Abstract
Experimental painful peripheral neuropathies produced by the chemotherapeutic drugs, paclitaxel and vincristine, are produced by relatively low doses that do not cause axonal degeneration in peripheral nerve. Using quantitative immunolabeling with the PGP9.5 antibody, we have investigated whether these painful neuropathies might be associated with degeneration that is confined to the region of the sensory fiber's receptor terminals in the skin. Because complete and partial nerve transections are known to cause an increase in PGP9.5 in epidermal Langerhans cells (LCs), we also examined whether this effect occurs in chemotherapy-treated animals.
At the time of peak pain severity, rats with paclitaxel- and vincristine-evoked painful peripheral neuropathies had a significant decrease (24% and 44%, respectively) in the number of intraepidermal nerve fibers (IENF) in the hind paw glabrous skin and an increase (217% and 121%, respectively) in the number of PGP9.5-positive LCs, relative to control. However, neither loss of IENF nor an increase in PGP9.5-positive LCs was found in rats with a painful peripheral neuropathy evoked by the anti-HIV agent, 2′,3′-dideoxycytidine. We also confirmed that there is a decrease in IENF and an increase in PGP9.5-positive LCs in rats with neuropathic pain following a partial nerve injury (CCI model) and in rats with a complete sciatic nerve transection.
Partial degeneration of the intraepidermal innervation suggests mechanisms that might produce chemotherapy-evoked neuropathic pain, and activation of cutaneous LCs suggests possible neuroimmune interactions that might also have a role.
Keywords: Chemotherapy, Degeneration, Dideoxycytidine, Langerhans cells, Neuropathic pain, Paclitaxel, Vincristine
Introduction
Taxane and vinca alkaloid chemotherapeutics are among the most effective agents against solid tumors. Their dose-limiting side effect is the production of peripheral neuropathy, which in many patients is accompanied by a chronic neuropathic pain syndrome (reviewed in Polomano et al., 2001; Dougherty et al., 2004). The anti-tumor action of these drugs is due to their binding to β-tubulin, which interferes with the dynamic assembly and disassembly of the mitotic spindle and leads to aborted cell division (Jordan and Wilson, 2004). Axonal microtubules also contain β-tubulin, and it has been thought that the neuropathy is secondary to paclitaxel and vincristine binding to microtubules and thereby impairing axoplasmic transport, with a consequent progressive, dying-back axonopathy. However, there is considerable evidence against this hypothesis (reviewed in Polomano et al., 2001; Flatters and Bennett, 2006).
Animal experiments using relatively high systemic doses of paclitaxel, or injections of paclitaxel directly into a peripheral nerve, have provided evidence of axonal degeneration, but in these experiments, the animals are hypoesthetic or anesthetic (e.g., Cliffer et al., 1998; Lauria et al., 2005a). In contrast, relatively low systemic doses of paclitaxel and vincristine produce signs of pain hypersensitivity, including allodynia and hyper-algesia (Aley et al., 1996; Authier et al., 1999, 2000, 2003; Polomano et al., 2001; Nozaki-Taguchi et al., 2001; Flatters and Bennett, 2006; Siau and Bennett, 2006). Light- and electron microscopic studies of peripheral nerves from rats with low-dose paclitaxel- and vincristine-evoked painful peripheral neuropathies have failed to find any axonal degeneration in peripheral nerve (Tanner et al., 1998a; Polomano et al., 2001; Topp et al., 2000; Flatters and Bennett, 2006). Thus, the cause of paclitaxel- and vincristine-evoked painful neuropathies is unknown, and the relation between the neuropathic pain state and the neuropathy per se is unclear.
The absence of axonal degeneration at the level of the peripheral nerve does not rule out the possibility of a degenerative process confined to the region of the sensory neuron's peripheral terminal arbors. In order to examine this possibility, we used immunocytochemical labeling (PGP9.5 antibody) to quantify the intraepidermal nerve fibers (IENF) in plantar hind paw skin of rats with paclitaxel- and vincristine-evoked painful peripheral neuropathies at the time of peak pain severity. We compared these animals to rats with a painful peripheral neuropathy produced by the anti-HIV false nucleoside, 2′,3′-dideoxycytidine (ddC), that produces similar symptoms in rats and man (Dubinsky et al., 1989; Dalakas, 2001; Joseph et al., 2004). As positive controls, we also examined rats with painful peripheral neuropathy secondary to nerve trauma (the CCI model of Bennett and Xie, 1988) and rats with a sciatic nerve transection.
Complete and partial transection of a peripheral nerve is known to increase PGP9.5 in epidermal Langerhans cells (LCs; Hsieh et al., 1996; Lin et al., 2001; Lindenlaub and Sommer, 2002). To determine whether paclitaxel, vincristine, and ddC have a similar effect, we counted PGP9.5-positive LCs and confirmed their identity using double labeling with an antibody (OX-6) that is specific for cells of the monocyte lineage.
Materials and methods
These experiments conformed to the ethics guidelines of the International Association for the Study of Pain (Zimmermann, 1983), the National Institutes of Health (USA), and the Canadian Institutes of Health Research. All experimental protocols were approved by the Facility Animal Care Committee of the Faculty of Medicine, McGill University in accordance with the regulations of the Canadian Council on Animal Care.
Animals
Adult male Sprague-Dawley rats (200 – 300 g, Harlan Inc., Indianapolis, IN; Frederick, MD breeding colony; N = 29) were housed in groups of three on sawdust bedding in plastic cages. Artificial lighting was provided on a fixed 12-h light–dark cycle with food and water available ad libitum.
Drug administration and surgery
Paclitaxel (Taxol®; Bristol-Myers-Squibb, Montreal; 6 mg/ml) was diluted with saline to a concentration of 2 mg/ml and injected IP (2 mg/kg) on 4 alternate days (days 0, 2, 4 and 6) as described previously (Polomano et al., 2001; Flatters and Bennett, 2004). Vincristine (Novopharm Ltd., Toronto) was diluted with saline to a concentration of 50 μg/ml and injected IP (50 μg/kg) for 10 consecutive days as described previously (Siau and Bennett, 2006). 2′,3′-Dideoxycytidine (ddC; 50 mg/ml; Sigma-Aldrich, Oakville, ON) was administered as a single IV bolus (50 mg/kg) via the tail vein as described by Joseph et al. (2004). Control animals received injections of the paclitaxel vehicle.
The chronic constriction injury (CCI) model was produced as described previously (Bennett and Xie, 1988) using isoflurane anesthesia. CCI rats received a contralateral sham operation (the nerve was manipulated but not ligated). Rats with a complete nerve transection were prepared using isoflurane anesthesia by cutting the common sciatic nerve at mid-thigh. Control animals for the two surgical preparations were anesthetized similarly but not operated on.
Behavioral testing
Animals were habituated to the behavioral testing environment, and three baseline measurements of mechanical sensitivity were taken prior to drug administration or surgery. The animals were placed on an elevated wire mesh floor and confined beneath overturned mouse cages made of clear plastic. von Frey filaments with bending forces of 4 g and 15 g were applied to the mid-plantar skin (avoiding the base of the tori) of each hind paw 5 times, with each application held for 5 s. Withdrawal responses to the von Frey filaments from both hind paws were counted and then expressed as an overall percentage response. Normal rats rarely withdraw from the 4 g stimulus; the increased level of responding seen after treatment is thus indicative of mechano-allodynia. Normal animals withdraw from the 15 g stimulus 10–20% of the time (e.g., Flatters and Bennett, 2004, 2006); the increased level of responding seen after treatment is thus indicative of mechano-hyperalgesia.
Immunocytochemistry
Except the cases with complete transection of the sciatic nerve, all the animals used in the immunocytochemical experiments had confirmed mechano-allodynia and mechano-hyperalgesia. The paclitaxel-, vincristine-, and ddC-treated animals were sacrificed at the approximate time of peak pain severity in each model: paclitaxel (day 31 (D31) after the first injection; Flatters and Bennett, 2006); vincristine (D16; Siau and Bennett, 2006); and ddC (D8; Joseph et al., 2004). Rats with complete sciatic nerve transection were sacrificed on D2 post-surgery, a time at which degeneration of the IENF is known to be nearly complete (Hsieh et al., 1996). For comparison to the complete nerve transection, the CCI rats were also sacrificed on D2; the CCI pain syndrome is present at this time (Bennett and Xie, 1988).
The rats were over-dosed with sodium pentobarbital (100 mg/kg; IP) and perfused transcardially with a vascular rinse (phosphate-buffered saline (PBS) containing 0.05% sodium bicarbonate and 0.1% sodium nitrite) for 1 min; followed by freshly prepared 4% paraformaldehyde in 0.1 M PB, pH 7.4, for 30 min. The hind paws were severed and post-fixed overnight, after which a block of glabrous skin was excised from the wide part of the plantar hind paw that lies distal to the calcaneous and proximal to the digital tori. Blocks of skin were cryoprotected in 30% sucrose solution at 4°C overnight and then embedded in Optimal Cutting Temperature (OCT) compound and stored at −80°C. Cryostat sections (30 μm) sections were collected in PBS containing 0.2% Triton-X 100 (PBS + T). Following a 1-h incubation in PBS + T containing 10% normal donkey serum (NDS; Jackson ImmunoResearch Laboratories; Mississauga, ON) at room temperature, sections were incubated in rabbit anti-human PGP9.5 primary antibody (Research Diagnostics; Flanders, NJ) diluted 1:6400 in PBS + T containing 5% NDS for 24 hr at 4°C. After rinsing in PBS + T, sections were incubated in donkey anti-rabbit IgG secondary antibody labeled with Cy3 (Jackson ImmunoReseach) diluted 1:200 for 1.5 h. Sections were then double labeled with mouse anti-rat MHC class II RT Ia (OX-6) primary antibody (Abcam; Cambridge, MA) diluted 1:400 for 1.5 h. After rinsing in PBS + T, sections were incubated in donkey anti-mouse IgG secondary antibody labeled with FITC (Jackson ImmunoReseach) diluted 1:30 for 1.5 h. Control sections incubated without primary antisera showed no specific staining.
Quantification of intraepidermal nerve fibers and Langerhans cells
All IENF and LC counts were done by an observer blind as to the animal's group assignment using a Zeiss Axioplan 2 Imaging fluorescence microscope. Using a 40× objective, all ascending nerve fibers that were seen to cross into the epidermis were counted; no minimum length was required and fibers that branched within the epidermis were counted as one (McCarthy et al., 1995; Lauria et al., 2005a). LCs were counted if the cell body was visible and double labeled with OX-6. A low magnification montage of each section was made, and the length of the epidermal border was measured (mean ± SD: 10.2 ± 1.8 mm). IENF and LC counts are expressed as the number per centimeter of epidermal border. Images for the illustrations were obtained with a Zeiss LSM 510 confocal microscope.
Sections from control rats (n = 4) were stained concurrently with the sections from the paclitaxel-, vincristine-, and ddC-treated rats (each group n = 4). Sections from a separate group of control rats (n = 4) were stained concurrently with the sections from the CCI (n = 4) and nerve transection groups (n = 5). The IENF and LC counts from these two control groups were nearly identical, and the data were thus combined for a single control group (n = 8).
Statistics
One-way ANOVAs were used to compare the mean IENF and LC counts for the 5 groups. Post hoc pair-wise comparisons to the control group were made with Dunnett's multiple comparison test. Differences were considered significant with P < 0.05.
Results
Intraepidermal nerve fibers
As described previously in both human and rat skin (e.g., Hsieh et al., 1996; McCarthy et al., 1995; Oaklander and Brown, 2004), IENFs emerged from cutaneous nerves and traveled vertically into the epidermis where they branched into terminal arbors consisting of multiple fine branches bearing terminal and en passant boutons (Fig. 1A).
Fig. 1.

(A) Confocal image of PGP9.5-stained intraepidermal nerve fibers in the glabrous hind paw skin of a normal rat. Arrows point to faintly stained Langerhans cells. Scale bar: 20 μm. (Column B) Confocal images of two double-labeled Langerhans cells from a normal rat. Scale bar: 10 μm. (Column C) Confocal images of three double-labeled Langerhans cells from a paclitaxel-treated rat. Scale bar: 10 μm.
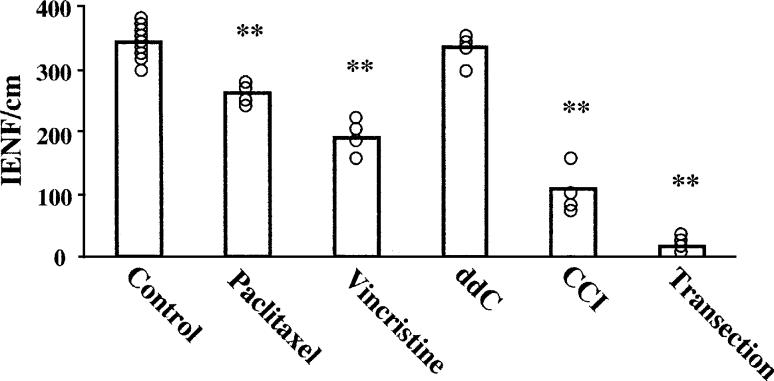
The IENF counts are summarized in Fig. 2. The group means were significantly different (ANOVA, P < 0.0001). Control rats had 343.1 ± 25.8 (mean ± SD) IENFs per centimeter, with little animal-to-animal variability. Paclitaxel-treated rats had 261.0 ± 25.2 IENFs per centimeter; a statistically significant (P < 0.01) reduction of 23.9% relative to control. Vincristine-treated rats had 190.9 ± 25.9 IENFs per centimeter; a statistically significant (P < 0.01) reduction of 44.4% relative to control. ddC-treated rats had 337.4 ± 26.5 IENFs per centimeter; a difference of −1.7% relative to control that is not statistically significant.
Fig. 2.
IENF counts for control, paclitaxel-treated, vincristine-treated, and ddC-treated rats; and for the ipsilateral side of CCI and sciatic nerve transection animals. Bars show the group means, open circles show values from each animal. **P < 0.01 relative to control.
As expected, CCI rats had a significant reduction in IENFs on the nerve-injured side (108.8 ± 41.6/cm); a significant (P < 0.01) decrease of 67.5% relative to control. This result is consistent with previous work (Basbaum et al., 1991; Munger et al., 1992; Ma and Bisby, 2000; Lin et al., 2001; Lindenlaub and Sommer, 2002).
Animals with a complete transection of the sciatic nerve had a near total loss of IENFs on the nerve-injured side (18.3 ± 12.2/cm); asignificant(P < 0.01) decrease of 94.5% relative to control.
There were no statistically significant changes in the numbers of IENFs contralateral to the CCI or complete nerve transection (369.2 ± 66.1/cm (+10.4% of control), and 331.2 ± 15.1/cm (−1.0% of control), respectively).
PGP9.5-positive Langerhans cells
As reported by others (Hsieh et al., 1996; Stankovic et al., 1999; Lin et al., 2001; Lindenlaub and Sommer, 2002), only low levels of PGP9.5 were detectable in LCs in the skin of normal rats when using antibody dilutions optimized for the demonstration of IENFs (Figs. 1A, B). Identification of these cells in normal animals was frequently difficult when viewing PGP9.5 staining alone. PGP9.5 immunoreactivity was generally faint and revealed only the cell body or the cell body with only short stretches of its primary branches. In these cases, the OX-6 staining was essential for cell identification (Fig. 1B). LCs in vincristine- and paclitaxel-treated rats had high levels of PGP9.5 immunoreactivity, with long stretches of primary, secondary, and sometimes even tertiary branches revealed clearly (Fig. 1C).
The LC counts are summarized in Fig. 3. The group means were significantly different (ANOVA, P < 0.0001). Control rats had 62.8 ± 13.7 (mean ± SD) PGP9.5-positive LCs per centimeter. Paclitaxel-treated rats had 197.2 ± 27.9 PGP9.5-positive LCs per centimeter; a statistically significant (P < 0.01) increase of 216.6% relative to control. Vincristine-treated rats had 137.7 ± 44.0 PGP9.5-positive LCs per centimeter; a statistically significant (P < 0.01) increase of 121.2% relative to control. The increase seen in the vincristine-treated rats was not significantly different than that seen in the paclitaxel-treated rats. ddC-treated rats had 60.8 ± 12.0 PGP9.5-positive LCs per centimeter, a difference of − 2.4% relative to control that is not statistically significant. The staining intensity of LCs in these animals was faint, similar to that seen in the control animals.
Fig. 3.
Counts of PGP9.5-labled LCs for control, paclitaxel-treated, vincristine-treated, and ddC-treated rats; and from the ipsilateral side of CCI and sciatic nerve transection animals. Bars show the group means, open circles show values from each animal. *P < 0.05; **P < 0.01 relative to control.
On the nerve injured side, CCI rats had 108.4 ± 18.8 PGP9.5-positive LCs cells per centimeter; a statistically significant (P < 0.01) increase of 75.6% relative to control. As in the chemotherapy-treated animals, the increase in LC number was accompanied by an increase in staining intensity. Contralaterally, CCI rats had 63.4 ± 12.7 PGP9.5-positive LCs cells per centimeter; a 2.8% increase that is not significantly different from the control value. On the nerve-injured side, rats with the sciatic nerve transection had 140.5 ± 50.8 PGP9.5-positive LCs cells per centimeter; a statistically significant (P < 0.05) increase of 127.7% relative to control. Contralaterally, nerve transected animals had 79.5 ± 24.1 PGP9.5-positive LCs per centimeter, a 28.8% increase relative to control that is not statistically significant.
OX-6-positive Langerhans cells
OX-6 staining was localized to the surface of the LC soma (Figs. 1B, C). All PGP9.5-positive LCs were also positive for OX-6, but many OX-6-positive LCs did not contain detectable PGP9.5. We compared the percentage of double-labeled LCs with the entire OX-6-positive population of LCs in paclitaxel-treated and control rats. Control rats had 282.0 ± 49.0 OX-6-positive LCs per centimeter, and paclitaxel-treated rats had 303.8 ± 63.0/cm; these values are not significantly different. There was no difference in the intensity of OX-6 staining in the two groups. The percentage of OX-6-positive cells that was double-labeled with PGP9.5 was 22.5% in the controls and 66.3% in the paclitaxel-treated rats. Thus, in paclitaxel-treated rats, there was an increase in the number of LCs expressing a detectable level of PGP9.5, but no change in the number of LCs in the tissue.
Discussion
We report here that paclitaxel- and vincristine-evoked painful peripheral neuropathies, but not ddC-evoked neuropathy, are accompanied by a partial degeneration of the sensory innervation of the epidermis and by an up-regulation of PGP9.5 in LCs.
Loss of innervation in paclitaxel- and vincristine-evoked painful peripheral neuropathies
This is the first unequivocal evidence of a neural lesion in these experimental models of chemotherapy-evoked painful peripheral neuropathy. Previous examinations of peripheral nerve axons from rats with paclitaxel- and vincristine-evoked painful peripheral neuropathies have found small increases in the average diameter of C fibers and in the incidence of microtubules with an atypical orientation in A and C fibers (Tanner et al., 1998a; Topp et al., 2000; Flatters and Bennett, 2006). The functional consequences of these changes are unclear. Prior efforts failed to find any axonal degeneration at the level of the peripheral nerve in vincristine-treated rats examined at an early time point in the course of the neuropathic pain syndrome (Tanner et al., 1998a; Topp et al., 2000). Moreover, Flatters and Bennett (2006) found no evidence of axonal degeneration at the level of the peripheral nerve in paclitaxel-treated rats examined at three time points: day 7 (preceding the onset of pain), day 27 (the time of approximate peak pain severity), and day 160 (when the pain state has resolved).
The mechanism by which paclitaxel and vincristine cause degeneration of the sensory terminal arbors is not known. We have shown that there is an increased incidence of abnormal mitochondria in sensory axons in rats with paclitaxel-evoked painful peripheral neuropathy (Flatters and Bennett, 2006). Impaired mitochondrial function might lead to degeneration of the axons' terminal arbors. Acetyl-l-carnitine, which can improve the function of impaired mitochondria (Virmani et al., 2004), prevents and reverses paclitaxel- and vincristine-evoked neuropathic pain (Ghirardi et al., 2005; Flatters et al., 2006).
ddC-evoked painful peripheral neuropathy
Rats with ddC-evoked painful peripheral neuropathy have sensory abnormalities that are very similar to those seen in paclitaxel- and vincristine-treated animals; however, we found no IENF loss in ddC-treated animals, and they did not have the increase in PGP9.5-positive LCs that was found in the other models. This suggests that ddC-evoked neuropathic pain is caused by a distinct mechanism, a possibility that is supported by other data (Aley and Levine, 2002; Joseph et al., 2004; Siau and Bennett, 2006).
Contralateral fiber loss after nerve lesion
At 2 days post-lesion, we found no statistically significant IENF loss contralateral to the complete sciatic nerve transection. Oaklander and Brown (2004) found a significant 54% contra-lateral loss after tibial nerve transection at 1 week post-lesion, but they did not examine earlier time points. Our finding suggests that contralateral IENF loss may occur with a distinct delay relative to that seen ipsilaterally.
Functional consequences of IENF loss
Accumulating evidence supports the idea that injury to somatosensory primary afferent neurons is necessary (although by no means sufficient) for the presence of painful peripheral neuropathy. For example, a loss of IENF like that reported here for paclitaxel- and vincristine-evoked pain has been documented in the neuropathic pain syndromes that accompany diabetes and glucose intolerance (Sumner et al., 2003), post-herpetic neuralgia (Oaklander, 2001), complex regional pain syndrome type-I (Albrecht et al., 2006; Oaklander et al., 2006), and burning mouth syndrome (Lauria et al., 2005b). We are still unable to identify the neural lesion that accompanies the painful peripheral neuropathy produced by ddC, but doses of ddC that are larger than those we tested do produce an anatomical lesion (Keswani et al., 2003). Why apparently identical neural lesions produce neuropathic pain in some patients but not in others is unknown.
It is probable that the fibers lost from the epidermis were largely Aδ and C fibers, including nociceptors, warming-specific, and cooling-specific fibers (McCarthy et al., 1995; Simone et al., 1998; Zylka et al., 2005). Normal rats do not have any sympathetic post-ganglionic fibers in the hind paw plantar epidermis (Yen et al., 2006).
At the time of peak pain severity, rats with paclitaxel- and vincristine-evoked painful peripheral neuropathies have an abnormal incidence (ca. 25%) of Aδ and C fibers with spontaneous discharge (Xiao and Bennett, 2005). The stimulus–response properties of Aδ and C fibers in paclitaxel-treated rats have not been examined. However, in rats with vincristine-evoked painful peripheral neuropathy, it has been found that about one-half of the C fibers have an abnormally large response to suprathreshold heat and mechanical stimulation (Tanner et al., 1998b, 2003).
It may be that spontaneous discharge and mechano/heat hyper-responsiveness are properties of fibers whose terminal arbors have degenerated or are in the process of degeneration. It is known that nociceptors whose axons have been transected acquire spontaneous discharge and that the proximal stump of the transected axon is mechano-sensitive (Devor and Seltzer, 1999). Axons whose terminal receptor arbors have degenerated might have similar spontaneous discharge and mechano-sensitivity. Nociceptor axons that are intact but travel in a peripheral nerve that contains degenerating axons also acquire spontaneous discharge (Wu et al., 2002); the same phenomenon might occur in terminal arbors that have degenerating neighbors. There is evidence that loss of Aδ cooling-specific fibers causes cold-allodynia (Ochoa and Yarnitsky, 1994). The loss of epidermal fibers in paclitaxel and vincristine rats might include a loss of Aδ cooling-specific fibers sufficient to evoke this phenomenon; thus accounting for the cold-allodynia seen in these animals (Polomano et al., 2001; Flatters and Bennett, 2004).
Langerhans cells
This is the first demonstration that PGP9.5 increases in LCs in paclitaxel- and vincristine-evoked painful peripheral neuropathies. We have confirmed earlier reports of increased PGP9.5 in LCs in rats after sciatic nerve transection (Hsieh et al., 1996; Stankovic et al., 1999) and in the CCI model (Lindenlaub and Sommer, 2002; Lin et al., 2001).
In the presence of IENF degeneration, it is conceivable that other kinds of dendritic cells and macrophages would invade the skin (reviewed in Valladeau and Saeland, 2005); these cells would also express the MHC class II antigen recognized by the OX-6 antibody (Otova et al., 1987; Bryan et al., 1988). We think it is very unlikely that the presence of other cell types led to a misidentification of LCs because the PGP9.5-positive cells exhibited the highly branched phenotype characteristic of LCs, and we found no increase in the number of OX-6-positive cells in paclitaxel-treated animals.
The up-regulation of PGP9.5 in LCs in those cases with IENF loss (paclitaxel- and vincristine-treatment, and the ipsilateral side of the CCI and nerve transection animals), but not in the cases without degeneration (ddC, and contralateral to CCI or transection), suggests that products of axonal degeneration may be the initiating signal for LC activation. If this were so, one might expect the largest increases in PGP9.5-positive LCs in cases with the greatest IENF loss. We found the opposite — the largest increases were in the chemotherapy cases, which had much less IENF loss than the animals with CCI or nerve transection. Hsieh et al. (1996) suggested that the PGP9.5 within LCs may represent protein that the LCs phagocytosed from degenerating nerve fibers. This seems an unlikely explanation in the case of the paclitaxel- and vincristine-treated animals where the IENF loss was considerably smaller than in the case of complete nerve transection.
Functional consequences of LC activation
A large body of evidence shows that LCs contribute to inflammatory cutaneous diseases, but a possible role for LCs in the mediation of neuropathic pain has received little attention. There is a report of an increase in the number of LCs containing the S100 antigen in patients with CRPS-I (Calder et al., 1998). Oaklander et al. (2003) found no increase in the number of LCs expressing the CD1a antigen in post-herpetic neuralgia patients.
Hsieh et al. (1996) detected increased levels of PGP9.5 transcripts in extracts of denervated skin, where LCs were the only cells present with PGP9.5 immunoreactivity. It thus seems likely that the up-regulated PGP9.5 immunoreactivity in LCs is due to authentic PGP9.5. PGP9.5 is an ubiquitin C-terminal hydroxylase (Wilkinson et al., 1989), a family of enzymes which cleave ubiquitinated conjugates in protein processing pathways. Its up-regulation in LCs thus suggests two possibilities. First, LCs are antigen-presenting cells and ubiquitin C-terminal hydroxylases are known to be involved in the processing of internalized antigenic proteins that precedes antigen presentation (Goldberg et al., 2002). Thus, the PGP9.5 increase in LCs may indicate increased antigen presentation. But if this is so, then it must be self-antigens that are being presented and there is no reason to suppose that chemotherapy-evoked neuropathy involves an autoimmune phenomenon. Second, ubiquitin C-terminal hydroxylases participate in protein synthesis (Elsasser and Finley, 2005). This suggests that the PGP9.5 up-regulation reflects an increased level of protein synthesis in LCs. Release of substances by activated LCs might affect nociceptor function.
LCs have close appositions with IENF terminals (Rice et al., 1993; Gaudillere et al., 1996; Hsieh et al., 1996). LCs express inducible nitric oxide synthase and release NO (Qureshi et al., 1996), which might sensitize remnant nociceptors. Moreover, there is evidence that activated LCs have increased synthesis of proinflammatory cytokines (Larrick et al., 1989; Matsue et al., 1992; Deng et al., 2003; Andersson et al., 2004), which are known to evoke ectopic spontaneous discharge and to sensitize C fiber nociceptors (Watkins and Maier, 2002). Hsieh et al. (1996) noted that following nerve transection, regenerating fibers that entered the epidermis appeared to travel towards the LCs, suggesting that LCs may release a neurotrophic factor (Torii et al., 1997).
In conclusion, paclitaxel- and vincristine-evoked painful peripheral neuropathies are associated with the degeneration of the intraepidermal terminal arbors of sensory fibers and the activation of the skin's resident immune cells. The next step is to determine whether these phenomena have a causal role in chemotherapy-evoked neuropathic pain.
Acknowledgments
Supported by the National Institutes of Health (R01-NS052255) and the Canada Foundation for Innovation. G.J.B. is a Canada Senior Research Chair. C.S. was a Research Fellow of the A*STAR program of Singapore. We thank A. L. Bailey and A. Ribeiro-da-Silva for their comments, A. L. Oaklander for advice, and L. Naso for assistance. Current address for C.S.: Department of Anesthesia, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119704; email: scinsg@yahoo.com.
References
- Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, Pare M, Davar G, Rice FL. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–266. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience. 2002;111:389–397. doi: 10.1016/s0306-4522(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Aley KO, Reichling DB, Levine JD. Vincristine hyperalgesia in the rat: a model of painful vincristine neuropathy in humans. Neuroscience. 1996;73:259–265. doi: 10.1016/0306-4522(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Andersson BU, Tani E, Andersson U, Henter JJ. Tumor necrosis factor, interleukin 11 and leukemia inhibitory factor produced by Langerhans cells in Langerhans cell histiocytosis. J. Pediatr. Hematol. Oncol. 2004;26:706–711. doi: 10.1097/00043426-200411000-00004. [DOI] [PubMed] [Google Scholar]
- Authier N, Coudore F, Eschalier A, Fialip J. Pain related behaviour during vincristine-induced neuropathy in rats. NeuroReport. 1999;10:965–968. doi: 10.1097/00001756-199904060-00013. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term taxol-induced nociceptive neuropathy in rats. Brain Res. 2000;887:239–249. doi: 10.1016/s0006-8993(00)02910-3. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. A new animal model of vincristine-induced nociceptive peripheral neuropathy. Neurotoxicology. 2003;24:797–805. doi: 10.1016/S0161-813X(03)00043-3. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Gautron M, Jazat F, Mayes M, Guilbaud G. The spectrum of fiber loss in a model of neuropathic pain in the rat: an electron microscopic study. Pain. 1991;47:359–367. doi: 10.1016/0304-3959(91)90229-Q. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bryan LA, Griebel PJ, Haines DM, Davis WC, Allen JR. Immunocytochemical identification of bovine Langerhans cells by use of a monoclonal antibody directed against class II MHC antigens. J. Histochem. Cytochem. 1988;36:991–995. doi: 10.1177/36.8.3292647. [DOI] [PubMed] [Google Scholar]
- Calder JS, Holten I, McAllister RMR. Evidence for immune system involvement in reflex sympathetic dystrophy. J. Hand Surg. (Br.) 1998;23B:147–150. doi: 10.1016/s0266-7681(98)80162-9. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, Zlotchenko E, Nguyen T, Garcia K, Tonra JR, Stambler N, Cedarbaum JM, Bodine SC, Lindsay RM, DiStefano PS. Physiological characterization of taxol-induced large-fiber sensory neuropathy in the rat. Ann. Neurol. 1998;43:46–55. doi: 10.1002/ana.410430111. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- Deng L, Ding W, Granstein RD. Thalidomide inhibits tumor necrosis factor-alpha production and antigen presentation by Langerhans cells. J. Invest. Dermatol. 2003;121:1060–1065. doi: 10.1046/j.1523-1747.2003.12565.x. [DOI] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Churchill Livingstone; Edinburgh: 1999. pp. 129–164. [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Dubinsky RM, Yarchoan R, Dalakas MC, Broder S. Reversible axonal neuropathy from the treatment of AIDS and related disorders with 2′,3′-dideoxycytidine (ddC) Muscle Nerve. 1989;12:856–860. doi: 10.1002/mus.880121012. [DOI] [PubMed] [Google Scholar]
- Elsasser S, Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell Biol. 2005;7:742–749. doi: 10.1038/ncb0805-742. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Ethosuximide has anti-allodynic and anti-hyperalgesic effects in rat models of paclitaxel and vincristine-induced painful peripheral neuropathies. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:247–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters SJL, Xiao W, Bennett GJ. Acetyl-l-carnitine prevents and reduces paclitaxel-induced painful neuropathy. Neurosci. Lett. 2006;397:219–224. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudillere A, Misery L, Souchier C, Claudy A, Schmitt D. Intimate associations between PGP9.5-positive nerve fibres and Langerhans cells. Br. J. Dermatol. 1996;135:343–344. doi: 10.1111/j.1365-2133.1996.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Ghirardi O, Vertechy M, Vesci L, Canta A, Nicolini G, Galbiati S, Ciogli C, Quattrini G, Pisano C, Cundari S, Rigamonti LM. Chemotherapy-induced allodynia: neuroprotective effect of acetyl-l-carnitine. In Vivo. 2005;19:631–637. [PubMed] [Google Scholar]
- Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol. Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Hsieh ST, Choi S, Lin WM, Chang YC, McArthur JC, Griffin JW. Epidermal denervation and its effects on keratinocytes and Langerhans cells. J. Neurocytol. 1996;25:513–524. doi: 10.1007/BF02284819. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev., Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Chander B, Hasan C, Griffin JW, McArthur JC, Hoke A. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann. Neurol. 2003;53:57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- Larrick JW, Morhenn V, Chiang YL, Shi T. Activated Langerhans cells release tumor necrosis factor. J. Leukoc. Biol. 1989;45:429–433. doi: 10.1002/jlb.45.5.429. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad neuropathologic–neurophysiologic correlation. J. Peripher. Nerv. Syst. 2005a;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- Lauria G, Majorana A, Borgna M, Lombardi R, Penza P, Padovani A, Sapelli P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain. 2005b;115:332–337. doi: 10.1016/j.pain.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Lin YW, Tseng TJ, Lin WM, Hsieh ST. Cutaneous nerve terminal degeneration in painful mononeuropathy. Exp. Neurol. 2001;170:290–296. doi: 10.1006/exnr.2001.7704. [DOI] [PubMed] [Google Scholar]
- Lindenlaub T, Sommer C. Epidermal innervation density after partial sciatic nerve lesion and pain-related behavior in the rat. Acta Neuropathol. (Berl.) 2002;104:137–143. doi: 10.1007/s00401-002-0527-7. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Calcitonin gene-related peptide, substance P and protein gene product 9.5 immunoreactive axonal fibers in the rat footpad skin following partial sciatic nerve injuries. J. Neurocytol. 2000;29:249–262. doi: 10.1023/a:1026519720352. [DOI] [PubMed] [Google Scholar]
- Matsue H, Cruz PD, Bergstresser PR, Takashima A. Cytokine expression by epidermal cell subpopulations. J. Invest. Dermatol. 1992;99:42S–45S. doi: 10.1111/1523-1747.ep12668619. [DOI] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- Munger BL, Bennett GJ, Kajander KC. The chronic constriction model of peripheral neuropathy: I. Axonal pathology in the sciatic nerve. Exp. Neurol. 1992;118:204–214. doi: 10.1016/0014-4886(92)90037-q. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001;93:69–76. doi: 10.1016/S0304-3959(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 2001;92:139–145. doi: 10.1016/s0304-3959(00)00481-4. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Brown JM. Unilateral nerve injury produces bilateral loss of distal innervation. Ann. Neurol. 2004;55:639–644. doi: 10.1002/ana.20048. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Stocks EA, Mouton PR. Number of Langerhans immune cells in painful and non-painful human skin after shingles. Arch. Dermatol. Res. 2003;294:529–535. doi: 10.1007/s00403-002-0362-7. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy) Pain. 2006;20:235–243. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Yarnitsky D. The triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain. 1994;117:185–197. doi: 10.1093/brain/117.1.185. [DOI] [PubMed] [Google Scholar]
- Otova B, Kren V, Bukovsky A, Pspichalova J. The cross-reaction patterns of the MRC OX-3, OX-6, and OX-17 monoclonal antibodies on rat inbred and congenic strains. Folia Biol. (Praha) 1987;33:363–366. [PubMed] [Google Scholar]
- Polomano R, Clark U, Mannes AJ, Bennett GJ. A painful peripheral neuropathy in rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Qureshi AA, Hosoi J, Xu S, Takashima A, Granstein RD, Lerner EA. Langerhans cells express inducible nitric oxide synthase and produce nitric oxide. J. Invest. Dermatol. 1996;107:815–821. doi: 10.1111/1523-1747.ep12330572. [DOI] [PubMed] [Google Scholar]
- Rice FL, Kinnman E, Aldskogius H, Johansson O, Arvidsson J. The innervation of the mystacial pad of the rat as revealed by PGP9.5 immunofluorescence. J. Comp. Neurol. 1993;337:366–385. doi: 10.1002/cne.903370303. [DOI] [PubMed] [Google Scholar]
- Siau C, Bennett GJ. Dysregulation of neuronal calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth. Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J. Neurosci. 1998;18:8947–8959. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic N, Johansson O, Hildebrand C. Increased occurrence of PGP 9.5-immunoreactive epidermal Langerhans cells in rat plantar skin after sciatic nerve injury. Cell Tissue Res. 1999;298:255–260. doi: 10.1007/s004419900083. [DOI] [PubMed] [Google Scholar]
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- Tanner KD, Levine JD, Topp KS. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J. Comp. Neurol. 1998a;395:481–492. [PubMed] [Google Scholar]
- Tanner KD, Reichling DB, Levine JD. Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J. Neurosci. 1998b;18:6480–6491. doi: 10.1523/JNEUROSCI.18-16-06480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner KD, Reichling DB, Gear RW, Paul SM, Levine JD. Altered temporal pattern of evoked afferent activity in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003;118:809–817. doi: 10.1016/s0306-4522(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Torii H, Yan Z, Hosoi J, Granstein RD. Expression of neurotrophic factors and neuropeptide receptors by Langerhans cells and the Langerhans cell-like cell line XS52 further support for a functional relationship between Langerhans cells and epidermal nerves. J. Invest. Dermatol. 1997;109:586–591. doi: 10.1111/1523-1747.ep12337516. [DOI] [PubMed] [Google Scholar]
- Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J. Comp. Neurol. 2000;424:563–576. [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin. Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Virmani A, Gaetani F, Binienda Z, Xu A, Duhart H, Ali SF. Role of mitochondrial dysfunction in neurotoxicity of MPP+: partial protection of PC12 cells by acetyl-l-carnitine. Ann. N. Y. Acad. Sci. 2004;1025:267–273. doi: 10.1196/annals.1316.033. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci. 2002;22:7746–7753. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Abstr. 11th World Congr. Pain. IASP Press; Seattle: 2005. Spontaneous discharge in primary afferent fibers in paclitaxel-evoked neuropathic pain in the rat; p. 426. [Google Scholar]
- Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J. Comp. Neurol. 2006;495:679–690. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]