Abstract
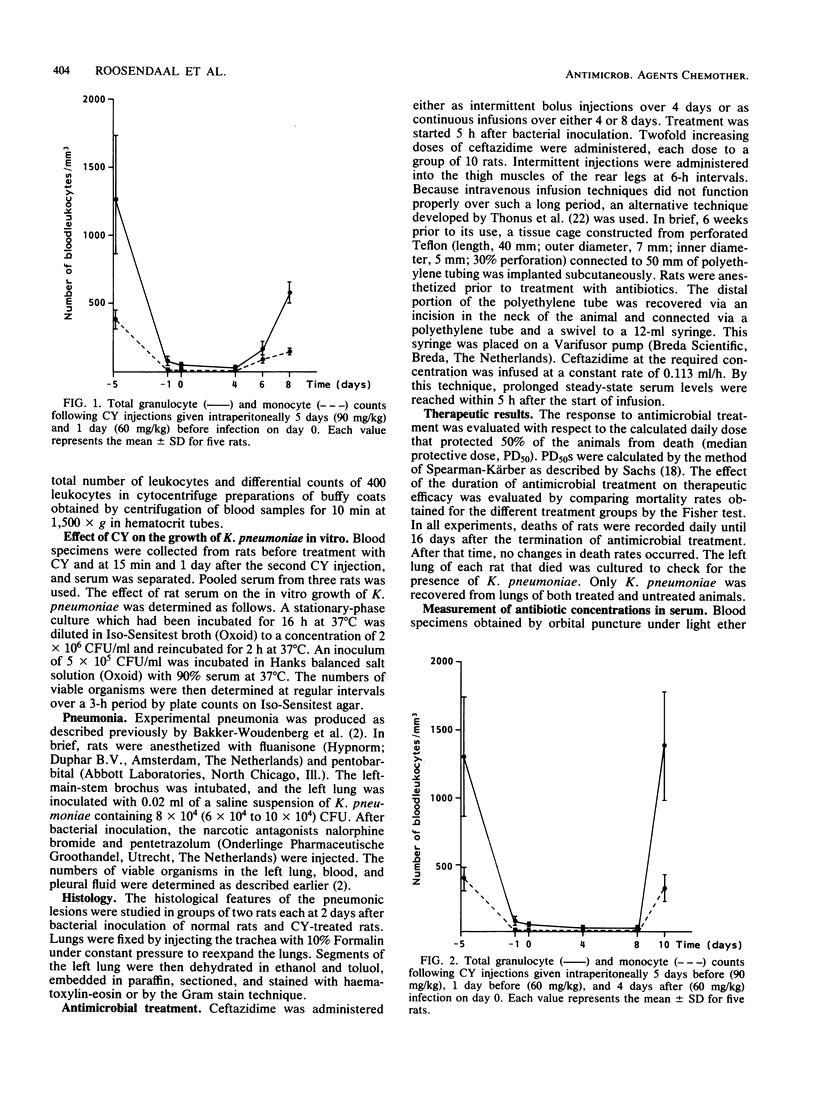
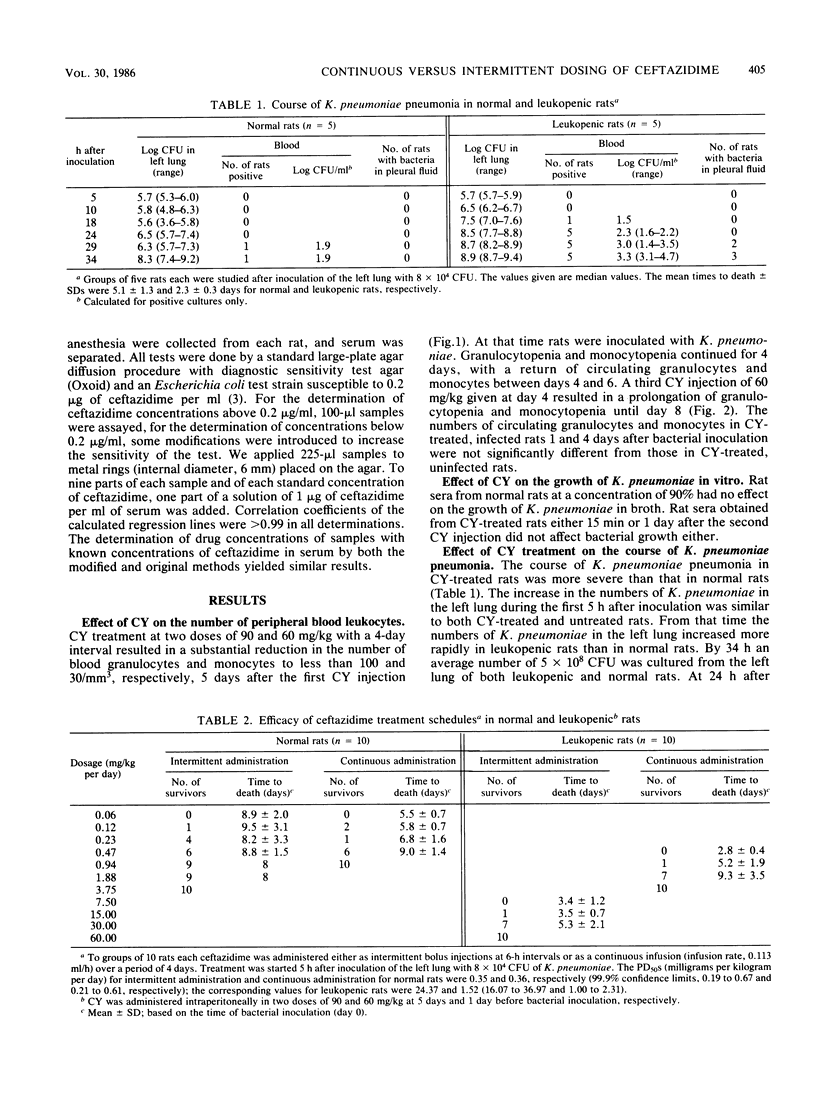
Experimental Klebsiella pneumoniae pneumonia was used to study the influence of cyclophosphamide-induced leukopenia on the relative therapeutic efficacy of continuous and intermittent (6-h intervals) administration of ceftazidime. The antimicrobial response was evaluated with respect to the calculated daily dose that protected 50% of the animals from death (PD50) until 16 days after the termination of a 4-day treatment. When ceftazidime was administered intermittently to leukopenic rats, the PD50 was 24.37 mg/kg per day, 70 times (P less than 0.001) the PD50 of 0.35 mg/kg per day for normal rats. Continuous administration of ceftazidime to leukopenic rats resulted in a PD50 of 1.52 mg/kg per day, four times (P less than 0.001) the PD50 of 0.36 mg/kg per day for normal rats. Continuous administration of ceftazidime in daily doses that protected 100% of normal and leukopenic rats from death resulted in serum levels of 0.06 and 0.38 micrograms/ml, respectively, whereas the MIC for the infecting K. pneumoniae strain was 0.2 micrograms of ceftazidime per ml. The effect of the duration of ceftazidime treatment by continuous infusion on the therapeutic efficacy in relation to the persistence of leukopenia was then investigated in leukopenic rats. The administration of 3.75 mg of ceftazidime/kg per day for 4 days protected all leukopenic rats from death, provided the circulating leukocytes returned at the end of antibiotic treatment. When leukopenia persisted for 8 days this ceftazidime treatment schedule resulted in the mortality of rats (P less than 0.05). However, when ceftazidime treatment was continued for 8 days, until the return of the leukocytes, there was no significant mortality (P greater than 0.05).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker-Woudenberg I. A., van den Berg J. C., Fontijne P., Michel M. F. Efficacy of continuous versus intermittent administration of penicillin G in Streptococcus pneumoniae pneumonia in normal and immunodeficient rats. Eur J Clin Microbiol. 1984 Apr;3(2):131–135. doi: 10.1007/BF02014330. [DOI] [PubMed] [Google Scholar]
- Bakker-Woudenberg I. A., van den Berg J. C., Michel M. F. Therapeutic activities of cefazolin, cefotaxime, and ceftazidime against experimentally induced Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother. 1982 Dec;22(6):1042–1050. doi: 10.1128/aac.22.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Ketchel S. J., Rodriguez V. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med. 1979 Oct;67(4):608–616. doi: 10.1016/0002-9343(79)90242-0. [DOI] [PubMed] [Google Scholar]
- Bundtzen R. W., Gerber A. U., Cohn D. L., Craig W. A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981 Jan-Feb;3(1):28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- Comber K. R., Boon R. J., Sutherland R. Comparative effects of amoxycillin and ampicillin on the morphology of Escherichia coli in vivo and correlation with activity. Antimicrob Agents Chemother. 1977 Dec;12(6):736–744. doi: 10.1128/aac.12.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutome T., Mitsuyama M., Takeya K., Nomoto K. Importance of antiserum and phagocytic cells in the protection of mice against infection by Klebsiella pneumoniae. J Gen Microbiol. 1980 Jul;119(1):225–229. doi: 10.1099/00221287-119-1-225. [DOI] [PubMed] [Google Scholar]
- Kramer B. S., Pizzo P. A., Robichaud K. J., Witesbsky F., Wesley R. Role of serial microbiologic surveillance and clinical evaluation in the management of cancer patients with fever and granulocytopenia. Am J Med. 1982 Apr;72(4):561–568. doi: 10.1016/0002-9343(82)90449-1. [DOI] [PubMed] [Google Scholar]
- Kunin C. M. Dosage schedules of antimicrobial agents: a historical review. Rev Infect Dis. 1981 Jan-Feb;3(1):4–11. doi: 10.1093/clinids/3.1.4. [DOI] [PubMed] [Google Scholar]
- Lavoie G. Y., Bergeron M. G. Influence of four modes of administration on penetration of aztreonam, cefuroxime, and ampicillin into interstitial fluid and fibrin clots and on in vivo efficacy against Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Sep;28(3):404–412. doi: 10.1128/aac.28.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikin D., Rolinson G. N. Antibiotic levels in experimentally infected mice in relation to therapeutic effect and antibacterial activity in vitro. J Antimicrob Chemother. 1979 Jul;5(4):423–429. doi: 10.1093/jac/5.4.423. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Current practices in antimicrobial dosing. Rev Infect Dis. 1981 Jan-Feb;3(1):12–18. doi: 10.1093/clinids/3.1.12. [DOI] [PubMed] [Google Scholar]
- Peterson L. R., Gerding D. N., Fasching C. E. Effects of method of antibiotic administration on extravascular penetration: cross-over study of cefazolin given by intermittent injection or constant infusion. J Antimicrob Chemother. 1981 Jan;7(1):71–79. doi: 10.1093/jac/7.1.71. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A., Robichaud K. J., Gill F. A., Witebsky F. G., Levine A. S., Deisseroth A. B., Glaubiger D. L., Maclowry J. D., Magrath I. T., Poplack D. G. Duration of empiric antibiotic therapy in granulocytopenic patients with cancer. Am J Med. 1979 Aug;67(2):194–200. doi: 10.1016/0002-9343(79)90390-5. [DOI] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I. A., van den Berg J. C., Michel M. F. Therapeutic efficacy of continuous versus intermittent administration of ceftazidime in an experimental Klebsiella pneumoniae pneumonia in rats. J Infect Dis. 1985 Aug;152(2):373–378. doi: 10.1093/infdis/152.2.373. [DOI] [PubMed] [Google Scholar]
- SCHMIDT L. H., WALLEY A. The influence of the dosage regimen on the therapeutic effectiveness of penicillin G in experimental lobar pneumonia. J Pharmacol Exp Ther. 1951 Dec;103(4):479–488. [PubMed] [Google Scholar]
- Sande M. A., Korzeniowski O. M., Allegro G. M., Brennan R. O., Zak O., Scheld W. M. Intermittent or continuous therapy of experimental meningitis due to Streptococcus pneumoniae in rabbits: preliminary observations on the postantibiotic effect in vivo. Rev Infect Dis. 1981 Jan-Feb;3(1):98–109. doi: 10.1093/clinids/3.1.98. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Weerts D., Klastersky J. Causes of death in febrile granulocytopenic cancer patients receiving empiric antibiotic therapy. Eur J Cancer Clin Oncol. 1984 Jan;20(1):55–60. doi: 10.1016/0277-5379(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Undeutsch C., Brunner H. Einfluss von Antikörpern auf die Phagozytose von Klebsiella pneumoniae durch Alveolarmakrophagen. Zentralbl Bakteriol A. 1981 Mar;249(1):43–52. [PubMed] [Google Scholar]
- Van Etta L. L., Kravitz G. R., Russ T. E., Fasching C. E., Gerding D. N., Peterson L. R. Effect of method of administration on extravascular penetration of four antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):873–880. doi: 10.1128/aac.21.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak O., Kradolfer F. Effects of subminimal inhibitory concentrations of antibiotics in experimental infections. Rev Infect Dis. 1979 Sep-Oct;1(5):862–879. doi: 10.1093/clinids/1.5.862. [DOI] [PubMed] [Google Scholar]



