FA values measured in equivalent control regions (0.447 vs 0.429). The differences in relative reductions in periplaque FA values are therefore likely related to differences in the technique of mapping the abnormal periplaque WM. Our technique of placing relatively large ROIs at predefined locations around the MS plaque left some areas uninterrogated between the oval ROIs. Additionally, averaging of FA values within relatively large ROIs may mask smaller regions of WM within ROIs that have far lower FA values. However, larger ROIs have the advantage of having less noise than smaller ROIs. For this reason, our technique may have been less sensitive to smaller focal areas of more profound FA reduction.
The pathophysiologic mechanism of FA reduction within MS plaques and the adjacent WM is likely related to a complex balance of perivascular inflammation, astrocytic proliferation, axonal destruction, and myelin loss (20,21). Variability in the degree of FA reduction is likely related to differing contributions of these various processes. Less FA reduction in the periplaque WM may be related to less advanced regions of tissue damage comprising mainly inflammatory material and astrocytic proliferation rather than to myelin and axonal loss. Some of these features (eg, myelin loss) may be reversible, while others (eg, axonal loss) may be irreversible. Future studies that address the relative contributions of these factors to the final FA measurements may therefore give more accurate prognostic information about disease progress and the effect of therapeutic agents.
For many of the plaques in our study (nine of 15), the FA values of the adjacent WM regions were reduced by less than 2 SD compared with the equivalent ROIs in healthy volunteers. The area of abnormal WM as determined with diffusion-tensor imaging was therefore the same as that seen on T2-weighted images in these patients. However, the measured anisotropy values in these periplaque regions were still substantially reduced (by a mean of 16%) when compared with analogous WM sites in healthy volunteers. It is likely that the smaller degrees of FA reduction seen in the periplaque regions in this subgroup represent areas of less severe myelin and axonal loss. Thus, the true area of abnormal WM is likely larger than that defined by using our thresholding technique. Further study will be required to determine a reliable threshold of FA reduction that can be used to give a clinically useful estimation of disease burden and response to therapy. It will also be important to determine the reversibility of FA reductions and whether alterations of FA in normal-appearing WM can predict future areas of T2 signal abnormality.
Wallerian degeneration has also been suggested as a possible mechanism of reductions in FA in normal-appearing WM distant from MS plaques (22). Antegrade axonal degeneration from more cephalad MS plaques could have affected the measured FA values in the periplaque WM of the plaques in our study. Because currently one cannot determine most specific WM pathways on FA maps, we could not assess this hypothesis. As fiber tract mapping techniques continue to improve, we hope to study this possible relationship between areas of FA reduction and the anatomy of WM tracts in the brain.
We found a significant difference between male and female subjects in both mean plaque FA values (P = .003) and mean percentage reductions in plaque FA compared with normal WM (P = .03). The higher P value obtained when comparing the percentage declines in FA suggests that much of the difference may be due to normal variability of FA values in different regions of the brain. Additionally, this subset analysis was based on a small sample size, and, therefore, the results should be interpreted with caution. Previous studies have shown no significant differences between male and female subjects at diffusion-tensor imaging (23). We believe that potential sex-based differences merit future study in a larger subject population.
This study had some evident limitations. We did not correlate alteration in FA with clinical manifestations of disease activity, and it will be important in future studies to determine whether these two features are correlated. Second, fiber tract mapping software was not available to us at the time of the study. Analysis of mean FA values in fiber tracts along important WM pathways might provide further insights into the role of diffusion-tensor imaging in assessing patients with MS and determining which role, if any, Wallerian degeneration plays in decreased FA values in normal-appearing WM.
Third, more than one plaque from the same patient was analyzed in two cases (three plaques in one patient and two in another). Plaques from the same subject may be more similar to each other than to plaques from different subjects; in the small number of such plaques included in this study, however, there was no evidence of such a difference. Finally, for technical reasons, we were unable to coregister the FA maps with the T2-weighted images; this limitation may have resulted in some error in ROI placement due to the poorer resolution of FA maps. When delineating the margin of signal intensity abnormality on the diffusion-tensor images, we deliberately included all areas with any elevation in signal intensity; this may have resulted in the inclusion, within the central ROI, of WM that was in fact normal appearing on T2-weighted images. An error of this type would be expected to result in an underestimation of the true FA reduction in plaques, suggesting that focal WM derangements in MS may be even greater than those that we saw in this study.
In conclusion, the extent of focal WM abnormality in MS in our study was frequently underestimated with standard clinical MR sequences. Diffusion-tensor MR imaging may represent a more sensitive method of locating areas of WM derangement and may have future applications in the assessment of disease burden and activity in demyelinating conditions. Further studies are necessary to determine whether serial changes in diffusion-tensor imaging measurements correspond to clinical status.
Abbreviations
- CI
confidence interval
- FA
fractional anisotropy
- MS
multiple sclerosis
- ROI
region of interest
- SD
standard deviation
- WM
white matter
Footnotes
Authors stated no financial relationship to disclose.
Author contributions:
Guarantor of integrity of entire study, J.M.P.; study concepts and design, all authors; literature research, S.M.K.; clinical studies, S.M.K., D.J.M., W.L.W., Y.K.; data acquisition, S.M.K., D.J.M., W.L.W., Y.K.; data analysis/interpretation, S.M.K., J.M.P., Y.K.; statistical analysis, S.M.K., Y.K.; manuscript preparation, editing, revision/ review, and final version approval, S.M.K., J.M.P.; manuscript definition of intellectual content, all authors
References
- 1.Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998;121:3–24. doi: 10.1093/brain/121.1.3. [DOI] [PubMed] [Google Scholar]
- 2.McDonald WI, Miller DH, Barnes D. The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol. 1992;18:319–334. doi: 10.1111/j.1365-2990.1992.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson VL, Miller DH, Rovaris M, et al. Primary and transitional progressive MS: a clinical and MRI cross-sectional study. Neurology. 1999;52:839–845. doi: 10.1212/wnl.52.4.839. [DOI] [PubMed] [Google Scholar]
- 4.Guo AC, MacFall JR, Provenzale JM. Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal appearing white matter. Radiology. 2002;222:729–736. doi: 10.1148/radiol.2223010311. [DOI] [PubMed] [Google Scholar]
- 5.Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 6.Waxman SG. Demyelinating diseases: new pathological insights, new therapeutic targets (editorial) N Engl J Med. 1998;338:323–325. doi: 10.1056/NEJM199801293380610. [DOI] [PubMed] [Google Scholar]
- 7.Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, et al. Investigation of normal appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56:926–933. doi: 10.1212/wnl.56.7.926. [DOI] [PubMed] [Google Scholar]
- 8.Rocca MA, Iannucci G, Rovaris M, Comi G, Filippi M. Occult tissue damage in patients with primary progressive MS is independent of T2-visible lesions. J Neurol. 2003;250:456–460. doi: 10.1007/s00415-003-1024-1. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 10.Papadakis NG, Xing D, Houston GC, et al. A study of rotationally invariant and symmetric indices of diffusion anisotropy. Magn Reson Imaging. 1999;17:881–892. doi: 10.1016/s0730-725x(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 11.Fu L, Matthews PM, De Stefano N, et al. Imaging axonal damage of normal appearing white matter in multiple sclerosis. Brain. 1998;121:103–113. doi: 10.1093/brain/121.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Davie CA, Barker GJ, Thompson AJ, Tofts PS, McDonald WI, Miller DH. 1H magnetic resonance spectroscopy of chronic white matter lesions and normal appearing white matter in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997;63:736–742. doi: 10.1136/jnnp.63.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leary SM, Davie CA, Parker GJ, et al. 1H magnetic resonance spectroscopy of normal appearing white matter in primary progressive multiple sclerosis. J Neurol. 1999;246:1023–1026. doi: 10.1007/s004150050507. [DOI] [PubMed] [Google Scholar]
- 14.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 15.Evangelou N, Esiri MM, Smith S, et al. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47:391–395. [PubMed] [Google Scholar]
- 16.Giordana MT, Richiardi P, Trevisan E, Boghi A, Palmucci L. Abnormal ubiquitination of axons in normally myelinated white matter in multiple sclerosis brain. Neuropathol Appl Neurobiol. 2002;28:35–41. doi: 10.1046/j.1365-2990.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 17.Arnold DL, Riess GT, Matthews PM, et al. Use of proton magnetic resonance spectroscopy for monitoring disease progression in multiple sclerosis. Ann Neurol. 1994;36:76–82. doi: 10.1002/ana.410360115. [DOI] [PubMed] [Google Scholar]
- 18.Guo AC, Jewells VL, Provenzale JM. Analysis of normal-appearing white matter in multiple sclerosis: comparison of diffusion tensor MR imaging and magnetization transfer imaging. AJNR Am J Neuroradiol. 2001;22:1893–1900. [PMC free article] [PubMed] [Google Scholar]
- 19.Kealey SM, Kim Y, Provenzale JM. Tensor diffusion imaging of demyelinating plaques and adjacent white matter in multiple sclerosis (abstr); Presented at the 41st Annual Meeting of the American Society of Neuroradiology; Washington, DC. April 26 to May 2, 2003.pp. 254–255. [Google Scholar]
- 20.Castriota Scanderbeg A, Tomaiuoulo F, Sabatini U, Nocentini U, Grasso MG, Caltagirone C. Demyelinating plaques in relapsing-remitting and secondary progressive MS: assessment with diffusion MR imaging. AJNR Am J Neuroradiol. 2000;21:862–868. [PMC free article] [PubMed] [Google Scholar]
- 21.Allen I, McKeown S. A histological, histochemical and biochemical study of the macroscopically normal white matter in MS. J Neurol Sci. 1979;41:81–91. doi: 10.1016/0022-510x(79)90142-4. [DOI] [PubMed] [Google Scholar]
- 22.Ciccarelli O, Werring DJ, Barker GJ, et al. A study of the mechanisms of normal appearing white matter damage in multiple sclerosis using diffusion tensor imaging. J Neurol. 2003;250:287–292. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- 23.Virta A, Barnett A, Pierpaoli C. Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magn Reson Imaging. 1999;17:1121–1133. doi: 10.1016/s0730-725x(99)00048-x. [DOI] [PubMed] [Google Scholar]


