Abstract
In eukaryotes, meiotic recombination events are distributed nonrandomly in the genome, with certain regions having high levels of recombination (hotspots) and others having low levels (coldspots). Species with similar DNA sequences (for example, chimpanzees and humans) can have strikingly different patterns of hotspots and coldspots. Below, by using a microarray analysis that allows us to measure the frequency of the meiosis-specific double-strand DNA breaks (DSBs) of all 6,000 yeast genes, we show that mutation of a single gene (SIR2), which encodes a histone deacetylase, significantly changes DSB frequencies of 12% of yeast genes, elevating DSBs of 5%, and reducing DSBs of 7%. Many of the genes with repressed recombination are located in large (50–100 kb) regions located near, but not at, the telomeres. Some of the genes with altered frequencies of DSBs (including the ribosomal RNA gene cluster) are known targets of Sir2p deacetylation in the wild-type strain.
Keywords: chromatin, meiosis, Sir2p, yeast
In the yeast Saccharomyces cerevisiae and in most other eukaryotes that have been examined, meiotic recombination is initiated by double-strand DNA breaks (DSBs) catalyzed by the topoisomerase-related Spo11p (1). The frequencies of DSBs vary widely at different sites in the yeast genome and are affected by both global and local factors (2). Examples of global effects are the suppression of recombination in large (50–100 kb) regions near the telomeres and centromeres (3–5). Examples of local effects are the preference for DSBs to occur within nuclease-sensitive regions of chromatin (6) at the 5′ ends of genes (3). Some hotspots (α hotspots) require the binding of transcription factors (2), whereas others (γ hotspots) are associated with local regions of high G + C base composition (3). Although the mechanistic explanations of these local effects are not clear, it has been suggested (3, 7, 8) that the preference for DSB formation to occur in regions of high G + C content is a consequence of cohesins blocking DSB formation at their AT-rich binding regions (3, 7–9).
No single well defined DNA sequence motif defines preferred sites for DSB formation in S. cerevisiae. This observation and the other observations described above indicate that the frequency of DSB formation is determined, at least in part, by chromatin structure rather than primary DNA sequence. In support of this conclusion, Yamada et al. (10) showed that deletion of a histone acetylase or an ATP-dependent chromatin remodeling factor resulted in a partial reduction in recombination activity at one hotspot in Schizosaccharomyces pombe. In this report, using DNA microarrays, we show that the DSB frequencies throughout the yeast genome are dramatically altered by a mutation in a single gene (SIR2) encoding a chromatin-modifying enzyme. This result demonstrates that one aspect of recombination is extremely malleable and is responsive to small changes in the global chromatin state.
Sir2p is a histone deacetylase, one of whose substrates is H4K16 (11). Sir2p binds near the telomeres and within the rRNA gene cluster (12), and Robyr et al. (13) showed that Sir2p-mediated modifications are concentrated near the telomeres. Sir2p is involved in silencing expression of telomeric genes, the silent mating type loci HML and HMR, and genes inserted within the rRNA gene cluster (14). Although no global analysis of the effects of the sir2 mutation on recombination have been reported previously, sir2 strains have elevated rates of intrachromosomal meiotic recombination in the rRNA genes (15) and elevated DSB formation in yeast artificial chromosomes containing mouse DNA (16), but reduced crossovers in one genetic interval near the end of chromosome I (17). As described below, we find that the sir2 mutation has a very pronounced effect on the distribution of meiosis-specific DSBs in S. cerevisiae, significantly elevating DSBs for 5% of the genes and significantly reducing DSBs for 7% of the genes.
Results
Genome-Wide Mapping of DSB Activity in sir2 Strains by DNA Microarrays.
To map the frequency of meiosis-specific DSBs throughout the genome in the sir2 strain MD311 [strain construction described in supporting information (SI) Table 1], we used the same method that we used previously to map DSBs in the wild-type strain JG169 (3, 5). In brief, we used chromatin immunoprecipitation to purify DNA that is covalently associated with Spo11p. These samples were labeled with a fluorescent probe and hybridized to a DNA microarray (containing all yeast genes and intergenic regions) along with total genomic DNA that was labeled with a different fluorescent probe. We will refer to the log2 of the hybridization ratio of (Spo11p-enriched DNA/total genomic DNA) as the DSB activity of the gene. Because the DNA fragments used for the microarray analysis were sheared to a size of ≈2 kb, the DSB activity is related to the frequency of local DSBs.
The DSB activities for each ORF and intergenic region derived from 13 sir2 microarrays and 11 isogenic wild-type microarrays (5) are shown in SI Table 2. Based on a two-tailed t test, comparing the wild-type and sir2 microarrays, we found 784 genes (12%) had significantly (P < 0.005) altered DSB activity (346 increased and 438 decreased). In all, we found that 3,441 ORFs had elevated DSBs in the sir2 strain and 2,946 had decreased DSBs, although many of these changes were not statistically significant. The summary of the median DSB activities for all ORFs is shown in SI Table 3.
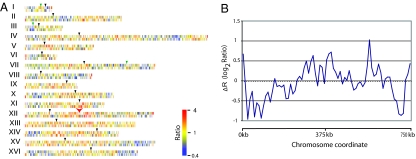
We also calculated the difference in DSB activities between sir2 and wild-type strains by subtracting the wild-type DSB activity from the activity observed in the sir2 strain. These values [termed ΔR, because the DSBs are the recombination (R)-initiating lesion] are shown for all elements on the microarray and for genes only are in SI Tables 3 and 4, respectively. In Fig. 1A, we depict ΔR for all genes on all chromosomes, showing DSB activities that are reduced (blue), increased (red), or unchanged (yellow) in the sir2 strain. These results, described in detail below, show that the Sir2p can act to suppress or promote DSB formation in different genomic regions, and can act either locally or regionally on the chromosome.
Fig. 1.
Depictions of the differences in DSB activities [log2(Spo11p-enriched DNA/total genomic DNA)] in a sir2 strain compared with a wild-type strain. For this analysis, the DSB activity in the wild-type strain was subtracted from the recombination activity in the sir2 strain, generating the ΔR values. (A) GeneSpring software was used to depict ΔR for all genes. Blue indicates a ΔR of <0 (fewer DSBs in the sir2 mutant than in wild-type), yellow indicates a ΔR of ≈0 (DSBs similar in sir2 and wild-type), and red indicates ΔR of >0 (DSBs higher in sir2 than in wild-type). Roman numerals specify each chromosome, black arrows show the position of the centromere, green arrows indicate the positions of genes in which the DSB frequencies were measured by using standard Southern blot analysis, and the red arrow indicates the position of the rRNA gene array. (B) The ChIPOTle software (18) was used to measure ΔR on chromosome X in a window of 20 kb, moved along the chromosome in intervals of 10 kb.
Genes and Genomic Regions with Elevated DSBs in the sir2 Strain.
We observed increased DSBs at the telomeres, within the rRNA gene cluster and its flanking sequences, and in genes scattered throughout the genome. Genes very close (within 10 kb) to the telomeres generally had elevated rates of DSBs in the sir2 strain (Fig. 1A). Fig. 1B shows the elevation of ΔR at the telomeres of chromosome X, as visualized by calculating ΔR along the chromosome in a moving window of 20 kb. Throughout the genome, the regions within 10 kb of the telomeres had an overrepresentation of genes with positive ΔR values (SI Table 5). Because Sir2p deacetylates H4K16 near the telomeres (13), we suggest that increased levels of acetylation in the sir2 strain results in a more open chromatin structure, allowing Spo11p and associated proteins increased access to the DNA.
In wild-type strains, recombination is suppressed within the rRNA genes (19) and flanking regions (4, 5), and loss of sir2 elevates the rate of intrachromosomal recombination in the rRNA gene cluster (15). Our analysis showed elevated DSBs within the rRNA gene cluster in the sir2 strain (Fig. 2), although these regions are still colder than the average gene. In addition, we found that the suppression of DSBs in genes flanking the cluster is partially relieved by the sir2 mutation (Fig. 2). Of the 146 genes in the region flanking the rRNA gene cluster (SGD coordinates 300–600 kb), 139 have elevated DSB activity and 7 have reduced DSB activity in the sir2 strain, a very significant (P < 0.0001) departure from the frequency of genes with elevated DSBs in the sir2 strain in the genome as a whole.
Fig. 2.
DSB activities in wild-type and sir2 strains on chromosome XII. This figure depicts the effect of the sir2 mutation on the rRNA gene cluster and its flanking sequences. Because only two of the ≈150 repeats are present on the microarray, the rRNA gene cluster is much larger than it appears in this representation. For all four graphs, DSB activities were measured in a window of 20 kb moved in intervals of 10 kb. (A) DSB activity in the wild-type strain JG169. (B) DSB activity in the sir2 mutant strain MD311. (C) Depiction of the overlap in the wild-type (red) and sir2 (blue) patterns of DSBs. (D) ΔR calculated along chromosome XII. The elevation in DSBs in the sir2 strain within the rRNA genes and flanking sequences is evident.
The final class of genes with elevated DSBs in the sir2 strain had no obvious common characteristic. One example is CYS3 on chromosome I (Fig. 3A). It is possible that the elevated recombination in these genes reflects a redistribution of recombination-promoting proteins from the regions proximate to the telomeres (which have suppressed DSBs in sir2 strains) to other regions in the genome.
Fig. 3.
Comparison of DSB activities by Southern blot analysis and microarray analysis. (A) The DSB activities along chromosome I (based on microarray data) were examined in a sliding window of 1 kb, moved in intervals of 250 bp. DSB formation is suppressed in the sir2 strain on the left (coordinates, 20–110 kb) and right (coordinates, 160–210 kb) arms of the chromosome; CEN1 is located at coordinate 151 kb. DSB formation at CYS3 (shown by an arrow) is elevated in the sir2 strain compared with wild-type. (B) Intact chromosomal DNA was isolated from wild-type (PG169) and sir2 (MD311) strains immediately after the cells were incubated in sporulation medium (0 h) or after 24 h in sporulation medium. Chromosomes were separated by pulse–field gel electrophoresis, and the separated chromosomes were examined by Southern blot analysis by using a chromosome I-specific probe (further details in SI Materials and Methods). DSBs in the left (arrow 4) and right (arrow 1) arms were reduced in the sir2 strain relative to DSBs near the middle of the chromosome (arrows 2 and 3). (C–E) We show standard Southern blots of DNA samples isolated from wild-type and sir2 strains hybridized to three different probes (enzymes used for DNA treatment shown in parentheses): HIS4 (BglII) (C), YGR177 (BglII) (D), and CYS3 (HindIII) (E); the specific probes used are described in SI Materials and Methods.
Genes and Genomic Regions with Suppressed DSBs in the sir2 Strain.
One salient feature of the sir2 strain is the increased suppression of DSBs in regions beginning ≈10–20 kb from the telomere and extending ≈100 kb toward the centromere (Figs. 1–4, SI Tables 5 and 6). One method of visualizing this suppression is to calculate ΔR along the chromosome in a moving window of 20 kb. For example, on chromosome X, the genes located within 20 kb of the telomere have slightly elevated DSB formation in the sir2 strain relative to wild-type, followed by the region of suppressed DSBs (Fig. 1B). We estimate that 21 of the 32 telomeres have large (≥50 kb) regions of suppressed DSBs that begin ≈20 kb from the chromosome end (SI Table 6). We term these areas of suppressed DSB formation “telomere-proximate suppressed regions” (TPSRs). The average size of TPSRs is 135 kb and the region telomere-distal to the TPSRs that escapes suppression averages ≈20 kb.
Fig. 4.
Differences in DSB formation between sir2 and wild-type strains (ΔR) as a function of distance from the telomere. For this analysis, we averaged ΔR in bins of 10 kb for all elements on the microarray for all chromosomes. Standard error bars are shown. The elevation of DSB formation within ≈10 kb of the chromosome end is evident, as is the suppression of DSBs that extends to ≈120 kb from the telomere.
In SI Table 5, we show the number of ORFs with increased and decreased recombination activity in the sir2 strain in 10-kb “bins” from the telomeres toward the centromere. There is a significant (P ≤ 0.0001) excess of ORFs with elevated DSBs in the 20 kb closest to the telomere and a significant (P < 0.0001) excess of ORFs with decreased DSBs for all ORFs between 30 and 90 kb from the telomere. Of the 438 ORFs with significantly reduced DSBs in the sir2 strain, 297 are in TPSRs. The elevation of DSBs very close to the telomeres and the suppression of DSBs in the regions between 20 and 150 kb from the telomeres averaged over all of the chromosomes are shown in Fig. 4. In addition to the TPSRs, there were two other large regions of reduced DSBs in the sir2 strain on chromosomes II and IV (Fig. 1A).
Although many of the genes with suppressed DSBs in the sir2 strain were located in TPSRs, there were also some isolated genes with reduced DSB formation, presumably reflecting a local alteration in chromatin structure. We looked for correlations between our ΔR data set and other global studies done in yeast, and found a correlation between genes that have reduced DSBs and genes that have reduced expression in sir2 haploids (SI Table 7). The interpretation of this correlation is discussed below.
Confirmation of the Microarray Results by Southern and Tetrad Analysis.
Our conclusions based on microarray studies were supported by Southern analysis and tetrad dissection. Fig. 3B shows a Southern analysis of chromosomal DNA samples (untreated with restriction enzymes), demonstrating meiosis-specific DSBs on chromosome I in the wild-type (JG169) and sir2 (MD311) strains. It is evident that DSBs near the ends of the chromosome are suppressed relative to DSBs near the middle in the sir2 strain. In the same pair of strains, we examined DSBs at the HIS4, YGR177C, and CYS3 loci by standard Southern analysis (Fig. 3 C–E). These loci represent ORFs that, by microarray analysis, had lower (HIS4) or higher (CYS3) DSB activities in sir2 strains compared with wild-type strains. YGR177C had very high levels of DSBs in both wild-type and sir2 strains, although the activity was slightly higher in the sir2 strain. For all loci examined, the Southern analysis was consistent with our conclusions from the microarrays.
In fungi, meiotic recombination activity can be monitored by the measuring the rate of aberrant segregation (gene conversion and related events) of a heterozygous marker (20, 21). Because the HIS4 gene is in a region of suppressed DSB formation in the sir2 mutant, we constructed wild-type (MD251) and sir2 (MD258) strains that were heterozygous for a mutation in HIS4, as well as a mutation in ARG4, a gene with a DSB that is not affected by the sir2 mutation. These two strains were sporulated and tetrads were dissected. The HIS4 aberrant segregation rate was significantly (P ≤ 0.001) reduced by the sir2 mutation, from 23% in MD251 to 8% in MD258 (total of 1,127 and 242 tetrads for MD251 and MD258, respectively). The aberrant segregation rate for ARG4 was not significantly affected (10% in MD251 and 8% in MD258). Thus, the tetrad analysis confirms the microarray analysis and demonstrates that the suppression of DSBs in TPSRs leads to an absolute reduction in recombination activity.
The strains MD251 and MD258 were heterozygous for markers allowing us to map crossovers in three intervals on chromosome III (CHA1-HIS4, HIS4-LEU2, and LEU2-CEN3) and between ARG4 and CEN8. The map lengths were significantly reduced by the sir2 mutation in the CHA1-HIS4 interval (from 41 cM in MD251 to 18 cM in MD258) and the HIS4-LEU2 interval (from 23 cM in MD251 to 14 cM in MD258). The map lengths in the other intervals were unaffected (SI Table 8). The microarray analysis indicates that the CHA1-HIS4 and the HIS4-LEU2 intervals are within a single TPSR on chromosome III, whereas the LEU2-CEN3 and ARG4-CEN8 intervals do not include large numbers of genes with altered DSB activities. Thus, these results support the conclusion that the alterations in DSB frequency detected by the microarrays in the sir2 strain affect the crossover frequencies and, therefore, the genetic map of S. cerevisiae.
Discussion
We show that the pattern of meiosis-specific DSBs in S. cerevisiae is very substantially altered by a mutation that results in loss of one of the yeast histone deacetylases. Winckler et al. (22) recently demonstrated that, despite ≈99% sequence conservation between human and chimpanzees, the positions of recombination hotspots are not conserved between these two genomes. Although there is evidence that sequence polymorphisms can influence the strength of local recombination events (a cis effect) in humans (23, 24), our results demonstrate that trans effects mediated by altering a chromatin-modifying protein can substantially change the genomic distribution of DSBs in budding yeast and the altered frequencies of DSBs associated with some genes affect the rates of crossing over. It is likely that similar types of alterations influence the differences in the human and chimpanzee genetic maps.
Of the 12% of the genes with significantly altered DSBs in the sir2 strains, ≈5% had elevated DSBs (positive ΔR) and 7% had reduced DSBs (negative ΔR). As described below, by correlating these ΔR values with various genomic data sets (studies of gene expression, protein binding, chromatin modifications, timing of replication, and other properties), we were able to distinguish a number of different classes within the positive and negative ΔR groups that presumably reflect different mechanisms. Most of our conclusions are based on the correlations shown in SI Table 7. Some of the details of the analysis, not discussed below, are provided in SI Materials and Methods.
Genes with Elevated DSBs in the sir2 Strain.
We can divide the genes with elevated DSBs into four classes: class 1, the genes located near (within 10 kb) of the telomeres; class 2, the rRNA genes; class 3, the genes flanking the rRNA gene cluster (within 150 kb of the cluster); class 4 all other genes with elevated DSBs. Telomeres bind Sir2p (12) and Sir2p represses gene expression at the telomeres (14), presumably as a consequence of its H4K16 deacetylation activity. It is likely that class 1 genes represent a direct effect of the enzymatic activity of Sir2p on DSB formation: elevated levels of H4K16 acetylation leading to a more open chromatin structure that allows Spo11p increased access to the telomeric DNA.
The DSB frequencies of the class 2 (rRNA gene cluster) and class 3 (genes flanking the cluster) were also elevated by the sir2 mutation (Fig. 2). In wild-type strains, recombination is suppressed within the rRNA genes (19) and flanking regions (4, 5). San-Segundo and Roeder (25) suggested that the increased level of recombination observed in sir2 strains reflects increased levels of Hop1p (a positive regulator of recombination) in the nucleolus, because Pch2p excludes Hop1p from the nucleolus, and Pch2p requires Sir2p for its nucleolar localization. Similarly, the elevated rates of DSBs in genes that flank the rRNA gene cluster in the sir2 strain could be the result of elevated levels of Hop1p extending from the rRNA gene cluster.
Class 4 genes had no common characteristic that we have been able to discern. These genes are not associated with any obvious structural element of the chromosome (centromeres, replication origins or repetitive DNA elements). No class of genes is very significantly (P ≤ 0.001) overrepresented among these genes (determined by using the Stanford Genome Database Gene Ontology Term Finder). Because introduction of a strong DSB site can repress recombination at neighboring sites (26–28), it is likely that loss of strong DSB sites in some sites will elevate DSB formation at other sites. Consequently, it is possible that the elevated DSBs in class 4 genes are a consequence of a redistribution of recombination-promoting proteins from the TPSRs to class 4 genes.
Genes with Suppressed DSBs in the sir2 Strain.
There are also several classes of genes that have reduced DSBs in the sir2 strains: class 1, ORFs located in TPSRs; class 2, ORFs that are associated with reduced gene expression in sir2 strains; class 3, all other ORFs. Of the 438 ORFs with significantly reduced recombination, approximately two-thirds are class 1 genes as defined in SI Table 6. In wild-type strains, we have previously shown that DSB formation is suppressed in regions extending ≈80 kb from the telomeres (5). In the sir2 strains, the suppression of DSBs is more pronounced and extends to ≈130 kb from the telomeres (Fig. 4). Although it is possible that the increased extent of suppression represents a mechanism qualitatively different from that observed in the wild-type strain, we prefer the simpler hypothesis that the increased suppression observed in sir2 strains in an enhancement of the suppression that occurs in wild-type strains.
The region of DSB suppression near the telomeres in the wild-type strain is similar to the location of the regions in which the level of H3K18 acetylation is regulated by Hda1p (13). Although Hda1p acts at many positions scattered throughout the genome, contiguous regions located between 20 and 100 kb from the telomeres (termed HAST domains) are modified by Hda1p (13). One explanation of our results is that Hda1p-mediated deacetylation in HAST domain suppresses DSBs in the wild-type strain. If Sir2p and Hda1p compete in their ability to act in these regions, deletion of Sir2p might allow Hda1p to act in a more extended region. Because both Sir2p and Hda1p are histone deacetylases, we also need to postulate that Hda1p-mediated deacetylation (targeting H3K18) more effectively suppresses DSB formation than Sir2p-mediated deacetylation (targeting H4K16).
A second class of ORFs that have reduced DSBs are those that have reduced expression in sir2 haploids. Because sir2 haploids express both mating types, many genes involved in mating (production or response to pheromones) and retrotransposition are repressed, directly or indirectly by the a1.α2 repressor (29) that is encoded by genes from both mating type loci. Of the 100 genes that have the greatest decrease in expression in the sir2 haploid strain, 36 are related to conjugation or retrotransposition, as expected because both conjugation and retrotransposition are repressed in a/α diploids or sir2 haploids (29, 30). Of these 36 genes, 26 have reduced DSBs in the sir2 strain and 10 have elevated DSBs, a significant (P = 0.003) enrichment in genes with reduced DSBs compared with the genome as a whole (2,946 ORFs reduced and 3,441 elevated).
Why is there an association between the gene expression levels in a sir2 haploid and DSB formation in a sir2 diploid? Previously, we showed that the binding of transcription factors stimulates local meiotic DSB formation, but transcription per se is not required for hotspot activity (2). Our interpretation of this result was that chromatin modifications related to transcription factor binding facilitate the association of both the transcription and recombination machinery. Because much of the repression of haploid-specific genes in the a/α diploid is a consequence of the a1-α2 repressor, one would not necessarily expect that the repression levels would differ in a wild-type diploid and a sir2 diploid. One possibility is that, as a consequence of expression of the silent mating type loci in the sir2 diploid, more a1-α2 repressor is produced in a sir2 diploid than in a wild-type diploid and, consequently, the genes normally regulated by the repressor are turned off more completely. Although this hypothesis requires additional experimental support, we suggest that class 2 genes may represent genes with increased levels of binding of a repressor that reduces both gene expression and DSB formation. The class 3 genes with reduced DSBs do not have a common feature that we have been able to identify.
A final correlation is between the level of DSBs in intergenic regions and the promoter activity of these regions. Of the 6,357 intergenic regions represented on our microarrays, 1,666 are bidirectional promoters (located between divergently transcribed genes), 3,112 are unidirectional promoters, and 1,579 are nonpromoters (located between convergently transcribed genes). The average ΔRs for these regions (95% confidence limits in parentheses) are: 0.05 (0.01–0.08) for the bidirectional promoters, −0.06 (−0.03 to −0.08) for unidirectional promoters, and −0.14 (−0.11 to −0.17) for nonpromoters. Thus, the nonpromoter regions have substantially reduced ΔRs. We noted previously (3, 5) that DSBs associated with recombination hotspots were preferentially associated with bidirectional promoters and had a negative correlation with nonpromoters. There is also a strong correlation between the binding of cohesin and intergenic regions that are nonpromoters (7). One explanation of our data, therefore, is that the sir2 mutation, directly or indirectly, increases the binding of cohesins in the nonpromoter regions, reducing the level of DSBs associated with these already-cold regions.
Summary.
No single known factor explains the difference in DSB formation between the wild-type and sir2 strains. This result is not surprising, because no single factor correlates with the pattern of recombination hotspots and coldspots in the wild-type strain, and because DSB frequencies are elevated for some genes and repressed for others in the sir2 strain. The elevated levels of DSBs at known targets of Sir2p-directed deacetylation (telomeres and the rRNA gene cluster) are likely to be fairly direct effects of loss of Sir2p. The very striking reduction in DSBs in the TPSRs may reflect an increased level of Hda1p activity, although many other possibilities exist. Whatever the mechanism(s) responsible for the altered patterns of recombination observed in the sir2 strain, it is clear that alteration of a single gene can dramatically change the distribution of DSBs of an organism without a substantial effect on fertility (spore viability).
Materials and Methods
Strains.
All diploid strains used in this study were constructed by crossing derivatives of the haploid strains AS4 and AS13, which we have used in many previous studies (5). Alterations in AS4 and AS13 were made by transformation as described in detail in SI Materials and Methods and SI Table 1. The isogenic wild-type strain JG169 (5) and the sir2 diploid MD311 were used in the microarray experiments. Both strains had an epitope-tagged version of Spo11p and the rad50S mutation, allowing the purification of DNA sequences located near the site of Spo11p-associated DSBs by immunoprecipitation (5).
Genetic Methods and Southern Analysis.
Methods for transformation and tetrad analysis were standard. For Southern blot analysis, DNA was isolated by using the procedure described by Nag and Petes (31). The hybridization probes were prepared by PCR and the primers used for their preparation are described in SI Materials and Methods.
Microarray Analysis of DSB Activities.
The methods that we used were the same as used previously (5). In brief, we isolated Spo11p-associated DNA from sporulated cultures of JG169 or MD311 by using IgG Sepharose beads. The Spo11p in JG169 and MD311 is tagged with a epitope (ZZ) that binds the beads. The experimental DNA samples were labeled with Cy3 and hybridized to microarrays along with total genomic DNA that had been labeled with Cy5; in some experiments, we labeled the experimental DNA with Cy5 and genomic DNA with Cy3. The hybridization images were acquired with a GenePix 4000B scanner and analyzed with GenePix Pro 5.0 software. Subsequent steps in the data analysis are described in SI Materials and Methods. Excel spreadsheets summarizing the analysis are in SI Tables 2–4 and 7, and the raw data for these experiments are available at the University of North Carolina Microarray Database (https://genome.unc.edu) and through GEO accession no. GSE6245.
Supplementary Material
Acknowledgments
We thank M. Grunstein, J. Rine, A. Johnson, J. Gerton, J. Kohli, and members of the T.D.P. and J.D.L. laboratories for useful discussions. We thank J. Gerton (Stowers Institute for Medical Research, Kansas City, MO) for supplying the wild-type strain JG169 used in the microarray experiments. This research was supported by National Institutes of Health Grants GM24110 (to T.D.P.) and GM72518 (to J.D.L.).
Abbreviations
- DSB
double-strand DNA break
- TPSR
telomere-proximate suppressed region.
Footnotes
The authors declare no conflict of interest.
Data deposition: The microarray data have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE6245).
This article contains supporting information online at www.pnas.org/cgi/content/full/0700412104/DC1.
References
- 1.Keeney S, Giroux CN, Kleckner N. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 2.Petes TD. Nat Rev Genet. 2001;2:360–369. doi: 10.1038/35072078. [DOI] [PubMed] [Google Scholar]
- 3.Gerton JL, DeRisi J, Shroff R, Lichten M, Brown PO, Petes TD. Proc Natl Acad Sci USA. 2000;97:11383–11390. doi: 10.1073/pnas.97.21.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borde V, Lin W, Novikov E, Petrini JH, Lichten M, Nicolas A. Mol Cell. 2004;13:389–401. doi: 10.1016/s1097-2765(04)00034-6. [DOI] [PubMed] [Google Scholar]
- 5.Mieczkowski PA, Dominska M, Buck MJ, Gerton JL, Lieb JD, Petes TD. Mol Cell Biol. 2006;26:1014–1027. doi: 10.1128/MCB.26.3.1014-1027.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu TC, Lichten M. Science. 1994;263:515–518. doi: 10.1126/science.8290959. [DOI] [PubMed] [Google Scholar]
- 7.Glynn EF, Megee PC, Yu H-G, Mistrot C, Unal E, Koshland DE, DeRisi JL, Gerton JL. PLoS Biol. 2004;2:1325–1339. doi: 10.1371/journal.pbio.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blat Y, Protacio RU, Hunter N, Kleckner N. Cell. 2002;111:791–802. doi: 10.1016/s0092-8674(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 9.Blat Y, Kleckner N. Cell. 1999;98:249–259. doi: 10.1016/s0092-8674(00)81019-3. [DOI] [PubMed] [Google Scholar]
- 10.Yamada T, Mizuno K-i, Hirtoa K, Kon N, Wahls W, Hartsuiker E, Murofushi H, Shibata T, Ohta K. EMBO J. 2004;23:1792–1803. doi: 10.1038/sj.emboj.7600138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauve AA, Wolberger C, Schramm VL, Boeke JD. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 12.Lieb JD, Liu X, Botstein D, Brown PO. Nat Genet. 2001;28:327–334. doi: 10.1038/ng569. [DOI] [PubMed] [Google Scholar]
- 13.Robyr D, Suka Y, Xenarios I, Kurdistani SK, Wang A, Suka N, Grunstein M. Cell. 2002;109:437–446. doi: 10.1016/s0092-8674(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 14.Rusche LN, Kirchmaier AL, Rine J. Annu Rev Biochem. 2003;72:481–516. doi: 10.1146/annurev.biochem.72.121801.161547. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb S, Esposito RE. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 16.Klieger Y, Yizhar O, Zenvirth D, Shtepel-Milman N, Snoek M, Simchen G. Mol Biol Cell. 2005;16:1449–1455. doi: 10.1091/mbc.E04-07-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Y, Barton AB, Kaback DB. Chromosoma. 2000;109:467–475. doi: 10.1007/s004120000098. [DOI] [PubMed] [Google Scholar]
- 18.Buck MJ, Nobel AB, Lieb JD. Genome Biol. 2005;6:R97. doi: 10.1186/gb-2005-6-11-r97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petes TD, Botstein DB. Proc Natl Acad Sci USA. 1977;74:5091–5095. doi: 10.1073/pnas.74.11.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petes TD, Malone RE, Symington LS. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Broach J, Jones E, Pringle JR, editors. Plainview, NY: Cold Spring Harbor Lab Press; 1991. pp. 407–521. [Google Scholar]
- 21.Fan Q-Q, Xu F, Petes TD. Mol Cell Biol. 1995;15:1679–1688. doi: 10.1128/mcb.15.3.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winckler W, Myers SR, Richter DJ, Onofrio RC, McDonald GJ, Bontrop RE, McVean GAT, Gabriel SB, Reich D, Donnelly P, et al. Science. 2005;308:107–111. doi: 10.1126/science.1105322. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Lazzeroni L, Qin J, Huang MM, Navidi W, Erlich H, Arnheim N. Am J Hum Genet. 1996;59:1186–1192. [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffreys AJ, Neumann R. Hum Mol Genet. 2005;14:2277–2287. doi: 10.1093/hmg/ddi232. [DOI] [PubMed] [Google Scholar]
- 25.San-Segundo PA, Roeder GS. Cell. 1999;97:313–324. doi: 10.1016/s0092-8674(00)80741-2. [DOI] [PubMed] [Google Scholar]
- 26.Wu TC, Lichten M. Genetics. 1995;140:55–66. doi: 10.1093/genetics/140.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Kleckner N. EMBO J. 1995;16:5115–5128. doi: 10.1002/j.1460-2075.1995.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Q-Q, Xu F, White MA, Petes TD. Genetics. 1997;145:661–670. doi: 10.1093/genetics/145.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson AD. Curr Opin Gen Dev. 1995;5:552–558. doi: 10.1016/0959-437x(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 30.Baur M, Esch RK, Errede B. Mol Cell Biol. 1997;17:4330–4337. doi: 10.1128/mcb.17.8.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nag DK, Petes TD. Mol Cell Biol. 1993;13:2324–2331. doi: 10.1128/mcb.13.4.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.