Abstract
The identity of cells that mediate positive selection of CD8+ T cells was investigated in two T cell receptor (TCR) transgenic systems. Irradiated β2-microglobulin mutant mice or mice with mutations in both the Kb and Db genes were repopulated with fetal liver cells from class I+ TCR transgenic mice. In the case of the 2C TCR, mature transgene-expressing CD8+ T cells appeared in the thymuses of the chimeras and in larger numbers in the peripheral lymphoid organs. These CD8+ T cells were functional, exhibited a naive, resting phenotype, and were mostly thymus-dependent. Their development depended on donor cell class I expression. These results establish that thymic hematopoietic cells can direct positive selection of CD8+ T cells expressing a conventional TCR. In contrast, no significant development of HY (male antigen)–TCR+ CD8+ T cells was observed in class I+ into class I-deficient chimeras. These data suggest that successful positive selection directed by hematopoietic cells depends on specific properties of the TCR or its thymic ligands. The possibility that hematopoietic cell-induced, positive selection occurs only with TCRs that exhibit relatively high avidity interactions with selecting ligands in the thymus is discussed.
Positive selection of self-MHC-restricted T cells is determined primarily by the interaction of immature cortical thymocytes with MHC molecules present on cortical thymic epithelial cells (1, 2). The key role of thymic epithelial cells in positive selection was suggested originally by the finding that most T cells that develop from stem cells in an MHC-different thymus are restricted to the MHC type of the thymus (reviewed in ref. 2). However, in most of these experiments a low but significant level of T cell activity could be detected that was restricted to the MHC type of the hematopoietic cell donor. These T cells may have represented cross-reactive clones that had been positively selected by interactions with MHC molecules on thymic epithelial cells. Alternatively, the positive selection of some of these cells may have been directed by MHC molecules on hematopoietic cells (2).
Evidence that nonthymic epithelial cells can direct positive selection of CD8+ T cells came from analysis of class I MHC-deficient [β2-microglobulin (β2m) mutant] mice. Very few CD8+ T cells develop in class I-deficient mice, but when these mice were heavily irradiated and reconstituted with fetal liver stem cells from class I+ mice, development of CD8+ T cells ensued, albeit inefficiently (3). These T cells were restricted to donor type class I MHC molecules. Development of CD8+ T cells also was observed in class I-deficient mice into which class I+ fibroblasts were injected intrathymically (4). These findings suggested that MHC molecules on hematopoietic cells and fibroblasts were capable of directing positive selection of CD8+ T cells. However, this conclusion has not been accepted universally (5, 6). Furthermore, although CD4+ T cells can be positively selected by class II molecules expressed by fibroblasts (7), CD4+ T cells did not develop in class II-deficient mice that had been reconstituted with class II+ hematopoietic stem cells (8). The latter observation could be explained by a difference in the requirements for development of CD4+ vs. CD8+ T cells or a difference in the pattern of expression of the relevant MHC/peptide complexes.
Although positive selection of CD8+ T cells mediated by hematopoietic cells was observed in the case in which polyclonal T cell specificities were available for selection (3), it was not detected in previous studies of the development of T cells expressing an HY (male antigen)-specific, transgenic T cell receptor (HY-TCR) in allogeneic bone marrow chimeras (9, 10). A possible explanation for this discrepancy is that positive selection of T cells expressing the HY-TCR requires an interaction with a peptide/class I complex(es) that is not found on hematopoietic cells, whereas the peptide/class I complexes required for selection of many other T cells are found on hematopoietic cells. Alternatively, positive selection mediated by hematopoietic cells may occur only when the TCR has some special property not shared by the HY-TCR, such as unusually high or low affinity for positively selecting peptide/class I complexes.
Which cell types direct positive selection and why is of interest because it is likely to provide clues concerning the nature of the mechanisms underlying positive selection. Originally, the key role of thymic epithelial cells in positive selection gave rise to the idea that these cells might present a unique set of MHC-bound peptides specialized to support positive selection (11). This idea has been largely abandoned in the face of a variety of recent data, including the evidence that cells other than thymic epithelial cells can induce positive selection under some circumstances (reviewed in refs. 1 and 2). The superior activity of thymic epithelial cells in positive selection, rather, may be due to their localization or to other hypothetical properties of these cells, such as their capacity to provide unique, maturation-inducing signals to developing T cells that synergize with TCR-mediated signals or their capacity to enhance the avidity of the interaction with developing thymocytes. Knowledge of the circumstances under which different cells induce positive selection ultimately will help to distinguish among these and other possibilities. The conditions under which hematopoietic cells induce positive selection are also relevant in evaluating the efficacy of fully allogeneic or xenogeneic bone marrow transplantation in humans. Selection of some T cells by hematopoietic cells may provide such transplant recipients with useful T cells that can respond to antigens presented by the available antigen-presenting cells, most of which are also donor-derived.
We have investigated the circumstances under which T cells expressing a transgenic TCR undergo positive selection directed by class I molecules expressed by hematopoietic cells. The results demonstrate that T cells expressing the 2C transgenic TCR are positively selected by hematopoietic cells, whereas T cells expressing the HY transgenic TCR are not. The results suggest that differences between T cell receptors, perhaps related to their avidity, determine whether they will undergo successful positive selection directed by hematopoietic cells.
MATERIALS AND METHODS
Mice.
HY-TCR (9), 2C-TCR (12), C57BL/6 β2m-deficient (H-2b β2m−/−), and double mutant KbDb−/− mice (13) all have been described. All these mouse lines were backcrossed to C57BL/6 (H-2b) mice at least five to six times. To obtain β2m−/− TCR transgenic mice, HY- and 2C-TCR transgenic mice were crossed with C57BL/6 β2m-deficient mice and subsequently intercrossed. C57BL/6J and B10.D2/oSnJ (H-2d) mice were obtained from The Jackson Laboratory.
Fetal Liver Chimeras.
Fetal liver chimeras were prepared by γ-irradiating (950 rad) 10- to 12-week-old recipient mice with a 137Cs source followed by i.v. injection of 5–10 × 106 day 16 fetal liver cells from TCR transgenic donors. Thymectomy was performed on 8- to 10-week-old H-2b β2m−/− mice 2 weeks before irradiation and reconstitution (ATx chimeras) by excising the thymus from the open chest cavity. Chimeras were analyzed 3–4 months later. Thymectomized chimeras were checked for thymic remnants, which were absent in the chimeras used for subsequent analysis. In the case of HY-TCR transgenic chimeras, fetal sex was determined after reconstitution by detecting Y chromosome-specific sry sequences in DNA of residual fetal liver cells as described (14).
Antibodies.
Anti-CD4-PE, anti-CD8α-FITC or Tricolor, anti-CD44-FITC or Biotin (IM7.8.1), anti-CD62L (l-selectin)-FITC or biotin (MEL-14), anti-CD8β-PE, anti-CD25/IL-2Rα-FITC (PC61.5.3.), and Streptavidin-Tricolor (SA1006) were obtained from Caltag (South San Francisco, CA). Anti-CD122/IL-2Rβ-FITC (TM-β1) was obtained from PharMingen, anti-CD8α-RED613 (53–6.7) was obtained from GIBCO, and Streptavidin-PE was obtained from Molecular Probes. Hybridomas producing anti-2C-TCR (1B2) were provided by D. Y. Loh (Washington University School of Medicine, St. Louis), and anti-HY-TCR (T3.70) was from H. von Boehmer (Institut Necker, Paris, France). Anti-CD8.2α (2.43), anti-HSA (M1/69.16.11.HL), and anti-NK1.1 (PK1.36) hybridomas were purchased from American Type Culture Collection (Manassas, VA). The antibodies were purified from culture supernatants by chromatography on Protein G or Protein A agarose and either biotinylated or FITC-conjugated.
Cell Preparations and Flow Cytometry.
Viable suspensions of thymic, mesenteric lymph node and splenic cells were prepared by Ficoll/Hypaque centrifugation. Cells (4–10 × 105) in PBS/5% FCS/0.02% NaN3 were treated with anti-FcRγ mAb 2.4G2 to block Fc receptors. For three-color analysis of β2m−/− chimeras, cells were stained in the first step with anti-CD4-PE and T3.70-Biotin or 1B2-Biotin and in the second step with anti-CD8α or CD8.2α-FITC and Streptavidin-Tricolor. Cells from KbDb−/− chimeras were stained with anti-CD4-PE, anti-CD8-Tricolor, and 1B2-FITC. For analysis of activation markers in β2m−/− chimeras, the cells first were stained with anti-CD8-Red613 and 1B2-Biotin and, subsequently, with Streptavidin-Tricolor and one of the following FITC-conjugated mAbs: anti-CD122, -CD62L, -CD44, -CD25, or -NK1.1. In the case of KbDb−/− chimeras, cells first were stained with anti-CD8-Tricolor, 1B2-FITC, and one of the following biotinylated mAb: anti-CD122, -CD62L, or -CD44. Subsequently, cells were stained with Streptavidin-PE. For four-color analysis, cells first were stained with anti-CD4-PE, anti-CD8-Red613, 1B2-Biotin, or T3.70-Biotin and, subsequently, with Streptavidin-Tricolor and anti-HSA-FITC. Cells (2–4 × 104) were analyzed on an EPICS XL flow cytometer (Coulter) by using forward and side scatter gating to exclude nonviable cells. Graphics were generated by using the winmdi program (John Trotter, Salk Institute, San Diego), and the data are displayed on a four-decade logarithmic scale. The data from multiple determinations were evaluated by using the two-tailed Student’s t test.
Proliferation Assays.
Nylon wool-passed T cells from 2C-TCR transgenic chimeras were prepared, and the proportion of total 1B2+ as well as CD8+ 1B2+ T cells was determined by flow cytometry. For antigen-specific stimulation, the cells were stimulated as described previously (15) except that we employed triplicate cultures containing only 1 × 104 responder cells in round-bottomed, 96-well cell plates (#3799; Costar). Cultures were incubated for 72 h and pulsed with 1 μCi [3H]thymidine for 12 h before harvesting.
RESULTS
Hematopoietic Cells Mediate Positive Selection of 2C-TCR+ Thymocytes.
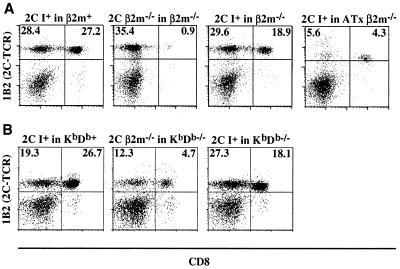
Lethally irradiated, age-matched class I+ and β2m−/− mice were reconstituted with day 16 fetal liver cells from 2C-TCR transgenic mice that were either class I+ or β2m−/−. The development of 2C-TCR+ CD8+ T cells in the periphery and the thymus was tested 3 months later, using the clonotypic 1B2 mAb. Strikingly, 1B2+CD8+ T cells were nearly as prevalent in the lymph nodes of β2m−/− recipients reconstituted with class I+ 2C-TCR transgenic stem cells as they were in the positive control (class I+ into class I+) chimeras (Fig. 1A and Table 1). Many fewer 1B2+CD8+ lymph node T cells developed in chimeras in which both the donor and recipient were β2m−/− (Fig. 1A and Table 1).
Figure 1.
Abundant 2C-TCR+ CD8+ T cells in lymph nodes of class I+ into β2m−/− fetal liver chimeras (A) and class I+ into KbDb−/− chimeras (B). Lymph node cells from representative fetal liver chimeras were analyzed for expression of CD8 and the 2C-TCR (with 1B2 mAb) by flow cytometry. The percentages of 1B2+ CD8− and 1B2+ CD8+ T cells of total live lymphocytes in the respective quadrants are indicated. The 1B2+ CD8− cells essentially are all CD4−CD8− T cells, as shown in separate analyses. Previous studies have shown that CD4−CD8− T cells expressing transgenic TCRs commonly appear in TCR transgenic mice (16, 17), though the origin of such cells has not been established. The expression level of the 2C-TCR on peripheral T cells derived from the ATx chimeras was marginally but reproducibly lower compared with those developed in euthymic chimeras: MFI (mean fluorescence intensity) = 46.9 (2C I+ in ATxβ2m−/−), 68.7 (2C I+ in β2m+), and 66.2 (2C I+ in β2m−/−).
Table 1.
Analysis of lymph node cells from TCR transgenic fetal liver chimeras
| Donor | Recipient | n | Cells/prep, ×10−6 | Clonotype-TCR+CD8+CD4− cells/prep
|
|
|---|---|---|---|---|---|
| % | Number × 10−6 | ||||
| 2C, I+ | β2m+ | 7 | 12.2 | 22.3 (2.5) | 3.2 (1.1) |
| 2C, β2m−/− | β2m−/− | 4 | 9.2 | 1.2 (0.4) | 0.1 (0.1) |
| 2C, I+ | β2m−/− | 8 | 19.3 | 19.0 (4.4)‡ | 3.7 (1.0)† |
| 2C, I+ | ATx, β2m−/− | 5 | 8.4 | 5.7 (0.8)† | 0.5 (0.2)* |
| 2C, I+ | KbDb+ | 9 | 20.1 | 30.8 (7.6) | 5.97 (2.1) |
| 2C, ß2m−/− | KbDb−/− | 9 | 11.4 | 5.9 (1.5) | 0.64 (0.4) |
| 2C, I+ | KbDb−/− | 8 | 19.3 | 16.6 (2.2)† | 3.55 (1.7)† |
| HY, I+ | β2m+ | 4 | 18.5 | 2.1 (1.3) | 0.43 (0.4) |
| HY, β2m−/− | β2m−/− | 2 | 16.5 | 0.13 (0.06) | 0.02 (0.01) |
| HY, I+ | β2m−/− | 7 | 18.9 | 0.3 (0.16)* | 0.06 (0.03)* |
Numbers represent the mean values (SD) of n determinations. n, number of chimeras analyzed.
Significance vs. corresponding class I− into class I− control group. *, P > 0.05 (not significant);
, P < 0.001;
, P < 0.0001.
We considered the possibility that positive selection in class I+ into β2m−/− chimeras was directed by class I molecules on the surface of β2m−/− thymic epithelial cells that had been reconstituted by free β2m originating from the donor hematopoietic cells. Therefore, we generated chimeras by using recipients with homozygous mutations in both the Kb and Db genes (KbDb−/− mice; ref. 13). The 2C-TCR is normally positively selected by the Kb molecule (18), which is not expressed in the KbDb−/− hosts. When class I+ 2C-TCR transgenic stem cells were transferred to KbDb−/− recipients, 1B2+CD8+ T cells were as numerous among lymph node cells as in the β2m−/− recipients (Fig. 1B and Table 1), arguing that β2m transfer to thymic epithelial cells cannot account for the appearance of 2C-TCR+ T cells in the chimeras. When the donor 2C transgenic stem cells were class I-deficient (β2m−/−) and the hosts were KbDb−/−, many fewer 1B2+CD8+ T cells appeared in the lymph nodes, although more than in β2m−/− recipients (see Discussion).
Along with the CD8+ population, we reproducibly detected CD8− 1B2+ cells (Fig. 1), that were also CD4− (data not shown). These CD4−CD8− 2C-TCR+ cells were not affected significantly by class I deficiency. In one experimental series, the percentage in chimeras constructed with class I+ donor and host cells averaged 17% ± 2.5%, whereas the percentage in chimeras constructed with β2m− 2C-TCR transgenic donor cells and KbDb−/− recipients averaged 20.6% ± 8.6%. The class I independence of CD4−CD8− TCRαβ transgene+ cells is in accord with previous evidence that they do not require positive selection by class I MHC molecules (16, 19), consistent with the idea that they represent aberrant γδ-lineage cells (19).
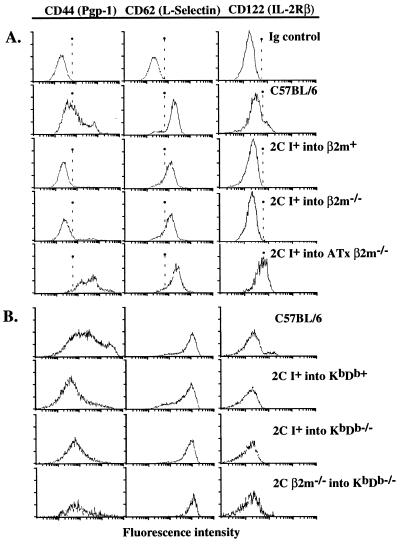
Most 1B2+ CD8+ T cells that developed in the class I+ into β2m−/− or class I+ into KbDb−/− chimeras exhibited a CD44− l-selectin+ IL-2Rβ− phenotype (Fig. 2) and were CD25− (data not shown), representing the phenotype of naive, resting CD8+ T cells. Their phenotype was indistinguishable from that of the corresponding cells in 2C-TCR transgenic class I+ into class I+ chimeras or nonchimeric 2C-TCR transgenic mice (Fig. 2 and data not shown). None of the 1B2+ CD8+ T cells in these or any other chimeras in this study expressed the NK1.1 antigen (data not shown).
Figure 2.
Phenotypes of 2C-TCR+ CD8+ T cells from fetal liver chimeras. Gated 2C-TCR+ CD8+ lymph node cells or gated CD8+ lymph node cells from control B6 mice were analyzed for expression of the indicated markers. The first row represents staining with isotype-matched control antibodies. Representative experiments are shown for chimeras and controls prepared with β2m−/− (A) and KbDb−/− (B) hosts. Most of the 2C-TCR+ CD8+ T cells from class Ι+ into both KbDb−/− and β2m−/− chimeras exhibited a resting, naive phenotype (CD44− l-selectin+ IL-2Rβ−), whereas the 2C-TCR+ CD8+ T cells from class I+ into ATx β2m−/− chimeras exhibited a phenotype consistent with past activation or memory status (CD44+ l-selectin+ IL-2Rβhi).
Positive selection of 1B2+ CD8+ cells mediated by class I+ hematopoietic cells also was evident in the thymus. The percentage or number of 1B2+CD8+CD4− thymocytes with the mature (HSA−) phenotype was significantly higher when β2m−/− recipients were reconstituted with class I+ transgenic stem cells than when these recipients were reconstituted with β2m−/− transgenic stem cells (Table 2). Similarly, in KbDb−/− recipients of class I+ transgenic stem cells there was an elevation in the number and frequency of both 2C-TCR+CD8+CD4− thymocytes and HSA−CD8+CD4− thymocytes (Table 2); in the few instances tested, most of the HSA−CD8+CD4− thymocytes expressed the 2C-TCR (data not shown). The number of mature CD8+ thymocytes in class I+ into class I-deficient chimeras, however, was reduced significantly compared with chimeras in which class I was expressed by both donor cells and host thymic epithelial cells. These data suggest that the relative efficiency of thymic positive selection by hematopoietic cells vs. thymic epithelial cells is less than would appear based on the lymph node data.
Table 2.
Analysis of thymocytes from TCR transgenic fetal liver chimeras
| Donor | Recipient | Cells/thymus, ×10−6 | Clonotype-TCR+CD8+CD4− cells
|
HSA− 2C-TCR+CD8+CD4− cells
|
||
|---|---|---|---|---|---|---|
| % of thymus | Per thymus × 10−6 | % of 2C-TCR+CD8+ cells | Per thymus × 10−3 | |||
| 2C, I+ | β2m+ | 14.9 | 18.9 (5.5) | 2.91 (2.3) | 19.7 (6.7) | 337.9 (465.0) |
| 2C, β2m−/− | β2m−/− | 31.9 | 1.3 (0.3) | 0.36 (0.1) | 0.3 (0.3) | 1.4 (2.1) |
| 2C, I+ | β2m−/− | 31.0 | 3.6 (1.1)¶ | 1.10 (0.4)§ | 10.8 (4.1)§ | 83.1 (55.5)‡ |
| HSA− CD8+CD4− cells* | ||||||
| % of CD8+ cells | Per thymus × 10−3 | |||||
| 2C, I+ | KbDb+ | 19.3 | 19.8 (5.7) | 3.94 (1.8) | 21.1 (7.5) | 881.8 (561) |
| 2C, β2m−/− | KbDb−/− | 35.9 | 1.2 (0.9) | 0.38 (0.3) | 5.9 (1.3) | 23.4 (18) |
| 2C, I+ | KbDb−/− | 37.6 | 3.8 (1.6)§ | 1.33 (0.59)§ | 12.9 (6.5)‡ | 118.5 (55)§ |
| HY, I+ | β2m+ | 36.5 | 13.8 (4.6) | 5.3 (3.6) | ND | ND |
| HY, β2m−/− | β2m−/− | 13.3 | 0.1 (0.1) | 0.015 (0.01) | ND | ND |
| HY, I+ | β2m−/− | 10.9 | 0.3 (0.2)† | 0.03 (0.02)† | ND | ND |
Numbers represent the mean values (SD) of four to nine determinations.
Significance vs. corresponding class I− into class I− control group.
P > 0.05 (not significant);
P < 0.05;
P < 0.01;
P < 0.002.
Determined from three-color staining. A few of the 2C, class I+ into KbDb−/− chimeras were analyzed by four-color staining; most of the HSA−CD8+CD4− cells in these animals were 2C-TCR+. ND, not determined.
The development of 2C-TCR+ CD8+ T Cells in Class I+ into Class I-Deficient Chimeras Is Thymus-Dependent.
To investigate whether the development of 2C-TCR+ T cells directed by hematopoietic cells is thymus-dependent, we thymectomized β2m−/− recipients before reconstitution with 2C-TCR transgenic stem cells from class I+ donors. Compared with the chimeras prepared with euthymic recipients, the number of 1B2+ CD8+ T cells in the lymph nodes of the thymectomized (“ATx”) chimeras was reduced by a factor of 8, and the percentage of these cells was reduced by more than a factor of 3 (Table 1 and Fig. 1A). This reduction was not due to surgical trauma, because two mock-thymectomized recipients yielded results similar to the unmanipulated euthymic recipients (included in the 2C, I+ into β2m−/− group in Table 1). Therefore, most of the T cells that developed under the influence of class I+ hematopoietic cells were thymus-dependent.
Although thymectomized β2m−/− recipients of class I+ stem cells contained many fewer 1B2+ CD8+ T cells than euthymic recipients, they consistently contained more of these cells than the β2m−/− into β2m−/− chimeras (Table 1). Hence, some 1B2+ CD8+ T cells apparently develop in the absence of a thymus. However, the phenotype of these cells was distinctive: the cells were mostly TCRlo, CD44hi, and IL-2Rβ+ (Fig. 2), and some of them (approximately 8%) expressed CD8αα homodimers (data not shown). This phenotype is characteristic of extrathymically derived T cells (20, 21). In contrast, virtually all 2C-TCR+ CD8+ T cells from euthymic chimeras expressed CD8αβ heterodimers (data not shown), and most of these cells were CD44− IL-2Rβ− (Fig. 2).
2C-TCR+ CD8+ T Cells from Class I+ into Class I− Chimeras Are Functional.
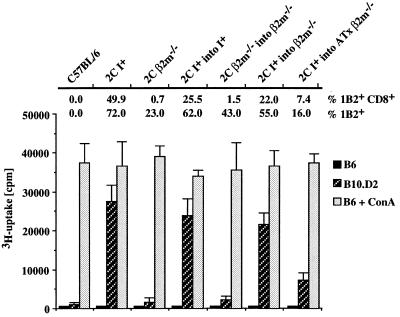
Peripheral T cells from the different transgenic chimeras were tested for their response to B10.D2 spleen cells, which express the cognate Ld antigenic complex for the 2C TCR (Fig. 3). Limiting numbers of responding T cells were employed in these reactions to minimize the contribution of alloreactive cells expressing endogenously encoded TCRs. Under these conditions, nontransgenic B6 T cells did not respond significantly to allogeneic B10.D2 stimulator cells (Fig. 3). The responses of the chimeric cells correlated well with the proportion of 1B2+ CD8+ T cells in the population. 2C-TCR transgenic, β2m−/− into β2m−/− chimeric T cells gave essentially no response, whereas 2C-TCR transgenic, class I+ into β2m−/− chimeric T cells responded nearly as well as 2C-TCR transgenic, class I+ into class I+ positive controls. Both of the latter chimeras contained a high percentage of 1B2+CD8+ T cells. Thymectomized β2m−/− recipients of class I+ transgenic stem cells gave a weaker but significant response, commensurate with the intermediate abundance of 1B2+ CD8+ T cells in these chimeras. 2C-TCR transgenic, class I+ into KbDb−/− chimeric T cells also responded well to B10.D2 stimulator cells, whereas 2C-TCR transgenic, β2m−/− into KbDb−/− chimeric T cells responded poorly (data not shown). The results indicate that the CD8+ T cells selected by class I+ hematopoietic cells are functional.
Figure 3.
2C-TCR+ CD8+ T cells from class I+ into β2m−/− chimeras respond to antigen-mediated stimulation. T cells from C57BL/6, 2C-TCR I+, or β2m−/− mice and from the various 2C-TCR+ fetal liver chimeras were stimulated with B10.D2 spleen cells, expressing the Ld alloantigen for the 2C-TCR, or with negative control C57BL/6 spleen cells. Samples of each cell preparation also were stimulated with Con A to assess mitogen responsiveness. The proportion of 1B2+ and 1B2+ CD8+ T cells in each cell preparation before stimulation is given above each set of columns. The Con A responses of the cells from 2C β2m−/− mice and 2C β2m−/− into β2m−/− chimeras probably is mediated by 2C-TCR+ CD4−CD8− cells, which fail to respond to Ld but can respond to more potent stimuli (22). The experiment was repeated once with similar results.
Class I+ Hematopoietic Cells Do Not Support Development of HY-TCR+ CD8+ Thymocytes.
We constructed chimeras in which fetal liver cells from female HY-TCR transgenic mice were used to repopulate female β2m−/− or class I+ recipients. As expected, HY-TCR+ (T3.70+) CD8+ T were prevalent in the thymuses of control HY-TCR class I+ into class I+ chimeras but not in β2m−/− into β2m−/− chimeras. However, in class I+ into β2m−/− chimeras, there was no significant increase in the number or percentage of HY-TCR+ CD8+ T cells in the thymus or periphery compared with β2m−/− into β2m−/− chimeras (Tables 1 and 2). The data suggest that class I molecules on hematopoietic cells are unable to support the maturation of HY-TCR transgenic T cells in class I-deficient recipients.
DISCUSSION
Positive Selection of 2C-TCR+ T Cells by Hematopoietic Cells.
The results demonstrate that successful positive selection of 2C-TCR transgenic T cells can occur in β2m−/− and KbDb−/− hosts if donor hematopoietic cells express class I molecules. Furthermore, approximately equal numbers of 2C-TCR+ CD8+ T cells developed when transgenic class I+ stem cells were used to repopulate β2m−/− or double mutant β2m−/−Tap-1−/− hosts (M.C. and D.H.R., data not shown). β2m−/−Tap-1−/− cells are known to have even lower levels of cell surface class I molecules than β2m−/− cells (23, 24). Successful selection in KbDb−/− hosts ruled out the possibility that selection was directed by Kb class I molecules on β2m−/− thymic epithelial cells, which had refolded in the presence of donor-derived β2m. The similarity of the phenotype of 2C-TCR+ CD8+ T cells that were selected on hematopoietic cells to those selected on thymic epithelial cells suggests that these cells represent typical CD8+ T cells rather than a special subset of CD8+ T cells. The relatively high expression of TCR and lack of NK1.1 expression by these CD8+ T cells clearly discriminates them from NK1.1+ T cells, which are positively selected by interactions with hematopoietic cells in normal mice (25, 26).
Positively selected 2C-TCR+ CD8+CD4−HSA−T cells also were evident in the thymus of class I+ into class I-deficient chimeras. In the thymus, however, class I+ into class I-deficient chimeras yielded substantially fewer mature 2C-TCR+CD8+ cells than observed in class I+ into class I+ chimeras, indicating that positive selection by hematopoietic cells is inefficient. This conclusion is consistent with previous results examining polyclonal T cell development in class I+ into class I-deficient chimeras (3). The greater relative prevalence of 2C-TCR+ T cells in the periphery compared with the thymus of class I-deficient recipients of transgenic stem cells suggests that the cells may accumulate in the periphery with time. The accumulation of these cells in the periphery probably does not involve substantial proliferation, judging by the naive phenotype of these cells.
The low efficiency of positive selection of CD8+ T cells mediated by hematopoietic cells may account for the failure to observe positive selection of thymocytes directed by hematopoietic cells in a thymic reaggregation culture system, which lasted only 5 days (6). Alternatively, the necessary hematopoietic cells may not have been available in the reaggregation cultures, or these cells may not have localized correctly under the conditions of reaggregation. Interestingly, it was reported that in vivo positive selection of 2C-TCR+ T cells did not occur when the Kb positive-selecting class I gene was expressed only in developing T cells because of its regulation by the CD2 promoter (27). This observation raises the possibility that non-T lineage hematopoietic cells are responsible for positive selection in our chimeras. Candidate cell types would include dendritic cells and macrophages.
The results with 2C transgenic mice indicate that T cells capable of being selected by hematopoietic cells are an overlapping set with those selected by thymic epithelial cells. The 2C-TCR arose in a normal BALB.B mouse (28) and is positively selected efficiently in a normal class I+ thymus as well as in the class I+ into class I− chimeras (12) (Fig. 1 and Table 1). Therefore, it cannot be argued that the T cells susceptible to positive selection by hematopoietic cells exhibit properties, such as TCR affinity for selecting ligands, that are outside of the range of T cells selected by cortical epithelial cells. On the other hand, we confirmed in our system the previous observation that HY-TCR transgenic T cells cannot be selected effectively by hematopoietic cells (9, 10). The data suggest that the 2C and HY TCRs, or their selecting ligands, differ in a critical property that influences the success of positive selection by hematopoietic cells. Previous studies have indicated that the 2C-TCR is selected more effectively than the HY-TCR in class I+ hosts, perhaps because of a higher avidity of the 2C-TCR for its selecting ligand (29). As support for the possibility that avidity is a determinant of successful selection by hematopoietic cells, we observed 10-fold enhanced selection of CD8+ HY-TCR transgenic thymocytes in β2m−/− hosts when the donor stem cells also contained an avidity-enhancing CD8.1 transgene, under the control of the CD2 promoter (data not shown). High avidity may enhance positive selection mediated by class Ilow thymic hematopoietic cells such as thymic macrophages or cortical thymocytes (30, 31). Another possibility is that peptide/class I MHC complex(es) necessary for positive selection of the HY-TCR are underrepresented on hematopoietic cells, resulting in a requirement for enhanced binding to achieve a sufficient stimulus for positive selection.
Our experiments do not challenge the well accepted notion that thymic epithelial cells play a critical role in controlling the developmental transition of thymocytes from the immature CD4+CD8+ stage to the mature CD8+CD4− stage. Although the hematopoietic cells in class I+ into class I− chimeras clearly provided the relevant class I molecules, the thymus apparently provided conditions favoring T cell development, because thymectomy of the class I− recipients resulted in an 8-fold reduction in the number of 2C-TCR+ CD8+ T cells in the lymph nodes (Table 1). The few 2C-TCR+ CD8+ T cells that remained in thymectomized class I− recipients exhibited a distinctive surface-marker profile characteristic of “extrathymic T cells” (20, 21): intermediate levels of the TCR, CD44+ CD25− IL-2Rβ+ NK1.1−, and some CD8αα+ cells. That thymic stromal cells generally are required for positive selection, but not necessarily for provision of peptide/MHC complexes, is consistent with the notion that thymic epithelial cells provide a unique, non-MHC-related stimulus to thymocytes, which is necessary for their maturation (32). In normal mice, the MHC-related stimulus and the putative, non-MHC-related stimulus presumably are both provided in most cases by thymic epithelial cells. In the case of positive selection mediated by class I molecules on hematopoietic cells, the two signals may be provided in trans by different cells. This could account, in part, for the low efficiency of positive selection mediated by hematopoietic cells.
The capacity of hematopoietic cells to inefficiently direct positive selection of CD8+ T cells in class I+ into class I− mice raises the possibility that this process also occurs to a limited extent in class I+ thymuses. Indeed, positive selection mediated by hematopoietic cells may contribute to the pool of T cells restricted to the class I molecules of the hematopoietic cell donor in fully allogeneic or F1 into parent bone marrow chimeras. On the other hand, there is abundant evidence that some TCRs can be triggered by different MHC molecules presenting the same, or different, peptide antigens (reviewed in ref. 2). Positive selection of such T cells by thymic epithelial cells is also likely to contribute to the pool of donor-restricted CD8+ T cells in MHC-different bone marrow chimeras. With the available data, it is difficult to gauge the relative contributions of these two mechanisms to the selection of donor-restricted CD8+ T cells.
Finally, a surprising observation was that more 2C-TCR+ CD8+ T cells developed when transgenic β2m−/− stem cells matured in KbDb−/− hosts vs. β2m−/− hosts. Unlike β2m−/− cells, KbDb−/− cells express β2m on the cell surface, associated with nonclassical class I molecules. It is possible that β2m from host cells can transfer to β2m−/− donor hematopoietic cells, resulting in the refolding and enhanced expression of Kb molecules on the donor hematopoietic cells. In this case, positive selection is mediated by hematopoietic cells. Alternatively, it is conceivable that the 2C-TCR can be selected weakly by a nonclassical class I molecule. Even if the latter is true, the enhanced selection of 2C-TCR+ cells when donor hematopoietic cells express Kb supports a role for class I molecules on hematopoietic cells in positive selection. Moreover, a putative, weakly selecting, nonclassical class I molecule is unlikely to explain the results observed in β2m−/− or β2m−/− TAP−/− recipients.
Concluding Remarks.
The findings that hematopoietic cells can inefficiently mediate positive selection of T cells with a “conventional” T cell receptor has implications for fully allogeneic or xenogeneic bone marrow transplants. The results suggest that CD8+ T cells that develop in such transplant recipients should include some cells that have been selected by class I molecules of the donor type and that these cells should be normal in their properties, though perhaps of relatively high avidity for donor class I molecules. These cells should be capable of responding to antigens presented by the available antigen-presenting cells, most of which are also of donor type. Such CD8+ T cells may provide protection against viruses and other infectious agents that infect hematopoietic cells, although they presumably would be ineffective in destroying infected, nonhematopoietic cells.
Acknowledgments
We thank P. Schow for expert flow cytometry, Dr. R. Hsiao for advice on thymectomy, and J. S. Kang, T. Hanke, and E. Robey for critical comments on the manuscript. This work was supported by fellowships from the Bundesministerium für Forschung und Technologie (to J.Z.) and from the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation (DRG-1485 to A.V.) and by grant AI30171 from the National Institutes of Health to D.H.R.
ABBREVIATIONS
- β2m
β2-microglobulin
- HY-TCR
HY antigen-specific, transgenic T cell receptor
References
- 1.Robey E, Fowlkes B J. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 2.Fink P J, Bevan M J. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 3.Bix M, Raulet D. Nature (London) 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 4.Pawlowski T, Elliott J D, Loh D Y, Staerz U D. Nature (London) 1993;364:642–645. doi: 10.1038/364642a0. [DOI] [PubMed] [Google Scholar]
- 5.von Boehmer H, Swat W, Kisielow P. Immunol Rev. 1993;135:67–79. doi: 10.1111/j.1600-065x.1993.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 6.Ernst B B, Surh C D, Sprent J. J Exp Med. 1996;183:1235–1240. doi: 10.1084/jem.183.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugo P, Kappler J, McCormack J, Marrack P. Proc Natl Acad Sci USA. 1993;90:10335–10339. doi: 10.1073/pnas.90.21.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz J, Auchincloss H, Grusby M, Glimcher L. Proc Natl Acad Sci USA. 1993;90:2779–2783. doi: 10.1073/pnas.90.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisielow P, Teh H S, Blüthman H, von Boehmer H. Nature (London) 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 10.Fort M M, Pardoll D M. Cell Immunol. 1996;171:74–79. doi: 10.1006/cimm.1996.0175. [DOI] [PubMed] [Google Scholar]
- 11.Marrack P, Kappler J. Science. 1987;238:1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- 12.Sha W C, Nelson C A, Newberry R D, Kranz D M, Russell J H, Loh D Y. Nature (London) 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 13.Perarnau B, Saron M-F, Martin B R S M, Bervas N, Ong H, Soloski M, Smith A G, Ure J M, Gairin J E, Lemonnier F A. Eur J Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Nature (London) 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 15.Sprent J, Schaefer M. J Exp Med. 1985;162:2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell J, Meleedy-Rey P, McCulley D, Sha W, Nelson C, Loh D. J Immunol. 1990;144:3318–3325. [PubMed] [Google Scholar]
- 17.von Boehmer H, Kirberg J, Rocha B. J Exp Med. 1991;174:1001–1008. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sha W, Nelson C, Newberry R, Pullen J, Pease L, Russell J, Loh D. Proc Natl Acad Sci USA. 1990;87:6186–6190. doi: 10.1073/pnas.87.16.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno L, Fehling H J, von Boehmer H. Immunity. 1996;5:343–352. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]
- 20.IIai T, Watanabe H, Seki S, Sugiura K, Hirokawa K, Utsuyama M, Takahashi-Iwanaga H, Iwanaga T, Ohteki T, Abo T. Immunology. 1992;77:556–563. [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. J Exp Med. 1995;182:759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Z, Kishimoto H, Brunmark A, Jackson M R, Peterson P A, Sprent J. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ljunggren H G, van Kaer L, Sabatine M S, Auchincloss H, Tonegawa S, Ploegh H L. Int Immunol. 1995;7:975–984. doi: 10.1093/intimm/7.6.975. [DOI] [PubMed] [Google Scholar]
- 24.Dorfman J R, Zerrahn J, Coles M C, Raulet D H. J Immunol. 1997;159:5219–5225. [PubMed] [Google Scholar]
- 25.Bix M, Coles M, Raulet D. J Exp Med. 1993;178:901–908. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendelac A, Killeen N, Littman D, Schwartz R. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 27.Schönrich G, Strauss G, Müller K P, Dustin L, Loh D Y, Auphan N, Schmitt-Verhulst A M, Arnold B, Hämmerling G J. J Immunol. 1993;151:4098–4105. [PubMed] [Google Scholar]
- 28.Kranz D M, Sherman D H, Sitkovsky M V, Pasternack M S, Eisen H N. Proc Natl Acad Sci USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robey E A, Ramsdell F, Kioussis D, Sha W, Loh D, Axel R, Fowlkes B J. Cell. 1992;69:1089–1096. doi: 10.1016/0092-8674(92)90631-l. [DOI] [PubMed] [Google Scholar]
- 30.van Ewijk W. Annu Rev Immunol. 1991;9:591–615. doi: 10.1146/annurev.iy.09.040191.003111. [DOI] [PubMed] [Google Scholar]
- 31.Surh C D, Gao E K, Kosaka H, Lo D, Ahn C, Murphy D B, Karlsson L, Peterson P, Sprent J. J Exp Med. 1992;176:495–505. doi: 10.1084/jem.176.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson G, Owen J, Moore N, Jenkinson E. J Exp Med. 1994;179:2027–2031. doi: 10.1084/jem.179.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]