Abstract
The inner ear is exposed to aminoglycosides or other drugs either intentionally or as a side effect of clinical treatments directed at other regions of the body. An understanding of the effects of drugs on the inner ear requires knowledge of the pharmacokinetics of the drug once it reaches the cochlear fluids, specifically how much of it reaches different parts of the ear and how long it stays there before disappearing. Accumulating data show that drug distribution in the inner ear is complex, especially for drugs applied locally to the ear's round window membrane. Locally applied drugs do not disperse rapidly, but instead spread very slowly through the fluid spaces by diffusion so that substantial differences in drug concentration occur in different regions of the ear. In some cases, the drug may leak from the inner ear to the blood as fast as it diffuses, meaning it may never become uniformly distributed even when applied for a long period. In recent years, experimental pharmacokinetic studies have become increasingly quantitative, permitting the results to be interpreted with computer models. Simulations of the drug distribution in animals have been used as a basis to predict the likely drug distribution in the larger, human inner ear. Such studies allow clinical drug delivery protocols to be optimized to minimize inadvertent hearing loss and to deliver therapeutic levels of the drug more effectively.
Introduction
The effects of drugs on the inner ear depend in large part on the pharmacokinetics of the drug within the fluids of the inner ear, specifically what drug concentrations reach different regions of the ear and how long the drug stays in each region before disappearing. For aminoglycosides, two routes of entry into the ear are important. The first is the systemic route, in which drugs circulate in the bloodstream and then penetrate the inner ear fluids. Because aminoglycosides given systemically can affect both hearing and balance, it is apparent that the drug enters both the cochlea (the auditory portion of the inner ear) and the inner ear's vestibular regions.
Other studies have shown that aminoglycosides applied locally to the inner ear may also suppress vestibular (balance) system function—with sometimes only minor effects on hearing. This has led to a second drug application method, one that has provided the basis of treatment for diseases such as Meniere's. The treatment consists of injecting solution containing aminoglycoside into the middle ear space so that it contacts the round window membrane (RWM) of the cochlea. Counterintuitively, applying aminoglycosides to the cochlea can result in greater suppression of the balance system than that of hearing. By understanding the pharmacokinetics of drugs applied by different methods, it is now possible to account for such results.
Local Application of Aminoglycosides to the Ear
Clinical Studies
Aminoglycosides were known to be toxic to the sensory cells of the inner ear and were first applied locally to human ears by Schuknecht (1956). This procedure was performed as an alternative to surgical labyrinthectomy and consisted of repeated injections of streptomycin into the middle ear space until vestibular symptoms developed. The method did not gain popularity until Lange (1977) reported finding that gentamicin was substantially less ototoxic than streptomycin. In Meniere's patients given gentamicin to suppress their vestibular symptoms, Lange reported that hearing could be preserved in 76% of cases. Since then, there have been numerous studies in which the applied gentamicin dose and the application protocol have been varied (as reviewed by Carey, 2004; see also Carey, 2005, this issue). From this pool of studies, it was deduced that a less-frequent dosing regimen resulted in better hearing preservation.
Subsequently, Toth and Parnes (1995) introduced the concept of a “titration protocol,” in which injections were given once a week until observation of the first symptoms of ototoxicity. Thus, the dosing and application protocols in use today were developed purely by empirical methods over a period of many years in numerous clinical studies. The actual gentamicin dose reaching the inner ear remains largely unknown, since the clinical method has no underlying quantitative rationale. Indeed, this trial-and-error approach in the optimization of delivery protocols—in the absence of a theoretical background—carries a substantial risk to patients. A recent example occurred with the advent of implantable catheters by which drugs could be delivered directly to a patient's RWM for a prolonged period. In one study using delivery via catheter, gentamicin was applied at a dose of 40 mg/day—common for a “one-shot” application, except that the dose was delivered continuously for a number of days until the patient experienced vestibular symptoms (Schoendorf, Neugebauer & Michel, 2001). In this study, nearly all the treated patients (80%) lost their hearing, presumably due to the higher intracochlear drug concentrations generated by the more efficient application system. Such a result should have been predictable, and serves to demonstrate that without a theoretical framework to quantify drug delivery protocols, trying to predict the outcome of protocol changes is a highly uncertain proposition.
The major variables that affect the amount of gentamicin entering the cochlea include:
Amount of drug applied, which depends on the concentration of drug in solution and on the volume of solution injected into the middle ear space.
Duration that the drug solution remains in contact with the RWM.
Number of times the drug application is repeated.
Method of application, specifically whether injection is performed “blindly” through the tympanic membrane; through a microotoscope (Plontke et al., 2002a) after visualizing the RWM and removing the “false” membranes that occur in 30% of patients (Alzamil & Linthicum, 2000); or by application through an implanted cannula.
Background medium of the drug-containing solution, which may potentially influence the permeability properties of the cells of the RWM. Properties such as the osmolarity, pH, and ionic composition are likely to influence the properties of cells that the solution contacts.
As noted above, the number of factors that can influence the drug level reaching the cochlear fluids is large and it is not possible to derive the influence of each of these factors in clinical studies.
Animal Studies
Experiments with animals allow some of the important factors determining intracochlear drug levels, and their effects on the ear, to be established. Interpretation is complicated, however, by differences in cochlear dimensions between humans and most experimental animals. Because of this size difference, if a treatment suitable for humans is applied to an animal, then the drug level in the cochlear fluids is likely to be far higher in the experimental animal than the human. Comparisons across species therefore require the differences in cochlear dimensions to be considered.
There are a number of studies in which morphological changes and/or functional changes in the ear have been documented following local gentamicin application. The applied gentamicin concentration plays a major role in the results. Wagner et al. (2005) report that a “one-shot” 10 mg/mL gentamicin dose caused only minor outer hair cell losses, while a 40 mg/mL dose caused substantial hair cell losses in the basal turns with less damage at the apex. Imamura and Adams (2003) similarly found extensive cellular damage with 40 mg/mL gentamicin, with organ of Corti degeneration most severe at the base and less at the apex. They reported that the pattern of damage depended markedly on the formulation of the applied drug. Extensive damage was found with commercial gentamicin solution, but considerably less damage occurred in many animals when the gentamicin was applied in a physiologic artificial perilymph solution. This suggests that the degree of toxicity of the applied solution to the RWM may influence its permeability properties and, therefore, the perilymph level of drug achieved. Using immunostaining methods, Imamura and Adams (2003) observed the distribution of gentamicin in cells of the ear and found a marked base-to-apical gradient of drug uptake that corresponded to the basal application site.
In other studies, inner ear changes resulting from sustained delivery of gentamicin have been observed. Wagner et al. (2005) compared gentamicin delivery to the RWM for 1 week by a mini-osmotic pump, with the total dosage matched to the “one-shot” application described above. With two applied concentrations (10 mg/mL and 40 mg/mL), the authors again found much greater damage with the higher applied drug level. They also found that hair cell losses were consistent across animals with the sustained drug delivery and that, compared to one-shot applications, more hair cell loss was produced at the base and less in the apical turns.
These studies confirm that the application protocol influences the outcome, even when the total amount of applied drug is the same. Okuda et al. (2004) similarly applied 4, 12, and 40 mg/mL solution to the round window (RW) niche with mini-osmotic pumps and noted decreasing damage in the apical turns compared to the base, with almost no damage in the second and third turns with the lowest applied dose.
The experimental studies described above demonstrate that: (1) damage to the cochlea is dose-dependent; (2) damage is greatest near the base of the cochlea and declines toward the apex; (3) damage depends on the application protocol (sustained delivery vs. “one-shot” application); and (4) damage depends on the background medium of the applied gentamicin solution.
Before discussing direct measurements of drug pharmacokinetics in the inner ear fluids, it is necessary to introduce the concept of “clearance.” When a drug is applied to one of the fluid spaces of the body (e.g., to the blood or perilymph), it does not stay there indefinitely; over time, it is lost to other spaces within the body. The loss from the specific compartment of interest is described as clearance. For example, if the kidneys remove a drug from the bloodstream and accumulate it in the urine, that would represent a clearance of drug from the bloodstream. In this example, the mechanism of clearance is well understood. Similarly, if a drug is applied to the inner ear, it will not stay there forever either. In this case, if the measured drug level in the cochlea declines with time, we again describe the loss as resulting from clearance. However, while perilymph concentration measurements may show that clearance is occurring, they commonly cannot define the mechanism of that clearance. The total clearance rate may represent the combined effects of many possible processes, including diffusion into adjacent compartments of the ear, loss across the vascular endothelial cells to the blood, or accumulation of the drug by tissues of the ear (hence removing the drug from the perilymph). Clearance by tissues may include accumulation in intracellular compartments, binding to receptors, or metabolism of the drug either intracellularly or by proteins in the fluid spaces. In addition to normal physiologic processes that contribute to clearance, there are also a variety of experimental procedures that can influence and contribute to clearance. For example, if a fluid without drug is washed through the middle ear spaces and contacts the RWM, then drug in the cochlear perilymph may diffuse out of the cochlea and into the fluid in the middle ear. This, too, would represent a clearance of drug from the inner ear fluid spaces. Fluid leaks from the cochlea can also contribute to clearance. Thus, establishing the rate, underlying mechanisms, and kinetic characteristics of clearance are of major importance in understanding the kinetics of drugs in cochlear fluids.
The gentamicin concentration and kinetics in the cochlear fluids have been investigated in a number of studies in which fluids of the ear were sampled and analyzed following local gentamicin delivery. The most extensive of these used the chinchilla as a model (Hoffer et al., 1997, 1999; Balough et al., 1998). Samples of perilymph were taken from the vestibule at various times following application of 5 mg/mL gentamicin to the RW niche. In these experiments, the drug solution was applied in the form of a gel made with fibrin glue—to stabilize the volume and keep it within the RW niche. The analysis of samples showed a slow entry of gentamicin into the vestibule, reaching a peak concentration of approximately 700 μg/mL after approximately 10 hours, before declining over a period of days. The amplitude of the peak represented approximately 14% of the applied concentration. In a subsequent study, Hoffer et al. (2001) analyzed perilymph samples taken from the RW, for the same, sustained release drug delivery protocol but at twice the concentration (10 mg/mL). Counterintuitively, they found a far lower and later peak concentration (300 μg/mL at 42–72 hours). This discrepancy probably arises from difficulties in sampling perilymph through the RWM without contamination by cerebrospinal fluid (CSF). Such contamination of the sample results in lower drug levels in the sample than appear to be actually present in the perilymph, which is discussed in more detail later.
Another study used a microdialysis technique to quantify the kinetics of gentamicin in the scala tympani (ST) of guinea pig perilymph following local application (Hibi, Suzuki & Nakashima, 2001). In this approach, a small probe containing a permeable membrane was sealed into ST and fluid was passed slowly through the probe. Fluid efflux from the probe, which had equilibrated with perilymph, was collected and analyzed for gentamicin concentration. A major advantage of this method is that many samples can be obtained over time from a single animal, allowing the entire time course of drug to be followed. Hibi et al. (2001) also observed that gentamicin entered ST rapidly, with concentration peaking within 100 minutes. In addition, they found that gentamicin was rapidly cleared from the perilymph, with a halftime of 75 minutes, which contrasts markedly with prior studies that indicated that gentamicin remained in the ear for more than a week after application. (Hoffer et al., 1999; Imamura & Adams, 2003). This anomalous finding likely results from methodological problems associated with the microdialysis technique. Specifically, it is the consequence of a failure to correct for the clearance of drug from perilymph caused by the dialysis procedure itself. As gentamicin diffuses into the dialysis system and is accumulated in the samples, the resulting loss from the cochlea represents a nonphysiological clearance of drug that would not occur in the absence of dialysis. In controlled studies, Hahn et al. (2005) demonstrated that microdialysis of small compartments or of ST of guinea pigs results in a decline of drug concentration in the compartment as a direct result of the dialysis procedure itself. In the presence of this clearance caused by the dialysis procedure itself, it is not possible to accurately quantify the true physiological clearance rate in the absence of dialysis. Thus, the high clearance rate reported in Hibi et al.'s (2001) study is not likely to represent the physiological rate.
The kinetics of gentamicin entry has also been measured in the chicken following local application of 200 mg/mL gentamicin (Bunting et al., 2004), with perilymph concentration peaking at approximately 50 μg/mL after four hours. This peak, at 0.03% of the applied dose, is far lower than that found in the chinchilla, and the authors point out that species differences are likely to be considerable. The decline of concentration in this study was found to be slow, with significant gentamicin still present after five days.
In another study, human perilymph was sampled from the vestibule following local application of a 40 mg/mL solution to the RWM (Becvarovski et al., 2002a). The concentration increased over the course of an hour, peaking at around 14 mg/mL, which is approximately 35% of the applied concentration. This suggests entry in the human occurs more readily than in animals.
Computer Simulations of Drug Movements in the Ear
A major part of our work in recent years has been to understand, in a quantitative manner, how drugs spread through the cochlea. Because the ear is a complex structure, the only way to do this with quantitative accuracy is through computer modeling. The first model of solute movements in the ear was originally developed to help interpret experiments in which the rate of longitudinal endolymph flow was measured (Salt et al., 1986). In these studies, the spread of chemical marker ions through the cochlear fluids was monitored with ion-selective microelectrodes. Ion electrodes measure concentration of substance at a fixed location over a period of time during the experiment. In our experiments, we could detect and monitor the time course of marker ion concentration increasing at the measurement site following application at a distant site. We needed to determine whether the maker was reaching our measurement electrodes by volume movements of the fluids, or whether the maker was simply diffusing passively through the compartment. Interpreting such measurements was impossible without some form of mathematical modeling. This problem was solved by developing a finite-element computer model that combined the basic processes of solute movements (passive diffusion and volume flow) so that marker spread as a function of both distance along the cochlea and time could be calculated. Initial simulations quickly showed that solute spread in most situations was accounted for by simple diffusion, with volume flow having negligible influence. Over time, the program became more sophisticated, taking into account the anatomic dimensions of the cochlea and a variety of anatomic communications, including the RWM, the helicotrema, the vestibule, inter-scala communications, and solute exchange with the blood. The basic processes underlying solute movements in the cochlea that were incorporated into the simulation program are illustrated in Figure 1. We quickly realized that this type of model had more general applications than simply interpreting flow measurements, and could be used to calculate the spread of any substance in the ear. In 1999, we added a more “user-friendly” visual interface to the program and made it publicly available via the Internet, available as a download at http://oto.wustl.edu/cochlea/. In the present version (v. 1.6h), cochlear dimensions can be changed to represent the ear of one of seven different species (human, gerbil, chinchilla, guinea pig, bat, rat, and mouse).
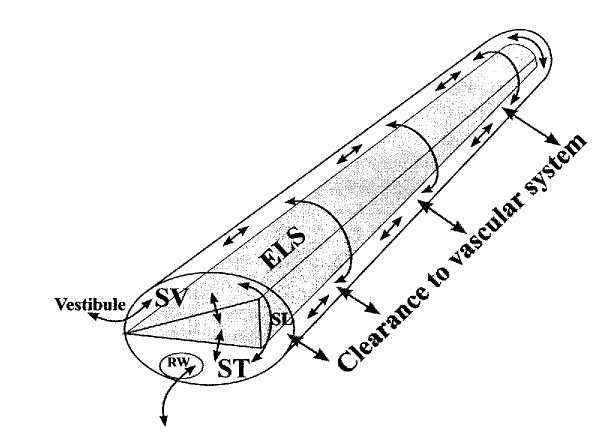
Figure 1.
Schematic of the important processes involved in the distribution of drugs in the ear following local application. The cochlea is shown uncoiled with scala vestibuli (SV) and scala tympani (ST) shown in white and the endolymphatic space (ELS) shown shaded. Communication with the middle ear and ST occurs via the round window (RW) membrane. Spaces of the vestibule communicate with SV. Communication between ST and SV can occur either via the ELS or through the extracellular spaces of the spiral ligament (SL). Substances are spread along the scalae by diffusion, some of which may be lost to the vascular system, a process known as clearance.
The details and capabilities of the simulator have been described in detail elsewhere (Salt, 2002). The value of such modeling is that it allows specific experiments to be interpreted, and in so doing to define the important parameters of drug movements such as the permeability of the RWM and the rate of clearance of the drug from the fluids. With these basic parameters available, it is then possible to calculate:
Concentration time courses occurring in different regions of the ear.
Distribution of drug along the length of the cochlear scalae at different times after application.
Changes in the drug distribution with distance and time for different delivery protocols.
Concentration gradients expected for similar treatments of the ears of different species.
For RW drug delivery, the model was initially validated experimentally using the ionic marker trimethylphenylammonium (TMPA). Solution containing TMPA was irrigated over the RWM, while the perilymph concentration of TMPA was monitored with ion selective electrodes sealed into the first or second turn of ST (Salt & Ma, 2001). Based on the recorded time course of TMPA at the two sites, it was possible to establish parameters representing the RWM permeability, the rate of longitudinal flow, and the rate of TMPA clearance from the scala, which allowed the model to closely fit the recorded data. It was also possible to extend the simulations for a longer period than the actual experiment to show that the concentration gradients of drugs along the cochlea do not decline with time. Instead, the gradient reaches a steady state in which diffusion of TMPA along the scala is balanced by the clearance of TMPA out of the scala. The TMPA therefore never reaches the apical part of the cochlea in the same concentration that it is at the base.
The demonstration that substantial drug gradients occur along the cochlea after local delivery is qualitatively consistent with the gradients of histologic damage seen following local gentamicin delivery (Wagner et al., 2005; Imamura & Adams, 2003; Okuda et al., 2004). It must be noted, however, that the magnitude of the drug gradient along the scala depends on two factors: the rate that the drug diffuses and the rate of clearance of the drug from perilymph. A diffusion coefficient defines how quickly the drug is diffusing and its value depends on molecular size, with smaller molecules diffusing faster (larger diffusion coefficients) and larger molecules diffusing more slowly (smaller diffusion coefficients). Therefore, larger drugs should result in steeper gradients.
The second factor affecting distribution is the rate of clearance. For drugs that are cleared rapidly from the scala, the longitudinal drug gradient will be large and the drug may never reach the apical regions of the cochlea. For substances that are cleared more slowly, the drug will diffuse further before it is cleared and the longitudinal gradients along the scala may be lower. For this reason, the rate of drug clearance by the cochlea is a very important parameter, since it not only influences the time the drugs stays in the ear but also how far the drug spreads with distance. The combination of these factors (diffusion coefficients and clearance rates) make predictions of drug concentrations along the cochlear duct very difficult. This can only be clarified through quantitative computer simulation.
This form of quantitative computer analysis has been applied to gentamicin concentration measurements in a study by Plontke et al. (2002b). The study simulated in detail the experimental studies of Hoffer et al. (1997), Balough et al. (1998), and Hoffer et al. (1999), including the drug delivery protocol and the procedures used to collect perilymph samples. In order to simulate the reported gentamicin time course, only a highly restricted set of parameters allowed the model to fit the experimental data. For example, it was found to be impossible for the drug to diffuse along ST, through the helicotrema, and then down scala vestibuli (SV) to the vestibule. By this route, the concentration obtained was too low and the time course far too slow. It was instead necessary to incorporate a so-called “radial” exchange between each of the scalae, (i.e., communication between the ST and scala media [SM], the SV and SM, and the SV and ST) within each segment of the cochlea. Radial communication has been demonstrated in prior studies (Saijo & Kimura, 1984; Salt, Ohyama & Thalmann, 1991), but its prominent influence on the movement of substance between ST and the vestibule had not previously been recognized. The rate of gentamicin clearance that allowed the model to best-fit the data was a half-time of 505 min (8.4 hours). Although this rate is consistent with some prior studies, it too cannot be regarded as the true rate that gentamicin is cleared from perilymph to blood by local cochlear mechanisms.
In Hoffer's experiments, the fluid volume applied to the middle ear was stabilized with fibrin glue and the gel stayed there for the duration of the experiment. Our analysis showed that in order to generate the observed time course, it was necessary to incorporate a process of middle-ear drug clearance, i.e., loss of drug to regions other than the cochlea (Plontke et al., 2002b). One consequence of this process occurs when the middle-ear concentration of gentamicin falls below that of the perilymph. Diffusion of gentamicin out of the cochlea occurs through the RWM in this situation. This loss of gentamicin again represents a significant source of clearance from the scala. In the analysis, it was not possible to distinguish the different sites of gentamicin clearance. Thus, our calculated rate represents the clearance only when there is a (nonphysiologic) fluid-filled RW niche. The clearance from perilymph when the middle ear is air-filled could be considerably lower. Analysis of both the dialysis and the sampling experiments thus serve to demonstrate how the precise details of an experiment can influence the outcome in unexpected ways. The clinical implications of these studies are that drugs delivered as gels in the middle ear may result in temporarily higher doses in the cochlea, but a prolonged fluid-filled middle ear may also contribute to increased drug clearance with time.
The calculations showing that drugs diffuse rather slowly along the cochlear scalae, while at the same time diffusing across the spiral ligament into the vestibule, account for how hearing can be preserved with a gentamicin dose that affects vestibular function. In the human, the region of maximum sensitivity to 4 kHz sounds is approximately 11.5 mm from the base of the cochlea. It can be calculated that with local RWM application, the drug concentration reaching the vestibule is comparable to that reaching the 4 kHz region, with lower gentamicin concentrations reaching the more apical regions sensitive to lower frequencies (Plontke et al., 2002b). There is no doubt that regions of high-frequency sensitivity at the base of the cochlea will be damaged by local gentamicin treatment, but those frequencies most important for speech discrimination can be spared.
Entry of Aminoglycosides Following Systemic Application
An understanding of how aminoglycosides enter the inner ear from the bloodstream requires knowledge of the nature of the so-called “blood-labyrinth barrier” (BLB) and how the endolymph and perilymph are maintained.
Perilymph Homeostasis Mechanisms
Because the ionic composition of perilymph is similar to that of blood plasma, it was initially believed that perilymph originated as an ultrafiltrate of blood (Schnieder, 1974). However, careful anatomic studies established that there were no fenestrations in the endothelial cells of the inner ear capillaries; instead, the cells formed a barrier with tight junctions morphologically similar to the blood-brain barrier (Jahnke, 1980). When combined with physiologic studies demonstrating that entry rates decreased for substances of greater molecular size (Juhn & Rybak, 1981), the existence of a functional BLB was established. This was further supported by studies showing that the profile of proteins in the perilymph was also markedly different from plasma (Thalmann et al., 1992). Ionic tracer studies have also demonstrated a tight barrier between blood and perilymph which shows a similar response to vascular hypertension as the blood-brain barrier (Inamura & Salt, 1992). When a period of acute hypertension was induced by epinephrine, the permeability of the BLB increased transiently. The anatomic site of the BLB is not well established, but is likely to include the endothelial walls of capillaries in the spiral ligament and the spiral vessels associated with the inner sulcus and organ of Corti. In addition, capillary beds of the spiral ganglion and modiolus may also contribute perilymph homeostasis. According to a recent study, there are numerous canaliculi in the bony walls that separate ST from the spiral ganglion through which communication may occur (Shepherd & Colreavy, 2004).
In many studies, it has been suggested that perilymph is partially derived from CSF. The anatomic basis of this concept is the cochlear aqueduct, which connects perilymph to the cranium. In the cochlea, the aqueduct opens into ST of the basal turn near the RWM. The concept of a CSF contribution to perilymph was originally supported by studies in which perilymph sampled from ST was found to have similar composition to CSF. Although this view remains widespread, we conclude that it largely arises from the technical difficulties of sampling pure perilymph from ST without artifactual CSF contamination. The difficulty occurs as soon as the bony otic capsule is perforated or as a fluid sample is withdrawn from ST. When any fluid volume is lost from the scala, it is replaced by CSF entering ST through the aqueduct. If the sample was taken from the base of ST, then it can easily become contaminated with a substantial amount of CSF. This can be mistakenly interpreted as demonstrating that CSF contributes to perilymph.
The influence of perilymph sampling procedures on sample composition and on the degree of sample contamination with CSF was demonstrated by Scheibe, Haupt & Bergmann (1984). Using careful sampling procedures, they were able to demonstrate systematic chemical differences between ST perilymph and CSF (Scheibe & Haupt, 1985). Hara, Salt and Thalmann (1989) also showed that the glycine composition of a sample taken from ST varied according to the sample volume taken, with samples larger than 0.2 μL showing lower glycine levels (consistent with CSF contamination). The degree of contamination of fluid samples with CSF was further quantified in a study where the concentration of the marker ion TMPA was monitored in ST with an ion selective microelectrode sealed into the scala (Salt, Kellner & Hale, 2003). The time course of TMPA concentration change in the scala was monitored before, during, and after fluid sampling through the RW or through the bony wall of the cochlea. The concentration of TMPA in the fluid sample was then compared with the perilymph content prior to sampling. Based on detailed computer simulations of the experiment, it was estimated that 1 μL samples taken from the basal turn of ST were contaminated with approximately 15% CSF, while 10 μL samples contained only 15% perilymph and 85% CSF. The use of such large samples taken from the basal turn is not unusual (Parnes, Sun & Freeman, 1999; Arnold et al., 2005).
When these findings are combined with measurements that show that perilymph does not flow appreciably along the scala when the cochlea is in its normal, sealed state (Ohyama et al., 1988; Salt & Ma, 2001), it can be concluded that the contribution of CSF to the normal perilymph composition and turnover is extremely small. Prior studies which have concluded that CSF contributes substantially to perilymph have often not taken adequate precautions to prevent CSF contamination of their samples. Others may have used species such as rats, in which the cochlear fluid volume is very small, so samples are more likely to be contaminated with CSF during sampling. In order to interpret any study based on perilymph sampling, it is very important to critically evaluate the precise methods that were used to obtain the fluid samples.
Our present understanding is the perilymph is not secreted in volume, but is instead maintained by transport or diffusion of solutes across the BLB with little apparent associated volume flow. The kinetics for drug entry from blood, or of drug clearance to blood, depends on the local BLB properties. For most purposes, we assume that the kinetics as a function of distance along the cochlea is similar in different cochlear turns. However, physical principles dictate that because the basal turn has larger scala cross-sectional areas and greater volume, we would expect slower kinetics in the basal turn. This would be true if the density of capillaries in apical and basal turns was the same. In contrast, if the capillary density was greater in the basal turn, the kinetics could be faster there. To our knowledge, there are no quantitative data to establish whether kinetics vary systematically in different turns of the cochlea, so we cannot exclude this possibility.
Endolymph Homeostasis Mechanisms
The origins of endolymph are perhaps better understood, partially because measurement of the ionic content of endolymph samples (with high potassium content and very low sodium content) has provided a robust “quality-control” index of sample purity. It is believed that cochlear endolymphatic ion homeostasis is dominated by the highly vascular stria vascularis in the lateral wall of the cochlea and by the dark cells in the vestibular system—the cells that contain a variety of K+, Na+, and Cl− transporters (Wangemann, 1995, 2002). The major ions appear to be recycled between the endolymph and perilymph (Spicer & Schulte, 1998), with ions such as K+ leaving the endolymph as part of the transduction current before being taken up and transported back into the endolymph. Ion recycling appears to occur without an appreciable net movement of water, since the rate of longitudinal endolymph flow is close to zero (Salt & Thalmann, 1989). It should not be assumed, however, that stria vascularis is the only tissue maintaining endolymph, since specific contributions to homeostasis may involve all other tissue types that contact the endolymph. Immunostaining studies have shown that other tissues comprising the endolymph boundary may contain specific transporters, such as the Ca-ATPases and H-ATPases found in the spiral limbus (Stankovic et al., 1997; Ichimiya, Adams & Kimura, 1994).
With regard to communication between the endolymph and vascular system, the endolymph has been regarded as a “deep” compartment, in which all communication occurs indirectly via the perilymph (Sterkers, Ferrary & Amiel, 1988). Although the major ions may be recycled between the endolymph and perilymph—supporting this “deep compartment” concept—that may not be true for all substances. It remains possible that substances in blood may pass through the endothelial cell walls of the capillaries in the intrastrial space, and from there traverse the marginal cells into the endolymph.
One factor affecting substance movements by these two routes may be the electrical charge of the substance. For substances with negative charge, the large positive endocochlear potential will result in a gradient for entry from the perilymph, so that the substance will be drawn into the endolymph. An example is the anionic marker AsF6, which when perfused through the perilymphatic space is drawn into the endolymph and remains there even when the perilymph concentration is subsequently reduced (Salt & DeMott, 1995). In contrast, cationic substances in the perilymph do not easily enter the endolymph, since entry is opposed by the positive endocochlear potential. This is demonstrated by the cationic marker TMPA, which does not enter the endolymph when perfused through the perilymphatic space (Salt et al., 1991). Movements of charged molecules between the endolymph and perilymph are therefore markedly influenced by the endocochlear potential.
The extent to which ionic movements between endolymph and the blood through the capillaries of stria vascularis are influenced by the endocochlear potential remains uncertain. It is known that capillaries in stria vascularis pass through the intrastrial space, an extracellular compartment that is positively polarized by the endocochlear potential. This raises the possibility that an electrical polarization of the capillaries could allow cationic substances (such as gentamicin) may move from plasma into the intrastrial space more readily as the electrical gradient opposing the movement would be less. There is, however, evidence that the endothelial cells of the capillaries are connected by gap junctions to the intermediate cell/basal cell complex (Takeuchi & Ando, 1998; Takeuchi, Ando, Sato, & Kakigi, 2001), which would not be consistent with the capillaries being electrically polarized. Nevertheless, the estimated incidence of gap junctions is not high (one per 0.03 mm2), and the electrical potential of the capillaries remains unknown. The characteristics of transport across the endothelial boundary into the intrastrial space remain undocumented at the present time, but a direct entry of gentamicin into endolymph by this route remains probable. The major communication processes between the cochlear fluids and the vascular system are summarized in Figure 2. Communication with the blood occurs primarily in the capillary beds of the lateral wall, which include elements in the spiral ligament and within the stria vascularis. Drugs can enter endolymph either indirectly via the perilymph or directly through the stria vascularis.
Figure 2.
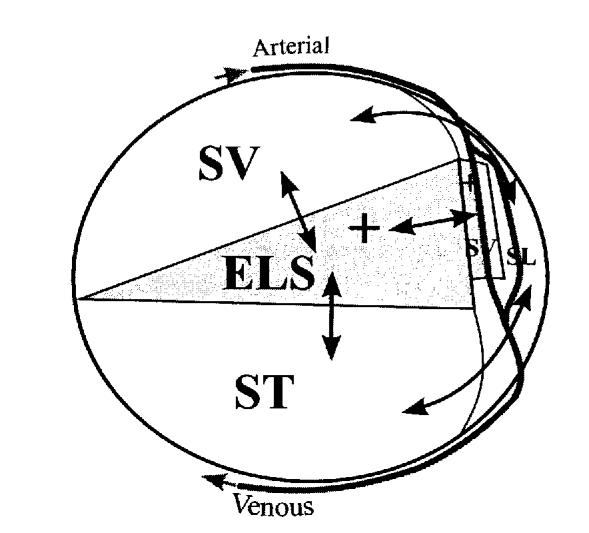
Schematic of a cross-section through one turn of the cochlea shows the major communication routes between the fluid spaces and the vasculature. Arterioles and venules pass around the scalae through channels in the bone and form capillary beds in both the stria vascularis (sv) and spiral ligament (SL) of the lateral wall. The perilymph of scala tympani (ST) and scala vestibuli (SV) readily communicate with fluid spaces of the spiral ligament. Drugs can enter the endolymphatic space (ELS) either by communication with the perilymphatic scalae or from capillaries that contact the positively polarized intrastrial space in stria vascularis.
Gentamicin Entry
The entry of gentamicin from blood into the fluids and tissues of the inner ear have been investigated by a variety of methods. Tran ba Huy et al. (1986) assayed fluid and tissue samples from rats at intervals after a single intravenous injection or sustained infusion of gentamicin at different dosages. They found that entry into the ear showed saturation kinetics, but concentrations in the ear never exceeded the level in plasma, demonstrating that gentamicin was not actively accumulated in the ear. Gentamicin concentrations in the endolymph were lower than those of the perilymph. It was also apparent that gentamicin entry into the ear occurred rapidly (over a 12-hour period) while the rate of loss of gentamicin from the ear occurred extremely slowly, with significant gentamicin levels still apparent after 24 days. This time course of entry into the ear contrasts with the functional effects of aminonglycosides, which typically require many days of treatment (typically 10–14) before substantial threshold elevations are observed. Entry of gentamicin into hair cells and subtle functional changes, consistent with suppression of the medial efferent system, do occur with a more rapid time course, which is consistent with the documented rate of drug entry into the perilymph (Hiel et al., 1993; Aran et al., 1999). The loss of hair cells and supporting cells is dose-dependent, decreasing from base to apex and from first to third rows of outer hair cells, consistent with a gradient of uptake across the rows (Hiel et al., 1993). With high doses given intraperitoneally, there was initially greater uptake in cells of the basal turn compared to the apex, but after four days this gradient declined (Imamura & Adams, 2003). Becvarovski et al. (2002b) sampled perilymph from humans at different times following intravenous administration and found perilymph levels comparable to the plasma levels for all but the shortest time intervals. The time course of cellular damage to the human cochlea has been studied in temporal bones and it has been shown that there is a decrease in the number of hair cells and in the cross-sectional area of stria vascularis within two weeks of the systemic aminoglycoside treatment (Kusunoki et al., 2004).
Facilitation of Gentamicin Entry with Diuretics
When given alone, systemic gentamicin does not cause threshold elevations until 10–14 days postdosage. In contrast, many studies have reported that aminoglycosides given systemically in combination with diuretics such as furosemide or ethacrynic acid result in hearing loss and cellular damage within a few days of the treatment (Brummett et al., 1975; McFadden et al., 2002). This effect appears to be specific to the cochlea with little or no damage produced in the vestibular system (McFadden et al., 2002). It is accounted for by an influence of the diuretic on the vascular permeability in the tissues of stria vascularis. Tran ba Huy et al. (1983) reported that endolymph levels of gentamicin were higher than those of perilymph in rats treated with gentamicin and ethacrynic acid, suggesting that the diuretic had facilitated the entry of gentamicin directly from blood into the endolymph. This is further supported by a tracer study (Naito & Watanabe, 1997) showing that horseradish peroxidase leaked from the capillaries of stria vascularis more readily after furosemide administration, probably as a result of the transient edema that occurs in stria when treated with diuretic. In an autoradiographic study using guinea pigs treated with tritiated gentamicin and ethacrynic acid (Hiel et al., 1992), gentamicin was first detected in the vessels of stria vascularis and in hair cells at the base of the cochlea after 1 hour. At later times (4 and 24 hours), the gentamicin level in stria vascularis was no longer detectable, although the concentration was increased in the hair cells.
If the diuretic is delayed a few hours after the gentamicin injection, then the degree of hair cell loss is reduced (McFadden et al., 2002). This is either because the plasma level of gentamicin has decreased by the time the barrier is made permeable, or because the reactive oxygen species (ROS) generated by each treatment are separated in time and do not overwhelm the antioxidant defense systems (McFadden et al., 2002). It has also been shown that if ethacrynic acid is given 12–18 hours after the gentamicin treatment, the gentamicin concentration in the perilymph is reduced and the cochlear hair cells are protected (Ding et al., 2003). This is explained by the diuretic increasing the permeability of the BLB, which in these circumstances allows the gentamicin that has accumulated in the perilymph to leak out.
Pharmacokinetic Approaches to Prevention of Aminoglycoside Ototoxicity
In recent years, there has been major interest in various approaches to protect the ear from damaging ototoxic substances, such as aminoglycosides or cisplatin. This is a complex issue, requiring not only an understanding of the pharmacokinetics of both the toxic agent and the protectant substance, but also the details of the underlying mechanism of protection and how the processes of toxicity and protection interact. Most of the studies published in this area fall into one of two categories.
The first is the protection from systemically delivered drugs that are used clinically to treat specific problems, but, as in the case of aminoglycosides or cisplatin, there is an undesirable ototoxic effect of the drug. If the ears of these patients could be protected from damage without blocking the primary action of the drug, it would have substantial clinical benefit. Most of the studies in this direction are therefore clinically directed. Protection of the ear from systemic drugs may occur through multiple mechanisms. Generally, treatments that affect the systemic level of drug, such as increasing clearance by the kidneys, are not appropriate, as they would attenuate the intended treatment. Protection of the ear could be afforded by: (1) a protective agent given systemically, or (2) a protective agent given locally by injection into the middle ear. To date, most studies have used the systemic delivery of protective agents. Substances delivered systemically having a protective effect on hearing loss or on histological damage following gentamicin include glutathione (Lautermann, McLaren & Schacht, 1995); salicylate (Sha & Schacht, 1999); dihydroxybenzoate (Sinswat, Wu, Sha & Schacht, 2000); the antioxidant alpha-tocopherol (Sergi et al., 2004; Fetoni et al., 2004); CEP–1347, a blocker of the c-Jun N-terminal kinase pathway (Ylokoski et al., 2002), trimetazine (Unal et al., 2005); and flavinoids (Long et al., 2004).
The second category of studies is the protection of the ear from the effects of locally applied drugs such as gentamicin. These studies are generally less clinically oriented, as the suppression of vestibular hair cell function is an intended purpose of the locally applied gentamicin. Instead, the majority of studies in this category have basic scientific goals as a general model for the prevention of cell death. Studies of this type have shown that the damage caused by locally applied aminoglycosides can be attenuated by locally applied alpha lipoic acid (Conlon & Smith, 2000), glutathione caspase inhibitors (Okuda et al., 2005), and Ginkgo biloba (Jung et al., 1998). The kinetics and distribution of the antioxidants D-methionine and thiourea have been studied in the rat using autoradiography and sampling by Laurell et al. (2002). Following a 1-hour application of thiourea or D-methionine, the peak levels were found to be 1.9% and 1.5% of the applied concentrations, respectively, followed by declines with half-times of 0.77 hours and 0.57 hours, respectively. The distribution of the substances in the tissues demonstrated highest concentrations in the spiral ligament, with the concentration declining from base to apex along the cochlea.
Another approach to providing antioxidant therapy has been reported by Kawamoto et al. (2003) and Kawamoto et al. (2004): the use of gene therapy to protect from ototoxicity. After transfection of cells with adenoviral vectors for overexpression of catalase or a Mn superoxide dismutase (SOD2; Kawamoto et al., 2004) or GDNF (Kawamoto et al., 2003), hair cells and hearing thresholds were significantly protected from aminoglycoside damage. The value of this approach is that the protective substance is far longer lasting than locally delivered drugs, since it is continually synthesized over time by cells of the cochlea.
Conclusion
In the past decade, we have developed an understanding of the basic pharmacokinetics of substances in the inner ear following local or systemic applications. The literature is complicated by the technical artifacts that can distort pharmacokinetic measurements. Nevertheless, the basic principles by which substances are distributed in the ear have been established and we are now in a position to quantify the drug levels achieved with different application protocols and for different experimental procedures.
Acknowledgements
This article was supported by NIH research grant RO1 DC01368 from the National Institute on Deafness and Other Communication Disorders (NIDCD).
References
- Alzamil KS, Linthicum FH., Jr. Extraneous round window membranes and plugs: Possible effect on intratympanic therapy. Annals of Otology, Rhinology, and Laryngology. 2000;109:30–32. doi: 10.1177/000348940010900105. [DOI] [PubMed] [Google Scholar]
- Aran JM, Erre JP, Lima da Costa D, Debbarh I, Dulon D. Acute and chronic effects of aminoglycosides on cochlear hair cells. Annals of the New York Academy of Sciences. 1999;28:60–68. doi: 10.1111/j.1749-6632.1999.tb08636.x. [DOI] [PubMed] [Google Scholar]
- Arnold W, Senn P, Hennig M, Michaelis C, Deingruber K, Scheler R, Steinhoff H, Riphagen F, Lamm K. Novel slow- and fast-type drug release round-window microimplants for local drug application to the cochlea: An experimental study in guinea pigs. Audiology & Neuro-otology. 2005;10:53–63. doi: 10.1159/000082575. [DOI] [PubMed] [Google Scholar]
- Balough BJ, Hoffer ME, Wester D, O'Leary MJ, Brooker CR, Goto M. Kinetics of gentamicin uptake in the inner ear of Chinchilla langier after middle-ear administration in a sustained-release vehicle. Otolaryngology and Head and Neck Surgery. 1998;119:427–431. doi: 10.1016/S0194-5998(98)70097-X. [DOI] [PubMed] [Google Scholar]
- Becvarovski Z, Bojrab DI, Michaelides EM, Kartush JM, Zappia JJ, LaRouere MJ. Round window gentamicin absorption: an in vivo human model. The Laryngoscope. 2002a;112:1610–1613. doi: 10.1097/00005537-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Becvarovski Z, Michaelides EM, Kartush JM, Bojrab DI, LaRouere MJ. Rapid elevation of gentamicin levels in the human labyrinth following intravenous administration. The Laryngoscope. 2002b;112:1163–1165. doi: 10.1097/00005537-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Brummett RE, Traynor J, Brown R, Himes D. Cochlear damage resulting from kanamycin and furosemide. Acta Otolaryngologica. 1975;80:86–92. doi: 10.3109/00016487509121305. [DOI] [PubMed] [Google Scholar]
- Bunting EC, Park DL, Durham D, Girod DA. Gentamicin pharmacokinetics in the chicken inner ear. Journal of the Association for Research in Otolaryngology. 2004;5:144–152. doi: 10.1007/s10162-003-4033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J. Intratympanic gentamicin for the treatment of Meniere's disease and other forms of peripheral vertigo. Otolaryngologic Clinics of North America. 2004;37:1075–1090. doi: 10.1016/j.otc.2004.06.002. Review. [DOI] [PubMed] [Google Scholar]
- Carey JP. Vestibulotoxicity and management of vestibular disorders. The Volta Review. 2005;105:251–276. this issue. [Google Scholar]
- Conlon BJ, Smith DW. Topical aminoglycoside ototoxicity: Attempting to protect the cochlea. Acta Otolaryngologica. 2000;120:596–599. doi: 10.1080/000164800750000397. [DOI] [PubMed] [Google Scholar]
- Ding D, McFadden SL, Browne RW, Salvi RJ. Late dosing with ethacrynic acid can reduce gentamicin concentration in perilymph and protect cochlear hair cells. Hearing Research. 2003;185:90–96. doi: 10.1016/s0378-5955(03)00258-2. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Sergi B, Ferraresi A, Paludetti G, Troiani D. Alpha-Tocopherol protective effects on gentamicin ototoxicity: An experimental study. International Journal of Audiology: Official Organ of the International Society of Audiology. 2004;43:166–171. doi: 10.1080/14992020400050023. [DOI] [PubMed] [Google Scholar]
- Hahn H, Kammerer A, DiMauro A, Zenner H-P, Salt AN, Plontke S. The use of microdialysis for quantification of dexamethasone and flourescein entry into scala tympani during round window application. 28th Midwinter Research Meeting of the ARO (Abstract) 2005;306 doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A, Salt AN, Thalmann R. Perilymph composition in scala tympani of the cochlea: Influence of cerebrospinal fluid. Hearing Research. 1989;42:265–272. doi: 10.1016/0378-5955(89)90150-0. [DOI] [PubMed] [Google Scholar]
- Hibi T, Suzuki T, Nakashima T. Perilymphatic concentration of gentamicin administered intratympanically in guinea pigs. Acta Otolaryngologica. 2001;121:336–341. doi: 10.1080/000164801300102699. [DOI] [PubMed] [Google Scholar]
- Hiel H, Erre JP, Aurousseau C, Bouali R, Dulon D, Aran JM. Gentamicin uptake by cochlear hair cells precedes hearing impairment during chronic treatment. Audiology: Official Organ of the International Society of Audiology. 1993;32:78–87. doi: 10.3109/00206099309072930. [DOI] [PubMed] [Google Scholar]
- Hiel H, Schamel A, Erre JP, Hayashida T, Dulon D, Aran JM. Cellular and subcellular localization of tritiated gentamicin in the guinea pig cochlea following combined treatment with ethacrynic acid. Hearing Research. 1992;57:157–165. doi: 10.1016/0378-5955(92)90148-g. [DOI] [PubMed] [Google Scholar]
- Hoffer ME, Allen K, Kopke RD, Weisskopf P, Gottshall K, Wester D. Transtympanic versus sustained-release administration of gentamicin: Kinetics, morphology, and function. The Laryngoscope. 2001;111:1343–1357. doi: 10.1097/00005537-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Hoffer ME, Balough B, Henderson J, DeCicco M, Wester D, O'Leary MJ, Kopke R. Use of sustained release vehicles in the treatment of Meniere's disease. Otolaryngologic Clinics of North America. 1997;30:1159–1166. [PubMed] [Google Scholar]
- Hoffer ME, Balough BJ, Kopke RD, Henderson J, DeCicco M, Wester DC, O'Leary MJ, Balaban C. Morphologic changes in the inner ear of chinchilla laniger after middle ear administration of gentamicin in a sustained-release vehicle. Otolaryngology and Head and Neck Surgery. 1999;120:643–648. doi: 10.1053/hn.1999.v120.a91762. [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. Journal of the Association for Research in Otolaryngology. 2003;4:176–195. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya I, Adams JC, Kimura RS. Immunolocalization of Na+, K(+)-ATPase, Ca(++)-ATPase, calcium-binding proteins, and carbonic anhydrase in the guinea pig inner ear. Acta Otolaryngologica. 1994;114:167–176. doi: 10.3109/00016489409126037. [DOI] [PubMed] [Google Scholar]
- Inamura N, Salt AN. Permeability changes of the blood labyrinth barrier measured in vivo during experimental treatments. Hearing Research. 1992;61:12–18. doi: 10.1016/0378-5955(92)90030-q. [DOI] [PubMed] [Google Scholar]
- Jahnke K. The blood-perilymph barrier. Archives of Otorhinolaryngology. 1980;228:29–34. doi: 10.1007/BF00455891. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Rybak LP. Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngologica. 1981;91:529–534. doi: 10.3109/00016488109138538. [DOI] [PubMed] [Google Scholar]
- Jung HW, Chang SO, Kim CS, Rhee CS, Lim DH. Effects of ginkgo biloba extract on the cochlear damage induced by local gentamicin installation in guinea pigs. Journal of Korean Medical Science. 1998;13:525–528. doi: 10.3346/jkms.1998.13.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Molecular Therapy. 2004;9:173–181. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Yagi M, Stover T, Kanzaki S, Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF. Molecular Therapy. 2003;7:484–492. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Kusunoki T, Cureoglu S, Schachern PA, Sampaio A, Fukushima H, Oktay MF, Paparella MM. Effects of aminoglycoside administration on cochlear elements in human temporal bones. Auris, Nasus, Larynx. 2004;31:383–388. doi: 10.1016/j.anl.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Lange G. The intratympanic treatment of Meniere's disease with ototoxic antibiotics. A follow-up study of 55 cases. Laryngo- Rhino- Otologie (Stuttgart) 1977;56:409–414. [PubMed] [Google Scholar]
- Laurell G, Teixeira M, Sterkers O, Bagger-Sjoback D, Eksborg S, Lidman O, Ferrary E. Local administration of antioxidants to the inner ear: Kinetics and distribution. Hearing Research. 2002;173:198–209. doi: 10.1016/s0378-5955(02)00613-5. [DOI] [PubMed] [Google Scholar]
- Lautermann J, McLaren J, Schacht J. Glutathione protection against gentamicin ototoxicity depends on nutritional status. Hearing Research. 1995;86:15–24. doi: 10.1016/0378-5955(95)00049-a. [DOI] [PubMed] [Google Scholar]
- Long M, Smouha EE, Qiu D, Li F, Johnson F, Luft B. Flavanoid of Drynaria fortunei protects against gentamicin ototoxicity. Phytotherapy Research: PTR. 2004;18:609–614. doi: 10.1002/ptr.1505. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Woo JM, Salvi RJ. Chinchilla models of selective cochlear hair cell loss. Hearing Research. 2002;174:230–238. doi: 10.1016/s0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- Naito H, Watanabe K. Alteration in capillary permeability of horseradish peroxidase in the stria vascularis and movement of leaked horseradish peroxidase after administration of furosemide. ORL; Journal for Oto-rhino-laryngology and its Related Specialties. 1997;59:248–257. doi: 10.1159/000276948. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Salt AN, Thalmann R. Volume flow rate of perilymph in the guinea pig cochlea. Hearing Research. 1988;35:119–130. doi: 10.1016/0378-5955(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Okuda T, Sugahara K, Shimogori H, Yamashita H. Inner ear changes with intracochlear gentamicin administration in guinea pigs. The Laryngoscope. 2004;114:694–697. doi: 10.1097/00005537-200404000-00018. [DOI] [PubMed] [Google Scholar]
- Okuda T, Sugahara K, Takemoto T, Shimogori H, Yamashita H. Inhibition of caspases alleviates gentamicin-induced cochlear damage in guinea pigs. Auris, Nasus, Larynx. 2005;32:33–37. doi: 10.1016/j.anl.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: An animal study followed by clinical application. The Laryngoscope. 1999;109:1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Plinkert PK, Plinkert B, Koitschev A, Zenner HP, Lowenheim H. Transtympanic endoscopy for drug delivery to the inner ear using a new microendoscope. Advances in Oto-rhino-laryngology. 2002a;59:149–155. doi: 10.1159/000059253. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Wood AW, Salt AN. Analysis of gentamicin kinetics in fluids of the inner ear with round window administration. Otology & Neurotology. 2002b;23:967–974. doi: 10.1097/00129492-200211000-00026. [DOI] [PubMed] [Google Scholar]
- Saijo S, Kimura RS. Distribution of HRP in the inner ear after injection into the middle ear cavity. Acta Otolaryngologica. 1984;97:593–610. doi: 10.3109/00016488409132937. [DOI] [PubMed] [Google Scholar]
- Salt AN. Simulation of methods for drug delivery to the cochlear fluids. Advances in Oto-rhino-laryngology. 2002;59:140–148. doi: 10.1159/000059251. [DOI] [PubMed] [Google Scholar]
- Salt AN, DeMott JE. Endolymph volume changes during osmotic dehydration measured by two marker techniques. Hearing Research. 1995;90:12–23. doi: 10.1016/0378-5955(95)00142-0. [DOI] [PubMed] [Google Scholar]
- Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hearing Research. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- Salt AN, Thalmann R. Rate of longitudinal flow of cochlear endolymph. In: Nadol JB, editor. Ménière's Disease. Kugler Press; Amsterdam: 1989. pp. 69–73. [Google Scholar]
- Salt AN, Kellner C, Hale S. Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hearing Research. 2003;182:24–33. doi: 10.1016/s0378-5955(03)00137-0. [DOI] [PubMed] [Google Scholar]
- Salt AN, Ohyama K, Thalmann R. Radial communication between the perilymphatic scalae of the cochlea. I. Estimation by tracer perfusion. Hearing Research. 1991;56:29–36. doi: 10.1016/0378-5955(91)90150-8. [DOI] [PubMed] [Google Scholar]
- Salt AN, Thalmann R, Marcus DC, Bohne BA. Direct measurement of longitudinal endolymph flow rate in the guinea pig cochlea. Hearing Research. 1986;23:141–151. doi: 10.1016/0378-5955(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H. Biochemical differences between perilymph, cerebrospinal fluid and blood plasma in the guinea pig. Hearing Research. 1985;17:61–66. doi: 10.1016/0378-5955(85)90131-5. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Bergmann K. On sources of error in the biochemical study of perilymph (guinea pig) Archives of Otorhinolaryngology. 1984;240:43–48. doi: 10.1007/BF00464343. [DOI] [PubMed] [Google Scholar]
- Schnieder EA. Contribution to the physiology of perilymph. Part I: The origins of perilymph. Annals of Otology. 1974;83:76–83. doi: 10.1177/000348947408300113. [DOI] [PubMed] [Google Scholar]
- Schoendorf J, Neugebauer P, Michel O. Continuous intratympanic infusion of gentamicin via a microcatheter in Meniere's disease. Otolaryngology and Head and Neck Surgery. 2001;124:203–207. doi: 10.1067/mhn.2001.112310. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Ablation therapy for the relief of Meniere's disease. The Laryngoscope. 1956;66:859–870. doi: 10.1288/00005537-195607000-00005. [DOI] [PubMed] [Google Scholar]
- Sergi B, Fetoni AR, Ferraresi A, Troiani D, Azzena GB, Paludetti G, Maurizi M. The role of antioxidants in protection from ototoxic drugs. Acta Otolaryngologica (Stockholm) 2004;552(Suppl):42–45. [PubMed] [Google Scholar]
- Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoxicity. Laboratory Investigation: A Journal of Technical Methods and Pathology. 1999;79:807–813. [PubMed] [Google Scholar]
- Shepherd RK, Colreavy MP. Surface microstructure of the perilymphatic space. Implications for cochlear implants and cell- or drug-based therapies. Archives of Otolaryngology—Head & Neck Surgery. 2004;130:518–523. doi: 10.1001/archotol.130.5.518. [DOI] [PubMed] [Google Scholar]
- Sinswat P, Wu WJ, Sha SH, Schacht J. Protection from ototoxicity of intraperitoneal gentamicin in guinea pig. Kidney International. 2000;58:2525–2532. doi: 10.1046/j.1523-1755.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Evidence for a medial K+ recycling pathway from inner hair cells. Hearing Research. 1998;118:1–12. doi: 10.1016/s0378-5955(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Stankovic KM, Brown D, Alper SL, Adams JC. Localization of pH regulating proteins H+ATPase and Cl−/HCO3− exchanger in the guinea pig inner ear. Hearing Research. 1997;114:21–34. doi: 10.1016/s0378-5955(97)00072-5. [DOI] [PubMed] [Google Scholar]
- Sterkers O, Ferrary E, Amiel C. Production of inner ear fluids. Physiological Reviews. 1988;68:1083–1128. doi: 10.1152/physrev.1988.68.4.1083. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Ando M. Dye-coupling of melanocytes with endothelial cells and pericytes in the cochlea of gerbils. Cell Tissue Research. 1998;293:271–275. doi: 10.1007/s004410051118. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Ando M, Sato T, Kakigi A. Three-dimensional and ultrastructural relationships between intermediate cells and capillaries in the gerbil stria vascularis. Hearing Research. 2001;155:103–112. doi: 10.1016/s0378-5955(01)00252-0. [DOI] [PubMed] [Google Scholar]
- Thalmann I, Comegys TH, Liu SZ, Ito Z, Thalmann R. Protein profiles of perilymph and endolymph of the guinea pig. Hearing Research. 1992;63:37–42. doi: 10.1016/0378-5955(92)90071-t. [DOI] [PubMed] [Google Scholar]
- Toth AA, Parnes LS. Intratympanic gentamicin therapy for Meniere's disease: Preliminary comparison of two regimens. Journal of Otolaryngology. 1995;24:340–344. [PubMed] [Google Scholar]
- Tran ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. Journal of Clinical Investigation. 1986;77:1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran ba Huy P, Manuel C, Meulemans A, Sterkers O, Wassef M, Amiel C. Ethacrynic acid facilitates gentamicin entry into endolymph of the rat. Hearing Research. 1983;11:191–202. doi: 10.1016/0378-5955(83)90078-3. [DOI] [PubMed] [Google Scholar]
- Unal OF, Ghoreishi SM, Atas A, Akyurek N, Akyo l G., Gursel B. Prevention of gentamicin induced ototoxicity by trimetazidine in animal model. International Journal of Pediatric Otorhinolaryngology. 2005;69:193–199. doi: 10.1016/j.ijporl.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Wagner N, Caye-Thomasen P, Laurell G, Bagger-Sjoback D, Thomsen J. Cochlear hair cell loss in single-dose versus continuous round window administration of gentamicin. Acta Otolaryngologica. 2005;125:340–345. doi: 10.1080/00016480510026881. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Comparison of ion transport mechanisms between vestibular dark cells and strial marginal cells. Hearing Research. 1995;90:149–157. doi: 10.1016/0378-5955(95)00157-2. [DOI] [PubMed] [Google Scholar]
- Wangemann P. K(+) cycling and its regulation in the cochlea and the vestibular labyrinth. Audiology & Neuro-otology. 2002;7:199–205. doi: 10.1159/000063736. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hearing Research. 2002;166:33–43. doi: 10.1016/s0378-5955(01)00388-4. [DOI] [PubMed] [Google Scholar]