Abstract
The effects of sodium lactate (NaL) and sodium chloride (NaCl), either alone (30 g/kg) or in combination (20+20 g/kg), on the microbiological and chemical quality of raw ground beef during vacuum-packaged storage at 2°C were investigated. The results showed that addition of NaL alone or in combination with NaCl significantly delayed the proliferation of aerobic plate counts, psychrotrophic counts, lactic acid bacteria and Enterobacteriaceae and extended the shelf life of the product up to 15 and 21 days, respectively, versus 8 days only for control. Over the storage time (21 days), NaL maintained the ground beef at almost constant pH, while the pH of control or NaCl-treated samples significantly decreased. Lipid oxidation (TBA value) was not affected by addition of NaL. At storage day 21 however, TBA values of both NaL-treated (0.309) and control (0.318) samples were significantly lower than those of samples treated with NaCl (0.463). The combination of NaCl with NaL significantly reduced the oxidative changes caused by NaCl (0.384 versus 0.463). Therefore, NaL alone or in combination with NaCl could be utilized successfully to reduce the microbial growth, maintain the chemical quality, and extend the shelf life of ground beef during refrigerated storage.
Keywords: Ground beef, Sodium lactate, NaCl, Microbial growth, Fat oxidation
1. Introduction
Fresh meat products are commonly marketed at refrigerated temperatures (2–5°C). However, many undesirable changes of the products can occur during refrigeration due to microbial growth and lipid oxidation, which give rise to quality reduction, meat spoilage, and economic loss.
Minimizing product contamination and delaying or inhibiting growth of spoilage and pathogenic organisms in the product are major keys for improving fresh meat shelf life and increasing consumer safety. While general cleanliness and proper sanitation are very effective, other means of controlling microbial growth in meat products may be prove useful.
Lactates are naturally present in meat. It had been permitted, as a natural preservative, by the USDA-Food Safety and Inspection Service (USDA-FSIS) at a level of up to 3 g/100 g meat (Bedie et al., 2001). Lactate at the level of 1.5–3.0 g/100 g of meat weight is being extensively used in the meat industry to improve various quality attributes of meat. Much research indicated that addition of sodium lactate (NaL) could improve flavor, color, tenderness, juiciness and cooking yields of ground beef and other meat products (Eckert, Maca, Miller, & Acuff, 1997; Maca, Miller, & Acuff, 1997; Vote et al., 2000). In addition to its desirable effect on sensory attributes, NaL has an antimicrobial effect. It has been shown to delay growth of meat spoilage organisms (Brewer, Rostogi, Argoudelis, & Sprouls, 1995; Maca, Miller, Bigner, Lucia, & Acuff, 1999; Vasavada, Carpenter, Cornforth, & Ghorpade, 2003) as well as food-borne pathogens (Miller & Acuff, 1994; Mbandi & Shelef, 2001; Bedie et al., 2001). Moreover, other authors reported that NaL has inhibitory effect on lipid oxidation (Nnanna, Ukuku, McVann, & Shelef, 1994; Walczycka, Kolczak, Kijowski, & Lacki, 1999). However, there is limited information on its effect when used in combination with table salt, which is normally added to the meat and meat products during processing or cooking.
Sodium chloride (NaCl) has been long used as a meat preservative. It is added to meats for its effects on sensory, functional and preservation properties. NaCl inhibit the microbial growth by restriction of the available water (i.e. lowers aw) in the meat products. However, its pro-oxidant activity accelerates the development of lipid oxidation in refrigerated meats (Rhee, 1988; Lee, Mei, & Decker, 1997). It had been reported that lactate reduced the pro-oxidant effect of NaCl in refrigerated and frozen meat products (Tan & Shelef, 2002).
The objectives of this study were to evaluate the effects of sodium lactate alone or in combination with sodium chloride on the microbiological and chemical quality of raw vacuum-packaged ground beef during refrigerated storage at 2°C.
2. Materials and methods
2.1. Sample preparation
Fresh coarse ground beef was obtained from a local meat retailer and immediately transported to the laboratory and prepared for testing on the day of purchase. NaL (Wako Pure Chemical Industries Ltd., Osaka, Japan) and NaCl (Katayama Chemical, Osaka, Japan) were the salts used for treatment of the ground beef. Ground meat was divided into four batches (2 kg each), which formulated to contain either NaL (30 g/kg), NaCl (30 g/kg), combination of NaL+NaCl (20 g+20 g/kg), or no additives (control). Salts were added to meat (w/w) on wet weight basis, and since aqueous solution of NaL was used, all other meat batches were formulated to contain the same amount of water. Salts were thoroughly mixed into the ground meat by hand, reground through a 0.3-cm grinder plate (Super grinder-MK-G3; Matsushita Electric Industrial, Osaka, Japan), and divided into 100-g samples. Each sample was vacuum-packaged in a polyethylene bags, labeled, and stored at 2°C. Ground beef was sampled at 3 days intervals during 21 days storage for microbiological and chemical analyses.
2.2. Microbiological analyses
Meat samples of 25 g were aseptically removed from the bags and homogenized in 225 ml of sterile buffered peptone water (1 g/l; Katayama Chemical, Osaka, Japan) for 1 min using a Stomacher 400 Lab Blender (Seward Medical, London, UK). From this homogenate, decimal serial dilutions were made in the same sterile peptone water and used for microbiological analyses of the ground beef samples at each of the appropriate time intervals during refrigerated storage.
2.2.1. Aerobic plate count
Aerobic plate counts (APC) were determined by inoculating 0.1 ml of the sample homogenate, at selected dilutions, onto duplicate sterile plates of pre-poured and dried Standard Method Agar (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) using the surface spread technique, then the plates were incubated for 48 h at 35°C.
2.2.2. Psychrotrophic count
Psychrotrophic counts (PTC) were determined in a similar method to that for APC except that plates were incubated at 7°C for 10 days. (Cousin, Jay, & Vasavada, 1992).
2.2.3. Lactic acid bacteria
For lactic acid bacteria (LAB) determination, diluted samples were plated on deMan, Rogosa, and Sharpe (MRS) agar (Merck, Darmstadt, Germany) and incubated at 30°C for 2–3 days in an anaerobic jars with disposable Anaerocult C bags (Merck, Darmstadt, Germany) for the generation of an anaerobic medium.
2.2.4. Enterobacteriaceae count
To determine Enterobacteriaceae counts (EBC), 1 ml of the appropriate dilution was inoculated by the pour-plated method on violet red bile agar (VRBA; Difco Laboratories Inc., Detroit, Michigan, USA) and overlaid with approximately 5 ml of the same growth medium (Jiménez, Salsi, Tiburzi, Rafaghelli, & Pirovani, 1999), then the plates were incubated at 35°C for 24 h.
2.3. pH measurement
Ten grams of sample were homogenized with 40 ml distilled water in a blender for 30 s. The homogenate was filtered and the pH value of the filtrate was determined using a digital pH meter (model HM-5S; TOA Electric Industrial Co. Ltd., Tokyo, Japan) standardized at pH 4 and 7.
2.4. Fat content
Before storage, fresh ground beef was analysed for its fat content according to the method of AOAC International (1999).
2.5. Lipid oxidation measurement
Lipid oxidation was determined by the 2-thiobarbi-turic acid (TBA) assay according to the procedure of Schmedes and Holmer (1989). Ground beef (10 g) was mixed with 25 ml of trichloroacetic acid (TCA) solution (200 g/l of TCA in 135 ml/l phosphoric acid solution) and homogenized in a blender for 30 s. After filtration, 2 ml of the filtrate were mixed with equal amount of aqueous solution of TBA (3 g/l) in a test tube. The tubes were incubated at room temperature in the dark for 20 h; then the absorbance was measured at 532 nm using UV-vis spectrophotometer (model UV-1200, Shimadzu, Kyoto, Japan). TBA value was expressed as mg malonaldehyde per kg of meat.
2.6. Statistical analysis
All measurements were carried out in triplicate, and all microbial counts were converted to base-10 logarithms of colony forming units per g of ground beef samples (log10 CFU/g). Data were analysed using analysis of variance (ANOVA) of the General Linear Models procedure of the Statistical Analysis System software (SAS Institute, Inc., 1990). Least significant differences were used to separate means at P<0.05.
3. Results and discussion
3.1. Microbiological evaluation
3.1.1. Aerobic plate count
As may be expected, the increase in storage time produced significant proliferations in APC, whatever the treatment conditions (Fig. 1). The initial (day 0) APC (log10 CFU/g) in ground beef ranged from 3.78 in NaL-treated meat to 4.15 in control samples. However by the day 9 of storage, APC of control (7.29) exceeded the maximal recommended limit of 7 log10 CFU/g for APC in raw meat (ICMSF, 1986), indicating a shelf life of about 8 days. While NaL-treatment significantly delayed the microbial growth and extended the shelf life of the product up to 15 days at which the APC was 6.73 versus 8.69 log10 CFU/g for control. Addition of NaL has been reported to produce significant reduction in growth of APC in refrigerated ground beef (Eckert et al., 1997; Maca et al., 1997) and ground pork (O’Connor, Brewer, McKeith, Novakofski, & Carr, 1993; Brewer et al., 1995) as well as in cooked beef products (Miller & Acuff, 1994; Maca et al., 1999).
Fig. 1.
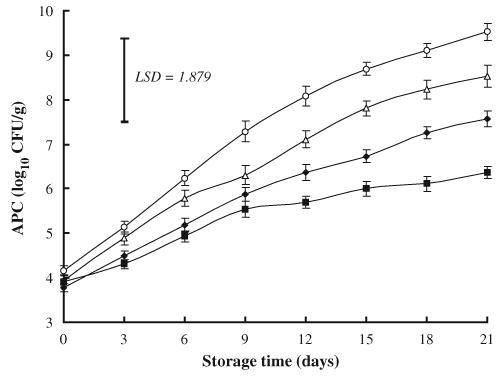
Effects of sodium lactate (NaL) and/or sodium chloride (NaCl) treatments on aerobic plate counts (APC) in ground beef during storage at 2°C. Values represent means±SE of three replicates; LSD is defined at P<0.05. (♦): NaL, 30 g/kg; (▵): NaCl, 30 g/kg; (▪): NaL+NaCl, 20 g+20 g/kg; (○): Control.
At day 21 of storage, samples containing combination of NaL with NaCL had a lower APC (6.37 log10 CFU/g) than the maximal recommended limit, while control samples exhibited a higher count of 9.53 log10 CFU/g, indicating that such combination is more effective than lactate alone. This result might have been due to the synergistic effect of the two adverse factors studied. This finding is consistent with Tan and Shelef (2002), who reported similar effect of lactates with NaCl combination on APC in ground pork stored at 2°C.
Addition of NaCl alone (30 g/kg) had no significant effect (P>0.05) on APC, although by day 12 of storage, NaCl-treated ground beef contained APC of 7.11 log10 CFU/g, which was about 1 log lower than that of control (8.09 log10 CFU/g). By day 21 however, APC in NaCl-treated samples (8.53 log10 CFU/g) was about 1 log higher than that of NaL-treated samples (7.57 log10 CFU/g) and 2 log higher (P<0.05) than that of samples treated with combination of NaL and NaCl (6.37 log10 CFU/g). These findings are in accordance with that of O’Connor et al. (1993), who claimed that ground pork formulated with NaCl (1.5–2.5 g/100 g) exhibited lower APC than controls up to day 14 of storage at 4°C. The present study indicated that NaL was more effective against microbial growth than NaCl.
3.1.2. Psychrotrorhic bacteria
At storage day 0, the initial PTC in ground beef samples ranged from 4.0 to 4.14 log10 CFU/g, and the changes in PTC were approximately similar to those of APC, with control also being the highest at day 21 (9.98 log10 CFU/g) followed by samples treated with NaCl (8.87 log CFU/g), while much lower counts was detected in samples treated with NaL either alone (7.79 log10 CFU/g) or in combination with NaCl (6.44 log10 CFU/ g) (Fig. 2). Significant differences were detected in PTC between samples treated with NaL either alone or in combination with NaCl and those of controls, also between samples treated with combination of NaL and NaCl and samples treated with NaCl alone.
Fig. 2.
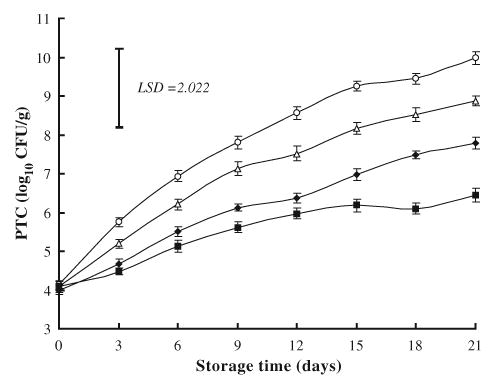
Effects of sodium lactate (NaL) and/or sodium chloride (NaCl) treatments on psychrotrophic counts (PTC) in ground beef during storage at 2°C. Values represent means±SE of three replicates; LSD is defined at P<0.05. (♦): NaL, 30 g/kg; (▵): NaCl, 30 g/kg; (▪): NaL+NaCl, 20 g+20 g/kg; (○): Control.
Length of refrigerated storage (2°C) had a significant (P<0.05) effect on PTC, which tended to increase as the storage time increased. However, Egbert, Huffman, Chen, and Jones (1992) claimed that the length of refrigerated storage (14 days at −2 to 0°C) or retail display (48 h at 5°C to 7°C) of low-fat ground beef had no effect on PTC, which remained between 7.8 and 7.9 log10 CFU/g within these periods.
3.1.3. Lactic acid bacteria
The initial count (log10 CFU/g) of LAB was ranged from 3.46 in NaL-treated samples to 3.58 in control meat. At storage day 21 however, significant lower count (5.3) was detected in samples treated with combination of NaL and NaCl when compared with control (8.36), NaCl-treated (7.83), or NaL-treated (7.28) samples, while addition of NaL alone did not produce significant reduction in LAB count (P>0.05) although it was about 1 log lower than control (Fig. 3). It has been documented that NaL is effective against LAB in meat products (Brewer, McKeith, & Sprouls, 1993; Sameshima et al., 1997). Conversely, It has been reported that LAB dominated the microbial flora in NaL-treated beef during vacuum-packaged storage at 0°C (Papadopoulos, Miller, Acuff, Vanderzant, & Cross, 1991) as well as in frankfurter-type sausage treated with NaL and stored at 0–4°C (Zivkovic, Radulovic, Ivanovic, Perunovic, & Dzinic, 2002).
Fig. 3.
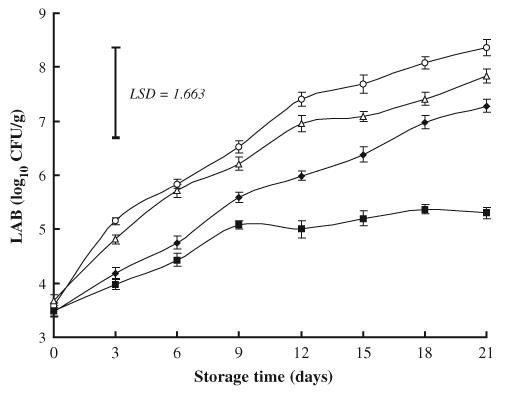
Effects of sodium lactate (NaL) and/or sodium chloride (NaCl) treatments on growth of lactic acid bacteria (LAB) in ground beef during storage at 2°C. Values represent means±SE of three replicates; LSD is defined at P<0.05. (♦): NaL, 30 g/kg; (▵): NaCl, 30 g/kg; (▪): NaL+NaCl, 20 g+20 g/kg; (○): Control.
3.1.4. Enterobacteriaceae
The growth of Enterobacteriaceae was slower than that of APC, PTC or LAB. The initial EBC increased from 1.8 log10 CFU/g in control samples at day 0 to a higher count of 7.39 log10 CFU/g by day 21 of storage, while it reached significant (P<0.05) lower counts of 5.19 or 3.22 log10 CFU/g in ground beef treated with either NaL or NaCl, respectively when compared with control (Fig. 4). This indicated that addition of NaCl was more effective (P<0.05) against Enterobacteriaceae than NaL. However, a combination of NaL and NaCl restricted the growth of the Enterobacteriaceae to a lower level of 1.66 log10 CFU/g, and appeared to be the most effective (P<0.05) among the other treatments against the growth of Enterobacteriaceae. These results are in accordance with those of Rondinini, Maifreni, and Marino (1996), who reported inhibitory effects of NaL against Enterobactericeae counts in cooked hams during vacuum-packaged storage for up to 9 week at 6°C, and also with Conner and Hall (1994), who reported a reduction in Escherichia coli population of chicken meat treated with combination of NaL and NaCl during frozen storage.
Fig. 4.
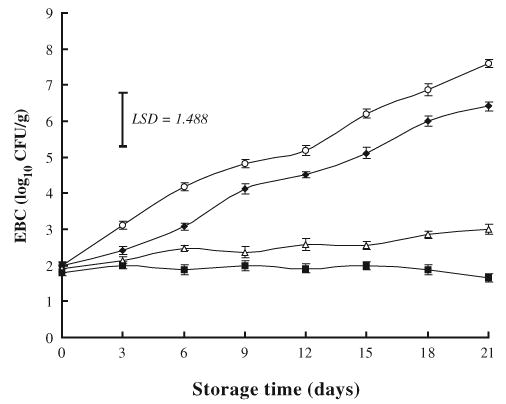
Effects of sodium lactate (NaL) and/or sodium chloride (NaCl) treatments on Enterobactericeae counts (EBC) in ground beef during storage at 2 °C. Values represent means±SE of three replicates; LSD is defined at P<0.05. (♦): NaL, 30 g/kg; (▪): NaCl, 30 g/kg; (▪): NaL+NaCl, 20 g+20 g/kg; (○): Control.
3.2. pH value
Changes in pH values in ground beef during storage at 2°C (Table 1) revealed that the initial pH value of control (5.80) was significantly (P<0.05) higher than those of samples treated with NaL (5.70), NaCl (5.65) or combination of NaL and NaCl (5.73). These findings disagree with Eckert et al. (1997) and Tan and Shelef (2002), who reported that NaL had no significant effects on initial pH of ground meat products. At day 21, however, no significant difference was observed in the pH value between control (5.72) and all other treatments except with samples treated with NaCl (5.51), which was also significantly lower than those treated with NaL (5.71) or combination of NaL and NaCl (5.74). These results are consistent with Brewer et al. (1995), who found ground pork treated with NaCl (1.5–3 g/100 g) had lower pH than those with NaL (2 or 3 g/100 g).
Table 1.
Effects of salt treatments and storage time on the pH of ground beef during refrigerated storage (2°C)
| Storage time (day) | Control | NaL (30 g/kg) | NaCl (30 g/kg) | NaL+NaCl (20+20 g/kg) |
|---|---|---|---|---|
| 0 | 5.80±0.01cz | 5.70±0.02axy | 5.65±0.03bx | 5.73±0.02by |
| 3 | 5.73±0.02by | 5.69±0.02axy | 5.63±0.02bx | 5.66±0.01ax |
| 6 | 5.70±0.04bxy | 5.72±0.03ay | 5.64±0.01bx | 5.69±0.03abxy |
| 9 | 5.61±0.02ax | 5.73±0.02az | 5.67±0.03by | 5.74±0.04bz |
| 12 | 5.62±0.01ax | 5.72±0.03ay | 5.56±0.02ax | 5.75±0.02by |
| 15 | 5.70±0.02by | 5.73±0.01ay | 5.57±0.01ax | 5.75±0.03by |
| 18 | 5.71±0.02by | 5.72±0.01ay | 5.55±0.02ax | 5.72±0.02aby |
| 21 | 5.72±0.03bcy | 5.71±0.02ay | 5.51±0.04ax | 5.74±0.02by |
Means±SE in the same column followed by different superscript are significantly different (P<0.05).
Means±SE in the same row followed by different superscript are significantly different (P<0.05).
NaL has been shown to stabilize pH during storage of meat products (Maca et al., 1997, 1999; Papadopoulos et al., 1991). Our results also showed that, over the storage time, addition of NaL maintained the ground beef at almost constant pH (5.69–5.73), while the pH of control or NaCl-treated samples significantly decreased.
3.3. Lipid oxidation
The fat content (g/100 g) in ground beef was 12.3±1.2. Changes in TBA values during refrigerated storage at 2°C are shown in Fig. 5. The initial TBA value of ground beef was ranged from 0.177 in NaL-treated meat to 0.196 in NaCl-treated samples. In all ground beef samples, storage time had a significant effect on TBA values, which tended to increase with storage. At storage day 21, TBA values of both NaL-treated (0.309) and control (0.318) samples were significantly lower than those of samples treated with NaCl (0.463). TBA value of ground beef was not affected by addition of NaL (P>0.05). This result is in agreement with that of many other researchers (Eckert et al., 1997; Lin & Lin, 2002; Vasavada et al., 2003), who found no difference in TBA values of meat products treated with NaL in comparison with controls. Reported effects of lactate on lipid oxidation (TBA) in stored meat products have varied. Some authors indicated that NaL had an antioxidant effect represented by decrease in TBA values (Maca et al., 1999; Nnanna et al., 1994; Walczycka et al., 1999). Conversely, pro-oxidative effects (significantly higher TBA values) of NaL were reported in precooked beef-carrageenan patties packaged with PVC film and stored at −20°C (Adams & Rhee, 1994), as well as in precooked reduced-fat pork sausage patties containing carrageenan and stored at 4°C or −20°C (Krahl, Rhee, Lin, Keeton, & Ziprin, 1995). It is possible that effects of NaL on lipid oxidation in meat products may be dependent on a variety of factors including the extent of microbial growth, the type and level of other nonmeat additives, packaging method, and storage time.
Fig. 5.
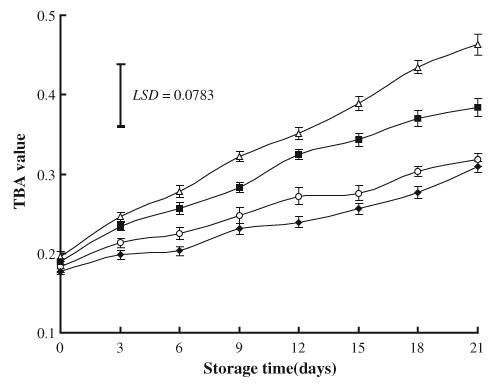
Effects of sodium lactate (NaL) and/or sodium chloride (NaCl) treatments on lipid oxidation (TBA value) in ground beef during storage at 2°C. Values represent means±SE of three replicates; LSD is defined at P<0.05. (♦): NaL, 30 g/kg; (▪): NaCl, 30 g/kg; (▪): NaL+NaCl, 20 g+20 g/kg; (○): Control.
NaCl enhanced lipid oxidation. The pro-oxidant effect of NaCl in meat products had been also reported by Rhee (1988) and Lee et al. (1997). However, the combination of NaCl with NaL, in this study, significantly reduced the oxidative changes caused by NaCl, which indicated by a reduction in TBA value from 0.463 to 0.384. This result is in accordance with Tan and Shelef (2002), who reported a significant (P<0.05) reduction of lipid oxidation in ground pork with NaCl stored at 2°C when it is used in combination with NaL.
TBA value is often used as an index of lipid oxidation in meat products during storage. Tarladgis, Watts, Younathan, and Dugan (1960) found that the TBA number at which rancid odor was first perceived was between 0.5 and 1.0. This threshold has served as a guide for interpreting TBA test results. Over 21 days storage in our study, both control and treated ground beef samples had TBA values less than 0.5 (mg malonaldehyde/kg meat).
4. Conclusion
Treatment of ground beef with sodium lactate alone or in combination with sodium chloride was effective against the proliferation of aerobic microorganisms, psychrotrophic bacteria, lactic acid bacteria and Enter-obacteriaceae. Both treatments maintained the pH at almost fixed values and did not affect lipid oxidation. Therefore, sodium lactate alone or in combination with sodium chloride could be utilized successfully to maintain the chemical quality, reduce the microbial growth, and extend the shelf life of ground beef during refrigerated storage.
References
- Adams SA, Rhee KS. Improvement of shelf-life and sensory properties of precooked low-fat ground beef patties. Paper no. 47D-17; Annual meeting of the institute of food technologists; June 25–29; Atlanta. 1994. [Google Scholar]
- Cunniff P, editor. AOAC International. Official methods of analysis of AOAC international. 16th ed. Gaithersburg, Maryland, USA: AOAC International; 1999. [Google Scholar]
- Bedie GK, Samelis J, Sofos JN, Belk KE, Scanga JA, Smith GC. Antimicrobials in the formulation to control Listeria monocytogenes postprocessing contamination on frankfurters stored at 4 degree C in vacuum packages. Journal of Food Protection. 2001;64:1949–1955. doi: 10.4315/0362-028x-64.12.1949. [DOI] [PubMed] [Google Scholar]
- Brewer MS, McKeith FK, Sprouls G. Sodium lactate effects on microbial, sensory, and physical characteristics of vacuum-packaged pork sausage. Journal of Muscle Foods. 1993;4:179–192. [Google Scholar]
- Brewer MS, Rostogi BK, Argoudelis L, Sprouls GK. Sodium lactate/ sodium chloride effects on aerobic plate count and color of aerobically packaged ground pork. Journal of Food Science. 1995;60:58–62. [Google Scholar]
- Conner DE, Hall GS. Efficacy of selected media for recovery of E. coli O157: H7 from frozen chicken meat containing sodium chloride, sodium lactate or poly phosphate. Food Microbiology. 1994;11:337–344. [Google Scholar]
- Cousin MA, Jay JM, Vasavada PC. Psychrotrophic microorganisms. In: Vanderzand C, Splittstoesser DF, editors. Compendium of Methods for the Microbiological Examination of Foods. 3rd ed. Washington, DC: American Public Health Association; 1992. pp. 153–168. [Google Scholar]
- Eckert LA, Maca JV, Miller RK, Acuff GR. Sensory, microbial and chemical characteristics of fresh aerobically stored ground beef containing sodium lactate and sodium propionate. Journal of Food Science. 1997;62:429–433. [Google Scholar]
- Egbert WR, Huffman DL, Chen CM, Jones WR. Microbial and oxidative changes in low-fat ground beef during simulated retail distribution. Journal of Food Science. 1992;57:1269–1274. 1293. [Google Scholar]
- ICMSF ‘‘International Commission on Microbiological Specification for Foods’’. Microorganisms in foods 2. Sampling for microbiological analysis: Principles and specific applications. 2nd ed. Toronto, Canada: University of Toronto Press; 1986. [Google Scholar]
- Jiménez SM, Salsi MS, Tiburzi RC, Rafaghelli RC, Pirovani ME. Combined use of acetic acid treatment and modified atmosphere packaging for extending the shelf-life of chilled chicken breast portions. Journal of Applied Microbiology. 1999;87:339–344. doi: 10.1046/j.1365-2672.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- Krahl LM, Rhee KS, Lin KW, Keeton JT, Ziprin YA. Sodium lactate and sodium tripolyphosphate effects on oxidative stability and sensory properties of precooked reduced-fat pork sausage with carrageenan. Journal of Muscle Foods. 1995;6:243–256. [Google Scholar]
- Lee SK, Mei L, Decker EA. Influence of sodium chloride on antioxidant enzyme activity and lipid oxidation in frozen ground pork. Meat Science. 1997;46:349–355. doi: 10.1016/s0309-1740(97)00029-6. [DOI] [PubMed] [Google Scholar]
- Lin KW, Lin SN. Effects of sodium lactate and trisodium phosphate on the physicochemical properties and shelf life of low-fat Chinese-style sausage. Meat Science. 2002;60:147–154. doi: 10.1016/s0309-1740(01)00116-4. [DOI] [PubMed] [Google Scholar]
- Maca JV, Miller RK, Acuff GR. Microbiological, sensory and chemical characteristics of vacuum-packaged ground beef patties treated with salts of organic acids. Journal of Food Science. 1997;62:591–596. [Google Scholar]
- Maca JV, Miller RK, Bigner ME, Lucia LM, Acuff GR. Sodium lactate and storage temperature effects on shelf life of vacuum packaged beef top rounds. Meat Science. 1999;53:23–29. doi: 10.1016/s0309-1740(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Mbandi E, Shelef LA. Enhanced inhibition of Listeria monocytogenes and Salmonella enteritidis in meat by combinations of sodium lactate and diacetate. Journal of Food Protection. 2001;64:640–644. doi: 10.4315/0362-028x-64.5.640. [DOI] [PubMed] [Google Scholar]
- Miller RK, Acuff GR. Sodium lactate affects pathogens in cooked beef. Journal of Food Science. 1994;59:15–19. [Google Scholar]
- Nnanna IA, Ukuku DO, McVann KB, Shelef LA. Antioxidant activity of sodium lactate in meat and model systems. Lebensmittel-Wissenschaft und-Technologie/Food Science and Technology (LWT) 1994;27:78–85. [Google Scholar]
- O’Connor PL, Brewer MS, McKeith FK, Novakofski JE, Carr TR. Sodium lactate/sodium chloride effects on sensory characteristics and shelf-life of fresh ground pork. Journal of Food Science. 1993;58:978–980. 986–986. [Google Scholar]
- Papadopoulos LS, Miller RK, Acuff GR, Vanderzant C, Cross HR. Effect of sodium lactate on microbial and chemical composition of cooked beef during storage. Journal of Food Science. 1991;56:341–347. [Google Scholar]
- Rhee KS. Enzymatic and non-enzymatic catalysis of lipid oxidation in muscle foods. Food Technology. 1988;42:127–130. [Google Scholar]
- Rondinini G, Maifreni M, Marino M. Application of sodium lactate to preservation of cooked ham. Ingegneria Alimentare le Conserve Animali. 1996;12:9–15. [Google Scholar]
- Sameshima T, Takeshita K, Miki T, Arihara K, Itoh M, Kondo Y. Effect of sodium nitrite and sodium lactate on the growth of lactic acid spoilage bacteria isolated from cured meat products. Japanese Journal of Food Microbiology. 1997;13:159–164. [Google Scholar]
- SAS. SAS/STAT user’s guide. Cary, NC: Statistical Analysis System Institute, Inc; 1990. [Google Scholar]
- Schmedes A, Holmer G. A new thiobarbituric acid (TBA) method for determination of free malonaldehyde (MDA) and hydroperoxides selectivity as a measure of lipid peroxidation. Journal of American Oil Chemistry Society. 1989;66:813–817. [Google Scholar]
- Tan W, Shelef LA. Effects of sodium chloride and lactates on chemical and microbiological changes in refrigerated and frozen fresh ground pork. Meat Science. 2002;62:27–32. doi: 10.1016/s0309-1740(01)00223-6. [DOI] [PubMed] [Google Scholar]
- Tarladgis BG, Watts BM, Younathan MT, Dugan LR., Jr A distillation method for the quantitative determination of malonaldehyde in rancid foods. Journal of the American Oil Chemists Society. 1960;37:44–48. [Google Scholar]
- Vasavada M, Carpenter CE, Cornforth DP, Ghorpade V. Sodium levulinate and sodium lactate effects on microbial growth and stability of fresh pork and turkey sausages. Journal of Muscle Foods. 2003;14:119–129. [Google Scholar]
- Vote DJ, Platter WJ, Tatum JD, Schmidt GR, Belk KE, Smith GC, Speer NC. Injection of beef strip loins with solutions containing sodium tripolyphosphate, sodium lactate, and sodium chloride to enhance palatability. Journal of Animal Science. 2000;78:952–957. doi: 10.2527/2000.784952x. [DOI] [PubMed] [Google Scholar]
- Walczycka M, Kolczak T, Kijowski A, Lacki J. Effect of sodium lactate and starter culture on minced beef during chilled storage. Polish Journal of Food and Nutrition Sciences. 1999;7(48):663–672. [Google Scholar]
- Zivkovic D, Radulovic Z, Ivanovic M, Perunovic M, Dzinic N. Influence of Na-lactate on shelf life and selected quality properties of frankfurter-type sausages. Technologija Mesa. 2002;42:129–135. [Google Scholar]


