Abstract
Chemotherapeutics in the taxane and vinca-alkaloid classes sometimes produce a painful peripheral neuropathy for which there is no validated treatment. Experiments with rat models of paclitaxel- and vincristine-evoked pain suggest that these conditions may not respond to all of the analgesics that have efficacy in other models of painful peripheral neuropathy. We tested gabapentin as a potential analgesic for paclitaxel- and vincristine-evoked pain. We used a repeated dosing paradigm because there are precedents showing that repeated drug exposure may be necessary to demonstrate analgesia in neuropathic pain models. Gabapentin is believed to work via binding to voltage-gated calcium channels that contain the alpha-2-delta type-1 (α2δ-1) subunit, and the expression of this subunit is known to be increased in some painful peripheral neuropathy models. Thus we also examined whether the paclitaxel-evoked pain syndrome was accompanied by an α2δ-1 increase, and whether gabapentin had any effect on subunit expression.
We found that the paclitaxel- and vincristine-evoked mechano-allodynia and mechano-hyperalgesia were significantly reduced by gabapentin, but only with repeated dosing. Paclitaxel-evoked painful peripheral neuropathy was associated with an increased expression of the α2δ-1 subunit in the spinal dorsal horn, but not in the dorsal root ganglia. The spinal cord increase was normalized by repeated gabapentin injections. Together, these findings suggest that repeated dosing with gabapentin may be beneficial in patients with chemotherapy-evoked painful peripheral neuropathy and that gabapentin's mechanisms of action may include normalization of the nerve injury-evoked increase in calcium channel α2δ-1 subunit expression.
Keywords: allodynia, hyperalgesia, paclitaxel, neuropathic pain, vincristine
Paclitaxel is one of the most effective and commonly used drugs for the treatment of solid tumors. Its dose-limiting side-effect is peripheral neuropathy which is usually manifest as loss of cutaneous sensations; however, this is accompanied by a chronic neuropathic pain syndrome in a significant minority of cases (for review see Dougherty et al., 2004). Other commonly used chemotherapeutics, e.g. vincristine and the platinum-complex agents, produce neuropathic pain syndromes with similar symptoms. There are no clinically proven analgesics for the treatment of these conditions. Chemotherapy-evoked neuropathic pain significantly limits what can be lifesaving therapy and it is a significant burden to many cancer patients.
Several lines of evidence suggest that drugs that modulate calcium channels may have analgesic efficacy in chemotherapy-evoked pain syndromes. Our laboratory has previously reported that ethosuximide, an anti-epileptic that blocks T-type calcium channels, produces excellent analgesia in animals with paclitaxel-evoked neuropathic pain (Flatters and Bennett, 2004). We have also shown that the intrathecal administration of calcium chelators produces analgesia in both paclitaxel- and vincristine-evoked painful peripheral neuropathies (Siau and Bennett, 2006). Gabapentin is known to bind to neuronal voltage-gated calcium channels that contain the alpha-2-delta type-1 subunit (α2δ-1; Gee et al., 1996; Field et al., 2000; Taylor, 2004; Bian et al., 2006; Löscher and Schmidt, 2006) and it has proven efficacy in both clinical and experimental painful peripheral neuropathies (reviewed in Wiffen et al., 2005; Gilron and Flatters, 2006). Accordingly, we examined whether gabapentin has an analgesic effect in rats with paclitaxel- and vincristine-evoked painful peripheral neuropathies. We used a repeated dosing paradigm because there is precedence for a delayed onset of analgesic efficacy in some cases (Shadiack et al., 1999; Hao et al., 2000; Xiao et al., unpublished observations).
Levels of α2δ-1 in the dorsal root ganglia (DRG) and dorsal spinal cord are known to be increased in rats in models of post-traumatic and diabetic painful peripheral neuropathies (reviewed in Luo et al., 2002). Thus, we also examined the expression of α2δ-1 in the DRG and spinal cord of paclitaxel-treated rats and determined whether gabapentin treatment had an effect on the subunit's expression.
EXPERIMENTAL PROCEDURES
These experiments conformed to the ethics guidelines of the International Association for the Study of Pain (Zimmermann, 1983), the National Institutes of Health (USA), and the Canadian Institutes of Health Research. All experimental protocols were approved by the Facility Animal Care Committee of the Faculty of Medicine, McGill University in accordance with the regulations of the Canadian Council on Animal Care.
Animals
Adult male Sprague–Dawley rats (220–300 g, Harlan Inc., Indianapolis, IN, USA; Frederick, MD breeding colony) were housed on sawdust bedding in plastic cages. Artificial lighting was provided on a fixed 12-h light/dark cycle with food and water available ad libitum.
Chemotherapy-evoked painful peripheral neuropathy models
Paclitaxel (Taxol®; Bristol-Myers-Squibb, Montreal, QC, Canada; 6 mg/ml) was diluted with saline to a concentration of 2 mg/ml. Paclitaxel (2 mg/kg) was injected i.p. on four alternate days (days 0, 2, 4 and 6) as described previously (Polomano et al., 2001). The time course of the paclitaxel-evoked pain syndrome has been described previously (Flatters and Bennett, 2006). Vincristine (Novopharm Ltd., Toronto, ON, Canada) was diluted with distilled water to a concentration of 50 μg/ml. Animals were injected i.p. with 50 μg/kg for 10 consecutive days as described previously (Siau and Bennett, 2006). The time course of the mechanoallodynia and mechano-hyperalgesia produced by this dose and dosing schedule was examined over the course of 42 days with comparison to a vehicle-injected control group. The results of a replicate time-course study were nearly identical to the results of the first study and so the data were combined.
Behavioral testing
Paclitaxel- and vincristine-treated rats have pronounced hyper-sensitivity to mechanical and cold stimulation, but little or no heat-hyperalgesia (e.g. Polomano et al., 2001; Flatters and Bennett, 2004); the same appears to be true in chemotherapy patients (Dougherty et al., 2004). We chose to examine gabapentin's effects on mechano-allodynia and mechano-hyperalgesia. The rats were habituated to the behavioral testing environment and baseline measurements of mechanical sensitivity were taken on three consecutive days with drug administration commencing on the day after the last baseline test. The rats were placed on an elevated wire mesh floor and confined beneath overturned mouse cages made of clear plastic. von Frey filaments with bending forces of 4 g, 8 g, and 15 g were applied to the mid-plantar skin (avoiding the base of the tori) of each hind paw five times, with each application held for 5 s. Withdrawal responses to the von Frey filaments from both hind paws were counted and then expressed as an overall percentage response. Normal rats hardly ever withdraw from the 4 g stimulus; the increased level of responding seen after chemotherapy is thus indicative of mechanoallodynia. Normal rats withdraw from the 8 g and 15 g stimuli 10–20% of the time; the increased level of responding to these hairs seen after chemotherapy is thus indicative of mechanohyperalgesia.
Drug administration
The gabapentin dose was 100 mg/kg, i.p., which has been shown to be effective in models of post-traumatic and diabetic painful peripheral neuropathies (e.g. Xiao and Bennett, 1996; Luo et al., 2002). Gabapentin/control treatments began at the approximate time of peak symptom severity in rats with confirmed neuropathic pain (26 days (D26) after the first paclitaxel-injection, and D17 after the first vincristine injection). Gabapentin- and vehicle (sa-line) -injected rats (n=12/group) were tested 1 h and 24 h after each of four daily injections. The 24 h test point served as a test for a persistent effect from the preceding injection; in the absence of a persistent effect, the 24 h test point served as a pre-injection baseline for the succeeding injection.
Roto-rod testing
Gabapentin's acute analgesic effects in animal neuropathic pain models are generally not considered to be confounded with motor or sedative side effects. However, we considered the possibility that such side effects might appear with the repeated dosing protocol used here. To investigate this possibility, rats were given three daily training sessions on a roto-rod task (Ugo Basile, Comerio, Italy; 7 cm diameter rod; constant speed of 20 revolutions per min., 60 s cutoff), treated with paclitaxel as described above, and then tested again on day 32, and 1 h after each of four consecutive daily injections of gabapentin (100 mg/kg, i.p.) or saline (n=8/group).
Quantification of α2δ-1 levels
Tissue was obtained from additional rats for quantification of α2δ-1 levels in the DRG and spinal cord dorsal horn. These rats were killed immediately after behavioral assays (4 g and 15 g stimuli) that were conducted 1 h after injection of saline or gabapentin. Separate groups (each n=7) were killed following the first injection of saline or gabapentin and following the third injection of saline or gabapentin. Naïve rats (neither paclitaxel nor gabapentin exposure) served as controls (n=7).
Animals were deeply anesthetized with sodium pentobarbital and guillotined. Spinal cords were quickly extruded through the cervical wound by high-pressure injection of ice-cold saline via a needle inserted intrathecally at the level of the cauda equina. The lumbosacral enlargement was excised and divided at the level of the central canal into dorsal and ventral halves. The dorsal half was frozen on dry ice and stored at −80 °C until assay. Extruding the spinal cord in this way tears the dorsal and ventral roots and leaves the DRG in situ. Immediately after obtaining the spinal cord, laminectomies were performed to expose the fourth and fifth lumbar spinal segment (L4 and L5) DRGs bilaterally; these were excised, frozen on dry ice, and stored at −80 °C until assay.
Western blot analysis
Protein expression levels were determined with Western blots as described previously (Luo et al., 2001). Briefly, frozen tissues were pulverized and extracted in 50 mM Tris buffer, pH 8.0, containing 0.5% Triton X-100, 150 mM NaCl, 1 mM EDTA, and protease inhibitors. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Protein extracts were separated by NuPAGE 3–8% Tris-acetate gel (Invitrogen, Carlsbad, CA, USA) electrophoresis under a reducing condition (0.05 M DTT), transferred onto a nitrocellulose membrane (Bio Rad, Hercules, CA, USA), and subsequently incubated in 5% blocking buffer (0.1% Tween 20 and 5% nonfat dry milk in PBS) for 1 h at room temperature. The membranes were then incubated with monoclonal antibodies against the calcium channel α2δ-1 proteins (1:1000 dilution; Sigma, St. Louis, MO, USA), or with an antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:10,000 dilution; Ambion, Austin, TX, USA) for 1 h at room temperature or overnight at 4 °C. GAPDH was used to verify equal protein loading. After washing, the membranes were incubated with anti-mouse IgG secondary antibodies conjugated to horseradish peroxidase (1:10,000 dilution; Cell Signaling Tech, Danvers, MA, USA) for 1 h at room temperature. The protein–antibody complexes were then visualized using enhanced chemiluminescent reagents (Super Signal West Pico; Pierce). Under reducing conditions, the delta peptide separates from the α2 subunit (Jay et al., 1991). Therefore, the primary antibody detected the α2 peptide only. Rat brain extracts containing high levels of α2δ proteins were used as a positive control. Densitometric analysis (Kodak Image Station 2000MM) of the immunore-active bands was performed within the linear range of the charge-coupled device camera and X-ray films. Comparisons in band densities between sample groups were performed after taking the ratio of the α2δ bands over the GAPDH bands for loading normalization.
Statistics
For the time-course and gabapentin challenge studies, pair-wise comparisons were made with Bonferroni-corrected unpaired t-tests. Where significant differences were found between gabapentin-and vehicle-treated groups, % anti-allodynic (or % anti-hyperalgesia) scores were computed: % anti-allodynia/hyperalgesia=[(vehicle group score)−(gabapentin group score)]÷[(vehicle group score)−(naïve baseline score)]×100. Densitometry values were compared with unpaired t-tests where significance changes were indicated by two-tailed P values <0.05.
RESULTS
Vincristine time course
In the time course study (Fig. 1), vincristine-treated rats developed statistically significant increase in responding to 4 g, 8 g, and 15 g von Frey hair stimulation by day 12 (day 9 was the last day of vincristine administration). Responses returned to near normal levels by days 32–38. The clear delay between the final vincristine injection and peak symptom severity is a consistent finding in our experience and has also been noted for the paclitaxel-evoked pain syndrome (Flatters and Bennett, 2006).
Fig. 1.
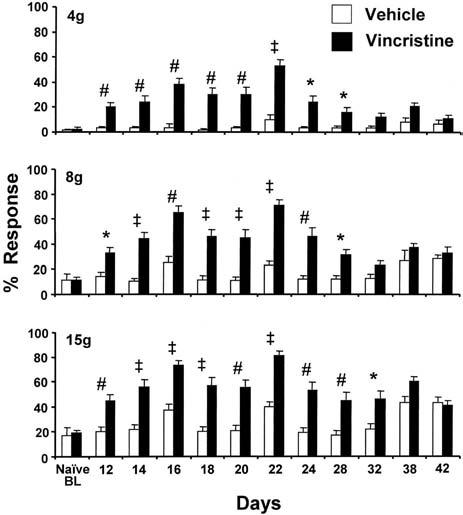
Time course of mechano-allodynia (responses to 4 g von Frey hair) and mechano-hyperalgesia (responses to 8 g and 15 g von Frey hairs) produced by 10 consecutive daily doses (starting on D0, ending on D9) of vincristine (50 μg/kg, i.p.). * P<0.05; # P<0.01; ‡ P<0.001. Vehicle group: n=6–13/time point; vincristine group: n=11–23/time point.
Effects of gabapentin in vincristine-treated rats
The vincristine-treated rats had statistically significant increased levels of responding to all three von Frey stimuli prior to drug challenge (Fig. 2). Injection of vehicle had no effect on responding at any time during the study. The first injection of gabapentin produced no significant change in responding to any of the stimuli. However, the 2nd, 3rd, and 4th, injections had robust, statistically significant analgesic effects for all three stimuli when tested 1 h post-injection. The degree of anti-allodynia and anti-hyperalgesia ranged from 34% to 66%. None of the analgesic responses persisted to 24 h post-injection.
Fig. 2.
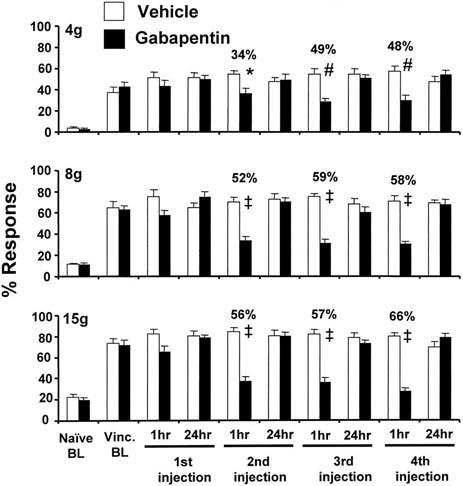
Responses to four consecutive daily injections of gabapentin (100 mg/kg, i.p.) in rats with vincristine-evoked neuropathic pain. Naïve BL: response level prior to receiving vincristine; Vinc. BL: post-vincristine baseline response level on D17 after the first injection of vincristine (one day before the start of gabapentin dosing). * P<0.05; # P<0.01; ‡ P<0.001.
Effects of gabapentin in paclitaxel-treated rats
The paclitaxel-treated rats had statistically significant increased levels of responding to all three von Frey stimuli prior to drug challenge (Fig. 3). Injection of vehicle had no effect on responding at any time during the study. The first injection of gabapentin had no effect. However, the 2nd, 3rd, and 4th, injections had robust, statistically significant analgesic effects on responding to the 8 g and 15 g stimuli when tested 1 h post-injection. Statistically significant suppression of responding to the 4 g stimulus was not seen until the 3rd injection. The degree of anti-allodynia and anti-hyperalgesia ranged from 34% to 59%. None of the analgesic responses persisted to 24 h post-injection.
Fig. 3.
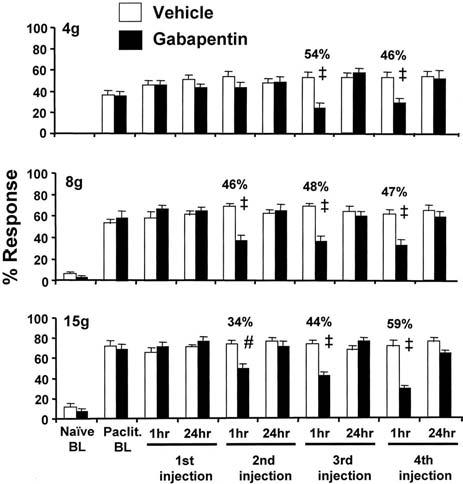
. Responses to four consecutive daily injections of gabapentin (100 mg/kg, i.p.) in rats with paclitaxel-evoked neuropathic pain. Naïve BL: response level prior to receiving paclitaxel; Pacli. BL: post-paclitaxel baseline response level on D26 after the first injection of paclitaxel (one day before the start of gabapentin dosing). * P<0.05; # P<0.01; ‡ P<0.001.
For both paclitaxel- and vincristine-treated rats the significant but clearly partial reversal of allodynia and hyper-algesia was comparable to what we have seen previously with gabapentin in rats with post-traumatic painful peripheral neuropathy (Xiao and Bennett, 1996).
Effects of gabapentin on roto-rod performance
All rats were able to meet the 60 s criterion on the roto-rod by the third training session. Post-paclitaxel performance assessed on day 32 (before giving gabapentin) was not significantly different from that seen at the end of the training sessions. Roto-rod performance 1 h post-injection in the saline-treated and gabapentin-treated groups was unchanged after each of the four daily injections (data not shown).
Effects of paclitaxel and gabapentin on α2δ-1 expression
Neither saline- nor gabapentin-treated rats had any significant change in α2δ-1 levels in the DRGs following the first or third injection (Fig. 4A).
Fig. 4.
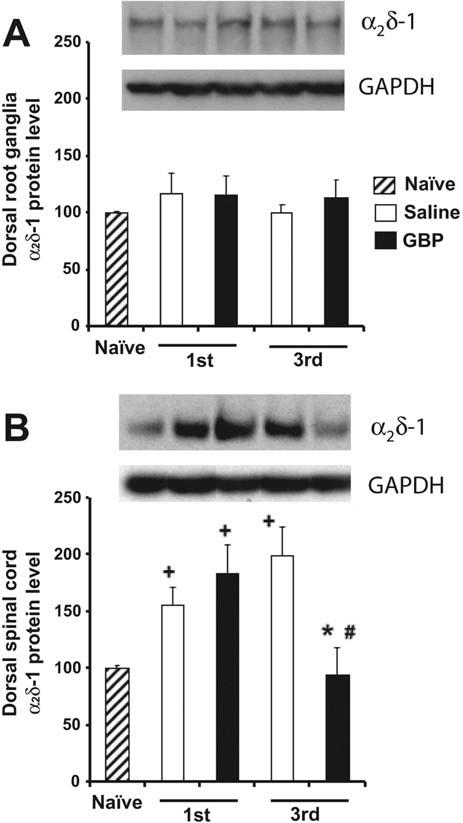
α2δ-1 Subunit protein levels in DRG (A) and dorsal spinal cord (B) of paclitaxel-treated animals after saline or gabapentin treatment (n=7/group). Samples in the representative Western blot in each panel are arranged in the same order as that indicated in the bar graph summaries. GAPDH bands were used as internal controls for protein loading. * P<0.05 compared with the third injection of saline group. # P<0.05 compared with the first injection of gabapentin group. + P<0.05 compared with naïve group.
As shown in Fig. 4B, rats with paclitaxel-evoked painful peripheral neuropathy that were killed 1 h after the first injection of saline or gabapentin had statistically significant increases of α2δ-1 subunit expression in the dorsal spinal cord (56–84% greater than the naïve control group mean). There was no significant difference in the magnitude of this increase in the saline-treated vs. gabapentin-treated groups. Consistent with the results of the previous experiment (Fig. 3), the first injection of gabapentin in these animals had no significant analgesic effect on responses to the 4 g and 15 g stimuli (5% and 1% changes, respectively).
Rats killed 1 h after the third injection of saline had a significant increase of α2δ-1 subunit expression in the dorsal spinal cord that was slightly larger than that seen after the first injection (99% greater than the naïve control group mean). However, the group that was killed after the third injection of gabapentin had a normal level of dorsal spinal cord α2δ-1 subunit expression (Fig. 5B). As in the previous experiment (Fig. 3), the third injection of gabapentin in these animals significantly reduced responding to the 4 g and 15 g stimuli (81% and 83% anti-allodynia/anti-hyperalgesia).
DISCUSSION
Analgesic efficacy
Gabapentin had significant analgesic action against the mechano-allodynia and mechano-hyperalgesia that are seen in paclitaxel- and vincristine-evoked painful peripheral neuropathies, but this action was clearly evident only after repeated dosing. A day or two of delay in the onset of analgesia would have little, if any, importance clinically. Thus, our results suggest that patients with paclitaxel- and vincristine-evoked pain may obtain relief with gabapentin. Encouraging results have been reported in open-label trials of gabapentin as adjunct therapy in cancer patients with neuropathic pain, but it is unclear whether the responders in these trials had chemotherapy-evoked neuropathic pain (Bosnjak et al., 2002; Caraceni et al., 2004). A preliminary report of a double-blind, randomized, placebo-controlled trial of gabapentin in cancer patients with chemotherapy-evoked pain found no analgesic efficacy, but the type of chemotherapy that these patients received has not been described and pain was assessed only via a rating of average daily pain intensity (Wong et al., 2005). Our results suggest that future clinical trials should specifically examine the patients' mechano-hypersensitivity as gabapentin may have symptom-specific activity (Woolf et al., 1998).
Matsumoto et al. (2006) found significant anti-allodynic and anti-hyperalgesic effects or rapid onset with 10–30 mg/kg gabapentin in a newly described mouse model of paclitaxel-evoked pain. There are two previous reports on gabapentin's actions on vincristine-evoked pain, and one on the effects of pregabalin. Each of these studies examined the effects of a single i.p. injection and each used the vincristine neuropathy model of Nozaki-Taguchi et al. (2001), where vincristine is administered via 14 days of continuous i.v. infusion, in contrast to our method where the drug is given as 10 consecutive i.p. boluses (Siau and Bennett, 2006). Lynch et al. (2004) demonstrated significantly reversed mechano-allodynia with an ED50 of 70 mg/kg (400 μmol/kg) gabapentin. In contrast, Luo et al. (2002) found no action against mechano-allodynia with 50–300 mg/kg gabapentin. Nozaki-Taguchi et al. (2001) found analgesic effects against mechano-allodynia with 80 mg/kg pregabalin. While differences between models, strain of rats used, and other variables may explain the discrepancies, our results suggest that more consistent results would be obtained with repeated dosing.
Delayed onset of analgesia
We found that significant analgesic effects were not clearly evident until gabapentin was administered repeatedly in both the paclitaxel and vincristine models. Similarly, Patel et al. (2001) noted a clear delay in the onset of gabapentin analgesia in a traumatic nerve injury model and Hao et al. (2000) reported a very pronounced delay in the onset of gabapentin analgesia in rats with neuropathic pain due to spinal cord injury. However, in general a delay in the onset of gabapentin-evoked analgesia is unusual. The analgesic effects of the same or lower doses of gabapentin are evident with a single injection in models of post-traumatic painful neuropathy and diabetic painful neuropathy (reviewed in Gilron and Flatters, 2006). Thus, we think it is unlikely that the delayed onset of activity is due to an inadequate dose. Moreover, we believe that the roto-rod results rule out any possible explanation based on cumulative sedation or motor dysfunction.
Neuropathic pain models and α2δ-1 levels
We found a statistically significant increase in α2δ-1 levels in the dorsal spinal cord at the time of peak-pain severity in paclitaxel-treated rats, but no change in levels in the DRG. Remarkably, repeated doses of gabapentin reduced α2δ-1 expression in the dorsal spinal cord to normal levels.
An increase in α2δ-1 mRNA and protein in the DRG has been reported in mice with paclitaxel-evoked pain, but spinal cord levels were not examined (Matsumoto et al., 2006). Diabetic painful peripheral neuropathy produces an increase in α2δ-1 proteins in the dorsal spinal cord of similar magnitude. The effects of diabetic neuropathy on levels in the DRG are unclear: Luo et al. (2002) found no change in protein levels, but Yusef et al. (2001) found increased mRNA levels. Luo et al. (2002) did not detect any change in α2δ-1 protein levels in dorsal spinal cord or DRG in rats with vincristine-evoked pain. An increase (ca. 70%) in α2δ-1 levels in the DRG has been reported in rats in a model of postherpetic neuralgia; dorsal spinal cord levels were not examined (Garry et al., 2005). In contrast, models involving traumatic nerve injury produce very large (500–1700%) increases in DRG protein levels and large increases (170%) in dorsal horn protein levels; large increases in mRNA levels have also been shown (Luo et al., 2001; Newton et al., 2001; Costigan et al., 2002; Wang et al., 2002; Valder et al., 2003).
The factor(s) that triggers α2δ-1 up-regulation in neuropathic pain syndromes is unknown. Axotomy is an obvious possibility in the nerve trauma models and axonal dieback may be a factor in diabetic painful peripheral neuropathy. Rats with paclitaxel-evoked neuropathic pain have primary afferent degeneration that is confined to the afferents' sensory terminal arbors (Flatters and Bennett, 2006; Siau et al., 2006). It is apparent that this distal degeneration is not a large enough insult to evoke α2δ-1 up-regulation in the DRG; it also does not trigger the expression of activating transcription factor 3 (ATF3), which appears in the nuclei of axotomized DRG neurons (Flatters and Bennett, 2006).
Mechanism of action of gabapentin's effects on neuropathic pain
Gabapentin's analgesic effects are generally easy to demonstrate in nerve trauma models where there are very large increases in α2δ-1 levels in both DRG and dorsal spinal cord. We have shown that repeated doses of gabapentin have efficacy in the paclitaxel model where there is a modest increase of only dorsal spinal cord α2δ-1 levels. Rats with painful diabetic neuropathy have a similar increase in spinal levels, but in the diabetic model most studies show gabapentin to be effective with even single dose administration (Field et al., 1999; Miki et al., 2001; Luo et al., 2002; Lindner et al., 2006). These observations suggest that neuropathic pain states derived from different pathological conditions may have distinct underlying mechanisms, and that gabapentin may have nerve injury type-specific (and perhaps species-specific) mechanisms of action within pain processing pathways. Moreover, in addition to acting on systems unrelated to the α2δ-1 subunit or its associated voltage-gated calcium channel (for review see Urban et al., 2005; Löscher and Schmidt, 2006), gabapentin may target pathways that regulate α2δ-1 subunit expression.
CONCLUSION
Previous work has suggested that the mechanism of action for gabapentin-evoked analgesia may differ in different neuropathic pain syndromes, and that the causal role of an increase in α2δ-1 expression in producing pain hypersensitivity may also differ in different neuropathic pain conditions (Luo et al., 2002; Li et al., 2006). Our results are consistent with these hypotheses. Our results suggest a previously unknown effect: gabapentin-induced reversal (normalization) of the increase in spinal α2δ-1 expression produced by damage to primary afferent sensory neurons. A clearer understanding of this phenomenon awaits more thorough studies. The cause of the delayed onset of activity for gabapentin's analgesic effect remains to be determined. It is of importance to note that the delayed onset of gabapentin's activity suggests that the use of a single injection of a potential analgesic may not be an adequate trial in at least some pain models.
UNCITED REFERENCES
Hwang JH, Yaksh TL (1997); Li CY, Song YH, Higuera ES, Luo ZD (2004); Luo ZD (2000).
Acknowledgments
Supported by the Mayday Fund, the Canada Foundation for Innovation, and the National Institutes of Health (G.J.B.: R01-NS052255; Z.D.L.: R01-NS40135). G.J.B. is a Canada Senior Research Chair. We thank Charles P. Taylor for pre-prints, Haiwei Jin for comments on the manuscript, and Chiang Siau and Lina Naso for assistance.
Abbreviations
- D (with number)
number of days
- DRG
dorsal root ganglion
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- α2δ-1
alpha-2-delta type-1
REFERENCES
- Bian F, Li Z, Offord JD, Davis MD, McCormick JA, Taylor CP, Walker LC. Calcium channel alpha2-delta type I subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdale, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res. 2006;1075:68–80. doi: 10.1016/j.brainres.2005.12.084. [DOI] [PubMed] [Google Scholar]
- Bosnjak S, Jelic S, Susnjar S, Luki V. Gabapentin for relief of neuropathic pain related to anticancer treatment: a preliminary study. J Chemother. 2002;14:214–219. doi: 10.1179/joc.2002.14.2.214. [DOI] [PubMed] [Google Scholar]
- Caraceni A, Zecca E, Bonezzi C, Arcuri E, Yaya Tur R, Maltoni M, Visentin M, Gorni G, Martini C, Tirelli W, Barbieri M, De Conno F. Gabapentin for neuropathic cancer pain: randomized controlled trial from the Gabapentin Cancer Pain Study Group. Cancer Treat Rev. 2004;31:58–62. [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Field MJ, Hughes J, Singh L. Further evidence for the role of the alpha(2)delta subunit of voltage dependent calcium channels in models of neuropathic pain. Br J Pharmacol. 2000;131:282–286. doi: 10.1038/sj.bjp.0703604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Ethosuximide has anti-allodynic and anti-hyperalgesic effects in rat models of paclitaxel and vincristine-induced painful peripheral neuropathies. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry EM, Delaney A, Anderson HA, Sirinathsinghji EC, Clapp RH, Martin WJ, Kinchington PR, Krah DL, Abbadie C, Fleetwood-Walker SM. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118:97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Gilron I, Flatters SJ. Gabapentin and pregabalin for the treatment of neuropathic pain: A review of laboratory and clinical evidence. Pain Res Manag. 2006;11(Suppl A):16A–29A. [Google Scholar]
- Hao JX, Xu XJ, Urban L, Wiesenfeld-Hallin Z. Repeated administration of systemic gabapentin alleviates allodynia-like behaviors in spinally injured rats. Neurosci Lett. 2000;280:211–214. doi: 10.1016/s0304-3940(00)00787-4. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg Anesth. 1997;22:249–256. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold M, Dickenson AH, Feng G, Luo ZD. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006 doi: 10.1016/j.pain.2006.04.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD, Bourin C, Chen P, McElroy JF, Leet JE, Hogan JB, Stock DA, Machet F. Adverse effects of gabapentin and lack of anti-allodynic efficacy of amitriptyline in the streptozotocin model of painful diabetic neuropathy. Exp Clin Psychopharmacol. 2006;14:42–51. doi: 10.1037/1064-1297.14.1.42. [DOI] [PubMed] [Google Scholar]
- Löscher W, Schmidt D. New horizons in the development of antiepileptic drugs: innovative strategies. Epilepsy Res. 2006;69:183–272. doi: 10.1016/j.eplepsyres.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD. Rat dorsal root ganglia express distinctive forms of the alpha2 calcium channel subunit. Neuroreport. 2000;11:3449–3452. doi: 10.1097/00001756-200011090-00010. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JJ, 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004;110:56–63. doi: 10.1016/j.pain.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxel-induced A-fiber-hypersensitization by gabapentin. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.106.103614. in press (doi: 10.1124/jpet.106.103614) [DOI] [PubMed] [Google Scholar]
- Miki S, Yoshinaga N, Iwamoto T, Yasuda T, Sato S. Antinociceptive effect of the novel compound OT-7100 in a diabetic neuropathy model. Eur J Pharmacol. 2001;430:229–234. doi: 10.1016/s0014-2999(01)01373-5. [DOI] [PubMed] [Google Scholar]
- Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res. 2001;95:1–8. doi: 10.1016/s0169-328x(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001;93:69–76. doi: 10.1016/S0304-3959(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Patel S, Naeem S, Kesingland A, Froestl W, Capogna M, Urban L, Fox A. The effects of GABA(B) agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflamma-tory pain in the rat. Pain. 2001;90:217–226. doi: 10.1016/S0304-3959(00)00404-8. [DOI] [PubMed] [Google Scholar]
- Polomano R, Clark U, Mannes AJ, Bennett GJ. A painful peripheral neuropathy in rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Molino LJ, Yagel SK. The novel anticonvulsant topiramate is antiallodynic in a rat model of neuropathic pain. Analgesia. 1999;4:173–179. [Google Scholar]
- Siau C, Bennett GJ. Dysregulation of neuronal calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siau C, Xiao WH, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.05.007. in press (doi:10.1016/j.expneurol.2006.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP. The biology and pharmacology of calcium channel alpha-2-delta proteins. CNS Drug Rev. 2004;10:183–188. doi: 10.1111/j.1527-3458.2004.tb00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Ren K, Park KT, Campbell B, Anker N, Sterans B, Aiyar J, Belley M, Cohen C, Bristow L. Comparison of the antinociceptive profiles of gabapentin and 3-methylgabapentin in rat models of acute and persistent pain: implications for mechanisms of action. J Pharmacol Exp Ther. 2005;313:1209–1216. doi: 10.1124/jpet.104.081778. [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem. 2003;87:560–573. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev. 2005;3:CD005452. doi: 10.1002/14651858.CD005452. [DOI] [PubMed] [Google Scholar]
- Wong GY, Michalak JC, Sloan JA, Mailliard JA, Nikcevich DA, Novotny PJ, Warner DO, Kutteh L, Dakhil SR, Loprinzi CL. A phase III double blinded, placebo controlled, randomized trial of gabapentin in patients with chemotherapy-induced peripheral neuropathy: A North Central Cancer Treatment Group study. J Clin Oncol. 2005;23(Suppl):8001. [Google Scholar]
- Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, Lipton R, Loeser JD, Payne R, Torebjork E. Towards a mechanism-based classification of pain? Pain. 1998;77:227–229. doi: 10.1016/S0304-3959(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Gabapentin has an antinociceptive effect mediated via a spinal site of action in a rat model of painful peripheral neuropathy. Analgesia. 1996;2:267–273. [Google Scholar]
- Yusef SP, Goodman J, Gonzales IM, Bramwell S, Pionnock RD, Dixon AK, Lee K. Streptozocin-induced neuropathy is associated with altered expression of voltage-gated calcium channel subunit mRNAs in rats dorsal root ganglion neurons. Biochem Biophys Res Comm. 2001;289:402–406. doi: 10.1006/bbrc.2001.5943. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


