Abstract
The antioxidant and antimicrobial effects of equivalent concentrations of fresh garlic (FG), garlic powder (GP) and garlic oil (GO) were investigated against lipid oxidation and microbial growth in raw chicken sausage during storage at 3°C. The antioxidant activities were compared to that of a standard synthetic antioxidant; butylated hydroxyanisole (BHA). The initial mean levels of thiobarbituric acid (TBA) value and peroxide value (POV) were 0.140 and 6.32, respectively. However after 21 days of storage, TBA and POV ranged from 0.151 to 4.92, respectively, in FG (50 g/kg) formulated samples to 0.214 and 8.64, respectively, in GO (0.06 g/ kg) formulation. Addition of either garlic or BHA (0.1 g/kg) significantly delayed lipid oxidation when compared with control. The antioxidant activities of the various materials added followed the order FG>GP>BHA>GO. On the other hand, the initial aerobic plate count (APC) in the samples was 4.41 log10 CFU/g. Addition of FG (30 g/kg) or GP (9 g/kg) significantly reduced the APC and, subsequently, the shelf-life of the product was extended to 21 days. However, addition of GO or BHA resulted in no significant difference in APC when compared with control. Sensory analysis indicated that FG had a significant stronger flavor than the other sausage formulations. The results suggest that fresh garlic and garlic powder, through their combined antioxidant and antimicrobial effects, are potentially useful in preserving meat products.
Keywords: Garlic, Antioxidant, Antimicrobial, Chicken sausage
1. Introduction
Chicken meat and its products have experienced increasing popularity and become widely spread all over the world. Chicken sausage is one of the popular foodstuffs among these products (Barbut, 2001). However during storage, quality attributes of the product deteriorate due to lipid oxidation and microbial growth. Lipids oxidation is responsible for reduction in nutritional quality as well as changes in flavor (Aguirrezábal, Mateo, Domínguez, & Zumalacárregui, 2000), while microbial contamination can precipitate major public health hazards and economic loss in terms of food poisoning and meat spoilage. Thus, application of suitable agents possessing both antioxidant and antimicrobial activities may be useful for maintaining meat quality, extending shelf-life and preventing economic loss (Yin & Cheng, 2003). Much research has indicated that lipid oxidation and microbial growth in meat products can be controlled or minimized by using either synthetic or natural food additives (Gray, Gomaa, & Buckley, 1996; Lee, Williams, Sloan, & Littell, 1997; Mielnik, Aaby, & Skrede, 2003). Various synthetic antioxidants, such as butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT), are commonly used to delay the development of rancidity in food products (Martinez-Tome et al., 2001; Ohshima, Yankah, Ushio, & Kiozumi, 1998). However, consumers are concerned about the safety of synthetic food additives. This concern has led to arouse a great interest in natural additives (Pokorný, 1991). Natural agents possessing antioxidant and antimicrobial properties have the advantage of being readily accepted by consumers, as they are considered natural.
Garlic is one of the most commonly used ingredients as a flavor enhancement for sausage. In addition to flavoring the foods, garlic is appreciated for its medicinal properties. Garlic has a wide spectrum of actions; not only antibacterial, antiviral, antifungal and antiprotozoal, but also has beneficial effects on the cardiovascular and immune systems (Harris, Cottrell, Plummer, & Lloyd, 2001). During the last decade, the antimicrobial activity of garlic and garlic-derived organosulfur compounds was widely investigated against both food spoilage bacteria and food-borne pathogens (Leuschner & Ielsch, 2003; Naidu, 2000; Unal et al., 2001). Besides its antimicrobial effect, garlic showed effective antioxidant activity in vivo and in vitro (Jackson et al., 2002; Prasad, Laxdal, Yu, & Raney, 1995). Garlic-rich organosulfur compounds and their precursors (allicin, diallyl sulfide and diallyl trisulfide) are believed to play a key role in these biological effects (Ankri & Mirelman, 1999; Kumar & Berwal, 1998).
To date, most previous studies have focused independently on either antioxidant or antimicrobial activities of garlic in meat products, and the objective of the present study was to investigate the antioxidant as well as the antimicrobial effectiveness of three garlic preparations, i.e. fresh, powder and oil at various concentrations, in preserving raw chicken sausage during refrigerated storage.
2. Materials and methods
2.1. Materials
Fresh garlic (FG) bulbs (Allium sativum, var. Chinese white garlic) were purchased from a local market. The dry skins of the bulb were removed before use; then the cloves were peeled and crushed finely by using a kitchen hand held grater. Garlic powder (GP) was purchased from Daiichi Foods Company (Daiichi Pharmaceutical, Japan). According to the manufacturer data, 3 kg of produced GP is weight equivalent to 10 kg of FG.
Garlic oil (GO) was prepared in the laboratory by steam distillation (Chen et al., 2001). Peeled garlic cloves (1 kg) were blended with 2 L of distilled water using a domestic blender (model MX-X61-W, National, Japan). The garlic oil was extracted by heating the homogenate during 3 h using a vertical steam distillation unit. The extract was dried over anhydrous sodium sulfate and then filtered through nitrocellulose acetate membrane. The oily product was designated GO. At the end of the extraction process, a final yield of 3 g of GO was obtained from 1 kg of garlic cloves, indicating that 0.03 g of the GO is weight equivalent to 10 g of the FG. The resulting GO was kept in a tightly closed opaque bottle at 4°C until use.
Butylated hydroxyanisole (BHA) was purchased from Wako (Wako Chemicals, Osaka, Japan) and used as a reference antioxidant. It was added to the sausage formulation at a concentration of 0.1 g/kg (USDA, 1999).
2.2. Preparation of chicken sausage
Frozen boneless chicken legs (thigh meat), purchased from a local commercial source, were thawed at 10°C just before sausage manufacture. The thigh meat and immediate subcutaneous fat, which was scraped from the outermost layer of skin, were ground together through a 0.4-cm grinder plate (Super grinder-MK-G3; Matsushita Electric Industrial, Japan). The ground meat was formulated to contain either FG (20, 30 or 50 g/kg), GP (6, 9 or 15 g/kg), GO (0.06, 0.09 or 0.15 g/kg), or BHA (0.1 g/kg). These corresponding levels of the three garlic forms are weight equivalent. All other ingredients were added in equal amounts (g/kg) to the various formulations of sausage meat: 100 g shattered ice, 20 g salt (sodium chloride), 1.5 g sodium polyphosphate, 1 g monosodium glutamate, 0.1 g sodium nitrate, and 6.2 g sausage seasoning (3.5 g white pepper, 1.5 g nutmeg, 0.7 g sage and 0.5 g allspice). The batter was mixed in an emulsifier (Kenmix Electronic, model FP800, Kenwood Ltd., Britain) for 3 min and the resulting mixture was stuffed tightly into hydrocellulose casings 1.5 cm in diameter (Viskase Corporation, Chicago, USA), which were subsequently divided into food-casing lengths of about 12 cm per unit. The sausage units were vacuum-packaged in polyethylene bags, labeled and stored at 3°C.
2.3. Compositional analysis and pH measurement
After processing and before storage, the sausage was analyzed for its moisture, protein and fat content according to the methods of AOAC International (1999). Analyses were conducted in triplicate; all reagents were of analytical grade. For determination of the pH, 10 g of sample were homogenized with 50 ml distilled water and pH value was measured by a digital pH-meter (HM-5S; TOA Electric Industrial Co. Ltd., Tokyo, Japan).
2.4. Assessment of lipid oxidation
2.4.1. Measurement of TBA value
The 2-thiobarbituric acid (TBA) assay was carried out according to the procedure of Schmedes and Holmer (1989). Sausage sample (10 g) was mixed with 25 ml of trichloroacetic acid solution (200 g/l of TCA in 135 ml/l phosphoric acid solution) and homogenized in a blender for 30 s. After filtration, 2 ml of the filtrate were added to 2 ml TBA solution (3 g/l) in a test tube. The test tubes were incubated at room temperature in the dark for 20 h; then the absorbance was measured at 532 nm by using UV–VIS spectrophotometer (model UV-1200, Shimadzu, Japan). TBA value was expressed as mg malonaldehyde per kg of sausage.
2.4.2. Measurement of peroxide value
Peroxide value (POV) was determined according to the AOAC International (1999). The sample (3 g) was weighed in a 250-ml glass stoppered Erlenmeyer flask and heated in a water bath at 60°C for 3 min to melt the fat, then thoroughly agitated for 3 min with 30 ml acetic acid–chloroform solution (3:2 v/v) to dissolve the fat. The sample was filtered under vacuum through Whatman filter paper to remove meat particles. Saturated potassium iodide solution (0.5 ml) was added to the filtrate, which was transferred into the burette of an automatic titrator (DL 25 Titrator, Mettler-Toledo AG, Greifensee, Switzerland) equipped with stirrer and pH electrode. The titration was allowed to run against standard solution of sodium thiosulfate (25 g/l). POV was calculated and expressed as milliequivalent peroxide per kg of sample:
where S is the volume of titration (ml), N the normality of sodium thiosulfate solution (N = 0.01) and W the sample weight (kg).
2.5. Aerobic plate count
Sausage sample (10 g) was homogenized with 90 ml of sterile peptone water (1 g/l) in a laboratory homogenizer (AM-5 Ace homogenizer, Nihonseiki, Japan) and serial dilutions were prepared, then 0.1 ml of each dilution was spread with a bent sterile glass rod on duplicate plates of pre-poured and dried standard plate count agar (Nissui Pharmaceutical, Japan). After 48-h incubation at 25°C, colonies were counted and results were expressed as log10 CFU/g of sausage sample.
2.6. Sensory evaluation
Representative samples of the different sausage formulations were cooked in hot water at 75°C for 25 min. The intensity of garlic flavor, tenderness and overall acceptability scores of the sausage were determined by 10 panelists at 0, 7, 14, and 21 days of storage. The panelists included staff members and students in the Department of Food Science, Faculty of Dairy Science, Rakuno Gakuen University. Samples were cut into uniform size (about 3 cm in length), and introduced to panelists in covered serving dishes coded with 3-digit random numbers. Control sausage samples as well as BHA- and GO-formulated samples were not evaluated for the sensory attributes after 14 days of storage due to their higher bacterial counts. An eight-point hedonic scoring scale (8=extremely intense/tender; 7=very intense/tender; 6=moderately intense/tender; 5= slightly intense/tender; 4=slightly bland/tender; 3=moderately bland/tender; 2=very bland/tender; 1=extremely bland/tender) was employed for garlic flavor intensity and tenderness of sausage, while a nine-point hedonic scale (9=like extremely; 8=like very much; 7=like moderately; 6=like slightly; 5=neither like nor dislike; 4=dislike slightly; 3=dislike moderately; 2=dislike very much; 1=dislike extremely) was used for evaluation of the overall acceptability.
2.7. Statistical analysis
All measurements were carried out in triplicate (n = 3), and results were subjected to analysis of variance (ANOVA) using SAS software (SAS Institute, 1990). Differences between means were determined by the least significant difference test, and significance was defined at P<0.05.
3. Results and discussion
3.1. Composition and pH value
The moisture, protein and fat contents (g/100 g) in the control sausage were 71.3 ± 1.23, 16.4 ± 0.57 and 7.98 ± 0.31, respectively. Addition of the different garlic forms did not cause any significant changes in these contents (data not shown).
The initial pH value ranged from 6.65 (in control samples) to 6.78 (in FG-formulated samples). In all sausage formulations, storage had a significant (P<0.05) effect on the pH values, which tended to increase with storage time. However, after 21 days of storage no significant difference was detected between pH of FG-formulated sausage (7.03) and any of the other sausage formulations, which were ranged from 6.85 to 6.90.
3.2. Antioxidant activity
Fig. 1 shows the effect of different concentrations of FG, GP, and GO on TBA values in the sausage during storage at 3°C. The initial TBA value was 0.140, and after 21 days of storage, it ranged from 0.151 to 0.175 in FG-formulation, 0.162 to 0.187 in GP-formulation, 0.198 to 0.214 in GO-formulation and averaged 0.191 in BHA-formulated samples. These values were significantly lower than that of control samples (0.278). A significant difference was also noted between FG- and GO-formulated samples, while TBA value in BHA-formulated samples were not significantly different from any of the various garlic formulations. The little increase in TBA values during the 21 days of storage was attributed to vacuum packaging, which creates less oxygen available for oxidation. TBA value is routinely used as an index of lipid oxidation in meat products in store (Raharjo & Sofos, 1993), and the rancid flavor is initially detected in meat products between TBA values of 0.5 and 2.0 (Gray & Pearson, 1987).
Fig. 1.

Effects of three equivalent concentrations of fresh garlic (FG), garlic powder (GP) and garlic oil (GO) as well as butylated hydroxyanisole (BHA) on 2-thiobarbituric acid (TBA) value in raw chicken sausage stored at 3°C (vertical bars represent SE; LSD defined at P<0.05). (A): Control (▪); FG 20 g/kg (○); GP 6 g/kg (♦); GO 0.06 g/kg (▵); BHA 0.1 g/kg (×). (B): Control (▪); FG 30 g/kg (○); GP 9 g/kg (♦); GO 0.09 g/kg (▵); BHA 0.1 g/kg (×). (C): Control (▪); FG 50 g/kg (○); GP 15 g/kg (♦); GO 0.015 g/kg (▵); BHA 0.1 g/ kg (×).
Fig. 2 shows changes in POV of garlic-formulated chicken sausage. The initial POV was 6.32, however after 21 days of storage, it ranged from 4.92 to 6.22 in FG-formulated samples, 5.68 to 6.91 in GP-formulations, and 7.74 to 8.88 in GO-formulations, while it was 7.21 in BHA-formulated samples. These values were significantly lower than that of the control (15.61). Moreover, significantly lower POV was noted in FG-formulated samples in comparison with GO formulations. The POV of the FG-formulated sample (50 g/kg) was also significantly lower than that of BHA-formulated samples. Results also showed that addition of FG at a concentration of 20 g/kg or higher significantly reduced POV in comparison with control (Fig. 2). Aguirrezábal et al. (2000) reported that in a Spanish dry fermented sausage, fresh garlic at 1% concentration had no marked effect on the POV. In the present study, POV in all samples were below 25 meq of active O2/kg, which is considered as limit of acceptability in fatty foods (Evranuz, 1993; Narasimhan, Raghuver, Arumngham, Bhat, & Sen, 1986).
Fig. 2.
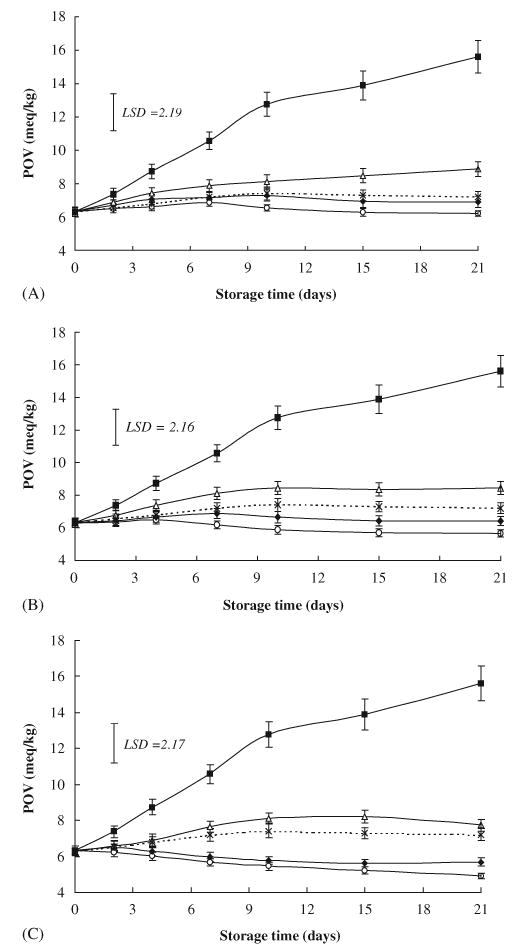
Effects of three equivalent concentrations of fresh garlic (FG), garlic powder (GP) and garlic oil (GO) as well as butylated hydroxyanisole (BHA) on peroxide value (POV) in raw chicken sausage stored at 3°C (vertical bars represent SE; LSD defined at P<0.05). (A): Control (▪); FG 20 g/kg (○); GP 6 g/kg (♦); GO 0.06 g/kg (▵); BHA 0.1 g/kg (×). (B): Control (▪); F G 30 g/kg (○); GP 9 g/kg (♦); GO 0.09 g/kg (▵); BHA 0.1 g/kg (×). (C): Control (▪); FG 50 g/kg (○); GP 15 g/kg (♦); GO 0.015 g/kg (▵); BHA 0.1 g/ kg (×).
Lipid oxidation represented by TBA and POV (Figs. 1 and 2) was reduced with the higher concentrations of each of the three forms of garlic. This result was in accordance with that of Yang, Yasaei, and Page (1993), who noted that the antioxidant activity of several compounds of garlic and garlic extracts was concentration dependent. The investigated antioxidant activity of the four materials added followed the order FG>GP>BHA>GO. The low antioxidant activity of GO in comparison with that of FG or GP could be attributed to the losses of volatile sulfur compounds during steam distillation. Some authors reported that garlic oil or steam-distilled garlic did not contain large amount of allicin, but contain various products of allicin transformation, none of which appears to have much biologic activity as either fresh garlic or garlic powder (Lawson, Wang, & Hughes, 1991; Miething, 1988). On the other hand, Sun, Ockerman, and Marriott (2000) claimed that addition of garlic did not afford any significant antioxidant effect in Chinese-style heat-dried (50–55°C/18 h) sausage product.
3.3. Aerobic plate count
Initial aerobic plate count (APC) in the sausage was 4.41 log10 CFU/g (Fig. 3); and during the first 10 days of storage the count in all sausage formulations remained below 7 log10 CFU/g which is the MPL (Maximal Permissible Limit) for APC recommended by ICMSF (1986). At storage day 21, sausage samples formulated with either FG (30 g/kg) or GP (9 g/kg) maintained lower APC (6.42 and 6.94 log10 CFU/g, respectively) than the MPL, while APC in each of control, BHA- and GO-formulated samples exceeded the limit of 7 log10 C-FU/g by 2.46, 2.23 and 1.12–1.64 logs, respectively. After 21 days storage, APC of both FG- and GP-formulated sausage were significantly lower than that of either the control or BHA-formulated samples. Moreover, FG-formulated samples showed significantly lower APC than those of the GO formulations. However, addition of garlic oil or BHA resulted in no significant difference in APC when compared with control samples (Fig. 3). The weak antimicrobial activity of GO may be due to the losses of sulfur compounds as mentioned above, and also due to the nature of GO itself, which is volatile and hydrophobic. The order of antimicrobial activity of the different materials added was FG>GP>GO>BHA. This observation supported the findings of Murray (1997), who claimed that only fresh garlic preparations provide the full range of beneficial compounds. However, we noted that GP shown a close activity to FG as an antimicrobial. To date, although the antimicrobial activity of garlic and garlic-derived organosulfur compounds were widely reported in culture media, few reports are available on its effect in meat products (El-Khatib & Abd El-Rahman, 1987; Yin & Cheng, 2003). However, Sun et al. (2000) reported that garlic added to heat-dried Chinese-style sausage did not produce significant differences in APC. Further studies are required to evaluate the antimicrobial activities of garlic added to sausage and other meat products in combination with heat treatment.
Fig. 3.
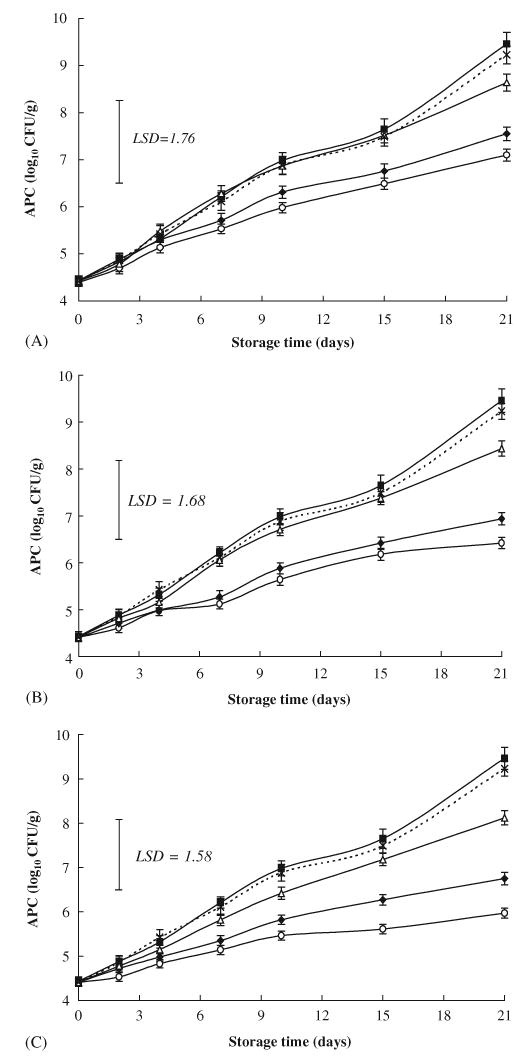
Effects of three equivalent concentrations of fresh garlic (FG), garlic powder (GP) and garlic oil (GO) as well as butylated hydroxyanisole (BHA) on aerobic plate count (APC) in raw chicken sausage stored at 3°C (vertical bars represent SE; LSD defined at P<0.05). (A): Control (▪); FG 20 g/kg (○); GP 6 g/kg (♦); GO 0.06 g/kg (▵); BHA 0.1 g/kg (×). (B): Control (▪); FG 30 g/kg (○); GP 9 g/kg (♦); GO 0.09 g/kg (▵); BHA 0.1 g/kg (×). (C): Control (▪); FG 50 g/kg (○); GP 15 g/kg (♦); GO 0.015 g/kg (▵); BHA 0.1 g/ kg (×).
3.4. Sensory evaluation
Storage time has no significant (P > 0.05) effect on the intensity of garlic flavor, tenderness or the acceptability scores of cooked chicken sausage. The intensity of garlic flavor, tenderness and overall acceptability (pooled data over the storage time up to 21 days) of chicken sausage as affected by different equivalent concentrations of FG, GP and GO are presented in Table 1. All the garlic formulations had more garlic flavor than the control and the BHA-formulated samples (P<0.05). Chicken sausage contained FG had a significant (P<0.05) stronger flavor than that contained GP or GO. No difference (P > 0.05) was detected in the tenderness between the different formulations of sausage. Control samples as well as BHA-formulated sausage showed the highest degree of acceptability, while samples contained FG at a concentration of 50 g/kg had the strongest flavor and the least acceptable scores. The acceptability of the taste of highly spiced food is transmitted both culturally and genetically, and the countries with hotter climates used spices more frequently and at much higher levels than countries with cooler climates (Billing & Sherman, 1998).
Table 1.
Mean scores of sensory characteristics of cooked chicken sausage treated with different equivalent concentrations of fresh garlic, garlic powder and garlic oil
| Sensory characteristic | C | Fresh garlic
|
Garlic powder
|
Garlic oil
|
BHA
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 (g/kg) | 30 (g/kg) | 50 (g/kg) | 6 (g/kg) | 9 (g/kg) | 15 (g/kg) | 0.06 (g/kg) | 0.09 (g/kg) | 0.15 (g/kg) | 0.1 (g/kg) | SEM | ||
| Garlic flavorx | 3.12a | 5.23d | 5.34d | 6.79e | 4.36bc | 4.45bc | 4.65c | 4.09b | 4.15b | 4.23b | 3.08a | 0.10 |
| Tendernessx | 7.08a | 6.78a | 6.73a | 6.66a | 6.74a | 6.89a | 6.62a | 7.03a | 6.87a | 6.95a | 6.94a | 0.08 |
| Overall acceptabilityy | 7.31d | 5.98b | 5.62b | 3.48a | 6.76cd | 6.58c | 6.23bc | 6.19bc | 5.86b | 5.72b | 7.23d | 0.12 |
C: Control; BHA: Butylated hydroxyanisole.
Means in the same row followed by different superscript are significantly different (P<0.05).
Eight-point hedonic scale: 8=extremely intense/tender; 7=very intense/tender; 6=moderately intense/tender; 5=slightly intense/tender, 4=slightly bland/tough; 3=moderately bland/tough; 2=very bland/tough; 1=extremely bland/tough.
Nine-point hedonic scale: 9=like extremely; 8=like very much; 7=like moderately; 6=like slightly; 5=neither like nor dislike; 4=dislike slightly; 3=dislike moderately; 2=dislike very much; 1=dislike extremely.
4. Conclusion
This study concluded that fresh garlic, garlic powder and garlic oil provide antioxidant and antimicrobial benefits to raw chicken sausage during cold storage (3°C) and the effects are concentration dependent. Among the garlic forms studied, fresh garlic at a concentration of 50 g/kg sausage demonstrated the most potent effect, but such a high concentration may not be acceptable by many people because of its strong flavor. However, addition of fresh garlic at 30 g/kg or garlic powder at 9 g/kg, did not result in a strong flavor and, at the same time, they produced significant antioxidant and antimicrobial effects and extended the shelf-life of the product up to 21 days. Therefore, it is suggested that garlic, as a natural herb, could be used to extend the shelf-life of meat products, providing the consumer with food containing natural additives, which might be seen more healthful than those of synthetic origin.
Acknowledgments
The authors would like to thank Dhanapati Neupany and Jin-bo Kim for their assistance. We also thank Prof. Nell Kennedy, Rakuno Gakuen University, for critical reading of the manuscript.
References
- Aguirrezábal MM, Mateo J, Domínguez MC, Zumalacárregui JM. The effect of paprika, garlic and salt on rancidity in dry sausages. Meat Science. 2000;54:77–81. doi: 10.1016/s0309-1740(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes and Infection. 1999;1:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Cunniff P, editor. AOAC International. Official methods of analysis of AOAC international. 16th ed. Gaithersburg, MD, USA: AOAC International; 1999. [Google Scholar]
- Barbut S. Poultry products processing: An industry guide. 1st ed. Boca Raton, FL, USA: CRC Press; 2001. [Google Scholar]
- Billing J, Sherman PW. Antimicrobial functions of spices: Why some like it hot. The Quarterly Review of Biology. 1998;73:3–49. doi: 10.1086/420058. [DOI] [PubMed] [Google Scholar]
- Chen HW, Yang JJ, Tsai CW, Wu JJ, Sheen LY, Ou CC, Lii CK. Dietary fat and garlic oil independency regulate hepatic cytochrome P450 2B1 and the placental form of glutathione S-transferase expression in rats. Journal of Nutrition. 2001;131:1438–1443. doi: 10.1093/jn/131.5.1438. [DOI] [PubMed] [Google Scholar]
- El-Khatib T, Abd El-Rahman H. Effect of garlic and Lactobacillus plantarum on growth of Salmonella typhimurium in Egyptian fresh sausage and beefburger. Journal of Food Protection. 1987;50:310–311. doi: 10.4315/0362-028X-50.4.310. [DOI] [PubMed] [Google Scholar]
- Evranuz ÖE. The effects of temperature and moisture content on lipid peroxidation during storage of unblanched salted roasted peanuts: shelf life studies for unblanched salted roasted peanuts. International Journal of Food Science and Technology. 1993;28:193–199. [Google Scholar]
- Gray JJ, Gomaa EA, Buckley DJ. Oxidative quality and shelf life of meats. Meat Science. 1996;43(Suppl):S111–S123. doi: 10.1016/0309-1740(96)00059-9. [DOI] [PubMed] [Google Scholar]
- Gray JI, Pearson AM. Rancidity and warmed-over flavor. In: Pearson AM, Dutson TR, editors. Advances in meat research. Vol. 3. NY, USA: Van Nostrand Company; 1987. pp. 221–269. [Google Scholar]
- Harris JC, Cottrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic) Applied Microbiology and Biotechnology. 2001;57:282–286. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- ICMSF. Microorganisms in foods. 2. Sampling for microbiological analysis: Principles and specific applications. 2nd ed. Toronto: University of Toronto Press; 1986. [Google Scholar]
- Jackson R, McNeil B, Taylor C, Holl G, Ruff D, Gwebu ET. Effect of aged garlic extract on casepase-3 activity, in vitro. Nutritional Neuroscience. 2002;5:287–290. doi: 10.1080/10284150290032012. [DOI] [PubMed] [Google Scholar]
- Kumar M, Berwal JS. Sensitivity of food pathogens to garlic (Allium sativum) Journal of Applied Microbiology. 1998;84:213–215. doi: 10.1046/j.1365-2672.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Lawson LD, Wang ZJ, Hughes BG. Identification and HPLC quantification of the sulfides and dialk(en)yl thiosulfides in commercial garlic products. Planta Medica. 1991;57:363–370. doi: 10.1055/s-2006-960119. [DOI] [PubMed] [Google Scholar]
- Lee TG, Williams SK, Sloan D, Littell R. Development and evaluation of a chicken breakfast sausage manufactured with mechanically deboned chicken meat. Poultry Science. 1997;76:415–421. doi: 10.1093/ps/76.2.415. [DOI] [PubMed] [Google Scholar]
- Leuschner RGK, Ielsch V. Antimicrobial effects of garlic, clove and red hot chilli on Listeria monocytogenes in broth model systems and soft cheese. International Journal of Food Sciences and Nutrition. 2003;54:127–133. doi: 10.1080/0963748031000084070. [DOI] [PubMed] [Google Scholar]
- Martinez-Tome M, Jimenez AM, Ruggieri S, Frega N, Strabbioli R, Murcia MA. Antioxidant properties of Mediterranean spices compared with common food additives. Journal of Food Protection. 2001;64:1412–1419. doi: 10.4315/0362-028x-64.9.1412. [DOI] [PubMed] [Google Scholar]
- Mielnik MB, Aaby K, Skrede G. Commercial antioxidants control lipid oxidation in mechanically deboned turkey meat. Meat Science. 2003;65:1147–1155. doi: 10.1016/S0309-1740(02)00345-5. [DOI] [PubMed] [Google Scholar]
- Miething H. HPLC—Analysis of the volatile oil of garlic bulbs. Phytotherapy Research. 1988;2:149–151. [Google Scholar]
- Murray MT. Which is better: Aged versus fresh garlic; glucosamine sulfate versus chondroitin sulfate. American Journal of Natural Medicine. 1997;4:5–8. [Google Scholar]
- Naidu AS. Natural food antimicrobial systems. Boca Raton FL, USA: CRC Press; 2000. [Google Scholar]
- Narasimhan S, Raghuver KG, Arumngham C, Bhat KK, Sen DP. Oxidative rancidity of groundnut oil evaluation by sensory and chemical indices and their correlation. Journal of Food Science and Technology. 1986;23:273–277. [Google Scholar]
- Ohshima T, Yankah VV, Ushio H, Kiozumi C. Antioxidizing potentials of BHA, BHT, TBHQ, tocopherol, and oxygen absorber incorporated in Ghanaian fermented fish product. Advances in Experimental Medicine and Biology. 1998;434:181–188. doi: 10.1007/978-1-4899-1925-0_15. [DOI] [PubMed] [Google Scholar]
- Pokorný J. Natural antioxidants for food use. Trends in Food Science and Technology. 1991;2:223–227. [Google Scholar]
- Prasad K, Laxdal VN, Yu M, Raney BL. Antioxidant activity of allicin, an active principle in garlic. Molecular and Cellular Biochemistry. 1995;148:183–189. doi: 10.1007/BF00928155. [DOI] [PubMed] [Google Scholar]
- Raharjo S, Sofos JN. Methodology for measuring malonaldehyde as a product of lipid peroxidation in muscle tissues: A review. Meat Science. 1993;35:145–169. doi: 10.1016/0309-1740(93)90046-K. [DOI] [PubMed] [Google Scholar]
- SAS. Statistical analysis system: User’s guide statistics. Cary, NC: SAS Institute, Inc; 1990. [Google Scholar]
- Schmedes A, Holmer G. A new thiobarbituric acid (TBA) method for determination of free malonaldehyde (MDA) and hydroperoxides selectivity as a measure of lipid peroxidation. Journal of American Oil Chemistry Society. 1989;66:813–817. [Google Scholar]
- Sun YM, Ockerman HW, Marriott NG. Garlic in Chinese sausage. Journal of Muscle Foods. 2000;11:35–43. [Google Scholar]
- USDA ‘‘United States Department of Agriculture’’. Nutrient database for standard reference, release 13. 1999. [Google Scholar]
- Unal R, Fleming HP, McFeeters RF, Thompson RL, Breidt F, Jr, Giesbrecht FG. Novel quantitative assays for estimating the antimicrobial activity of fresh garlic juice. Journal of Food Protection. 2001;64:189–194. doi: 10.4315/0362-028x-64.2.189. [DOI] [PubMed] [Google Scholar]
- Yang GC, Yasaei PM, Page SW. Garlic as anti-oxidant and free radical scavengers. Journal of Food and Drug Analysis. 1993;1:357–364. [Google Scholar]
- Yin MC, Cheng WS. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Science. 2003;63:23–28. doi: 10.1016/s0309-1740(02)00047-5. [DOI] [PubMed] [Google Scholar]


