Abstract
Effects of trisodium phosphate (TSP) and/or sodium chloride (NaCl) dipping on microbial quality and shelf life of chicken breasts were investigated during refrigeration. Chicken breasts were dipped in aqueous solution (w/v) of 10% TSP, 10% NaCl, combination of TSP and NaCl (7.5% + 7.5%) or distilled water (control) for 10 min, followed by tray-packaging storage at 2°C. During storage, chicken breasts dipped in TSP maintained almost constant pH, while pH of control or NaCl-treated samples significantly increased (P<0.05). TSP dipping resulted in initial reduction of 0.48 and 0.91 log10 CFU/g in aerobic plate counts and Enterobacteriaceae count, respectively, when compared with control. By storage day 6, APC of control chicken breasts reached 6.91 log10 CFU/g, while TSP-treatment either alone or in combination with NaCl significantly delayed microbial growth (P<0.05) and extended shelf life of refrigerated chicken breasts up to 12 days, at which APC were 6.87 and 6.39, respectively, versus 9.58 log10 CFU/g for control. Significant reductions in psychrotrophic and Enterobacteriaceae count were detected at the end of storage period in chicken breasts treated with TSP alone or in combination with NaCl, whereas such treatments had no significant effects on lactobacilli or mold and yeast populations.
Keywords: trisodium phosphate, NaCl, chicken breasts, shelf life, microbial growth
Introduction
Consumption of poultry meat has increased remarkably over the past two decades due to the perception that it is "healthier" alternative to red meats. However, increase in the consumption of poultry products has been accompanied by an increase in food-borne illnesses associated with poultry. Chicken and other types of poultry have higher pathogenic and spoilage bacterial counts than most other foods (1). Pathogenic bacteria associated with poultry include Salmonella spp., Campylobacter jejuni, Staphylococcus aureus and Listeria monocytogenes. In fact, poultry represents the single most important food source of Salmonella (2, 3) and C. jejuni (4, 5).
In addition to general hygienic practice, the use of chemicals as decontaminating agents for reduction or elimination of spoilage and pathogenic organisms from poultry meat and for extension of shelf life had been reported. Organic acids, such as lactic, citric, acetic, and propionic acids and/or their salts were evaluated, both at the laboratory level and in processing plants (6–10). Phosphates have been used as antimicrobial surface treatment agents to decrease populations of pathogens, prevent growth of spoilage microorganisms, and extend the shelf life of fresh poultry (11–13). In particular, trisodium phosphate (TSP) treatment yields superior antimicrobial effect compared to other phosphates (11, 14).
TSP is generally recognized as safe by the US Food and Drug Administration and has been approved by the US Department of Agriculture-Food Safety and Inspection Service (USDA-FSIS) at levels of 8–12% as an antimicrobial agent on raw chilled poultry carcasses that have been passed for wholesomeness. Carcasses are either sprayed with or dipped in the TSP solution for up to 15 s at a solution temperature of 13–17°C (15).
Treatment of poultry carcasses with TSP was effective in reducing populations of food-borne pathogens including Salmonella, Campylobacter, Escherichia coli O157:H7, Listeria, Staphylococcus aureus as well as spoilage bacteria including Pseudomonas and Lactobacillus (16–18). At present, the mechanism of TSP action is not completely understood; however, at the cell membrane level, TSP at a high pH (pH 12) helps to remove fat films and exerts surfactant or detergent effect (16). Sampathkumar et al. (19) demonstrated loss of cell viability and membrane integrity, and disruption of cytoplasmic and outer membranes of S. enteritidis strains treated with different concentrations of TSP at pH 10 to 11. Moreover, It has been indicated that treatment of poultry carcasses with 8 to 12% TSP did not produce any negative effect on the organoleptic quality of the treated poultry meat (13, 20, 21).
Sodium chloride (NaCl), a naturally occurring mineral, acts as a preservative and flavor enhancer. The use of NaCl in meat to increase shelf life and enhance flavor is an old practice. Addition of NaCl to meat has been associated with antimicrobial properties. It is also used to improve water-holding capacity and results in subsequent improvements in purge loss and cooking yield. Little is known, however, on the possible synergistic antimicrobial effect of TSP when used in combination with NaCl. Therefore, this work was aimed to study the effects of TSP dipping either alone or in combination with NaCl on the microbial growth and shelf life of tray-packaged chicken breasts stored at 2°C.
Materials and Methods
Sample preparation
Fresh boneless chicken breasts with skin (6 kg in 20 portions) were purchased on five occasions from a local commercial source on the day of slaughtering and immediately transported in a cooler containing crushed ice to the meat science laboratory in the Department of Food Science at Rakuno Gakuen University (Ebetsu, Hokkaido, Japan). Chicken breasts were prepared for treatment within 1 hr of arrival. TSP (Kanto Chemical Co., Inc., Tokyo, Japan) and NaCl (Katayama Chemical, Osaka, Japan) were the salts used for the treatment of chicken breasts. Chicken breast samples were divided into four batches (five portions per batch, of about 300 g per portion), which were dipped for 10 min in 3 L aqueous solution of either 10% TSP (w/v; pH 12.45), 10% NaCl (w/v; pH 6.02), combination of TSP and NaCl (7.5% + 7.5 % w/v; pH 11.80) or distilled water (control), and gently swirled with a sterile glass rod. They were then removed from the solution and allowed to drain on a stainless wire mesh screen for 5 min. Subsequently, the samples were placed on Styrofoam trays, over-wrapped with polyvinyl chloride film, labeled, and stored at 2°C. Chicken breasts were sampled at storage days 0, 3, 6, 9, and 12 for microbiological analyses and pH measurement.
pH measurement
Ten grams of each sample was homogenized with 50 mL distilled water in a blender for 30 s. The homogenate was filtered, and pH of the filtrate was determined using a digital pH meter (model HM-5S; TOA Electric Industrial Co. Ltd., Tokyo, Japan) standardized at pH 4 and 7.
Microbiological analyses
Composite chicken samples of 25 g (about 15 g of meat and 10 g of skin) were aseptically excised from the trays and homogenized in 225 mL of sterile buffered 0.1 % peptone water (Katayama Chemical, Osaka, Japan) for 1 min using a Stomacher 400 Lab Blender (Seward Medical, London, UK). From this homogenate, decimal serial dilutions were made in the same sterile peptone water and used for microbiological analyses of the chicken breast samples at appropriate time intervals during refrigerated storage. Aerobic plate counts (APC) were determined by surface spreading 0.1 mL of the sample homogenate, at selected dilutions, onto duplicate sterile plates of pre-poured and dried Standard Plate Count Agar (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan), and incubating the plates at 35°C for 48 hr. Psychrotrophic counts (PTC) were determined in a similar method to that for APC except that plates were incubated at 7°C for 10 days (22). For lactic acid bacteria (LAB) determination, diluted samples were plated on deMan, Rogosa, and Sharpe (MRS) agar (Merck, Darmstadt, Germany) previously incubated at 30°C for 2–3 days in an anaerobic jar with disposable Anaerocult C bag (Merck, Darmstadt, Germany) for the generation of an anaerobic medium. To determine Enterobacteriaceae counts (EBC), 1 mL of the appropriate dilution was inoculated by the pour-plated method on violet red bile agar (VRBA; Difco Laboratories Inc., Detroit, MI, USA) and overlaid with approximately 5 mL of the same growth medium (7). The plates were then incubated at 35 °C for 24 hr. Mold and yeast counts (MYC) were determined by cultivation on acidified (pH 3.5) potato dextrose agar (Elken Chemical Co., Ltd., Tokyo, Japan), followed by incubation at 25°C for 5 days (23).
Statistical analysis
All measurements were carried out in triplicates, and all microbial counts were converted into base-10 logarithms of colony forming units per g of chicken breast samples (log10 CFU/g). Data were subjected to analysis of variance (ANOVA) using the General Linear Models procedure of the Statistical Analysis System software of SAS Institute (24). Least significant differences were used to separate means at P < 0.05.
Results and Discussion
pH value
Changes in pH values of chicken breasts during storage at 2°C are shown in Table 1. TSP dipping resulted in a significant increase of the initial pH. In addition, during the first 6 days of storage, pH values of the samples dipped in TSP, either alone or in combination with NaCl, were significantly (P<0.05) higher than those of control and NaCl-dipped samples. This result was attributed to the high alkalinity of TSP. At day 12, however, no significant difference (P>0.05) was observed in the pH value among all treated samples. Kim and Kim (12) repotted that pH values of chicken legs treated with TSP (5–15%) gradually increased during 16 days storage at 4°C. On the contrary, our findings indicated that, over the storage time, dipping in TSP either alone or in combination with NaCl maintained the chicken breasts at almost constant pH (Table 1). However, pH of control and NaCl-treated samples showed no significant changes (P>0.05) for 6 and 9 days of storage, respectively, while by day 12 of storage, it significantly (P<0.05) increased. This increase in pH of control and NaCl-treated samples by the end of storage time may be attributed to their higher bacterial population (> 8 log10 CFU/g for APC and PTC), which may include higher proportion of proteolytic bacteria, which are capable of releasing basic amine compounds and free ammonia responsible for increasing the product pH (25). Most likely, TSP suppressed distinctly different bacterial groups than did NaCl.
Table 1.
Effects of salt dipping and storage time on the pH of chicken breast during refrigerated storage (2°C)
| Storage time (day) | Control | 10% TSP | 10% NaCl | 7.5% TSP + 7.5% NaCl |
|---|---|---|---|---|
| 0 | 6.25a;x | 7.10a;y | 6.10a;x | 6.75a;y |
| 3 | 6.31ab;x | 7.05a;y | 6.07a;x | 6.72a;y |
| 6 | 6.36ab;xy | 6.91a;z | 6.13a;x | 6.70a;yz |
| 9 | 6.63bc;xy | 6.90a;y | 6.32ab;x | 6.77a;y |
| 12 | 6.94c;x | 7.08a;x | 6.65b;x | 6.88a;x |
Means in the same column followed by different superscript are significantly different (P<0.05)
Means in the same row followed by different superscript are significantly different (P<0.05)
Microbiological evaluation
As expected, increase in storage time produced significant proliferations in APC, regardless of the treatment conditions (Fig. 1). Initial (day 0) APC (log10 CFU/g) of chicken breasts ranged from 3 in TSP-dipped samples to 3.48 in the control. This indicated that TSP dipping initially did not result in drastic reduction of APC (0.48 log10 CFU/g). Yang et al. (26) reported an initial decrease of 0.74 log10 CFU/chicken carcass sprayed inside and outside with 10% TSP. On the other hand, little changes were noticed in initial APC of chicken carcasses dipped in 10% TSP solution for 15 min (27), as well as in sliced pork loin chops dipped in 10% TSP for 30 sec and stored at 2°C (28). By day 6 of storage, APC of control chicken breasts reached 6.91 log10 CFU/g, which is closed to the maximal recommended limit (7 Iog10 CFU/g) set by ICMSF (29) for APC of raw poultry. While TSP-treatment, either alone (10%) or in combination with NaCl (7.5% + 7.5%), significantly (P<0.05) delayed the microbial growth and extended the shelf life of the refrigerated chicken breasts up to 12 days, at which APC were 6.87 and 6.39, respectively, versus 9.58 log10 CFU/g for the control. Dipping of chicken breasts in NaCl alone (10%) had no significant effect (P>0.05) on APC, although its count was 1 log lower than the control (6.98 versus 7.98 Iog10 CPU/g) by day 9 of storage. This result was in agreement with that of Kim and Marshall (13), who reported that dipping of chicken legs in 5–10% TSP solution for 10 min significantly reduced APC and extended their shelf life to approximately 12–16 days at 4°C.
Fig. 1. Effects of TSP and/or NaCl dipping on aerobic plate counts (APC) in chicken breasts during storage at 2°C.
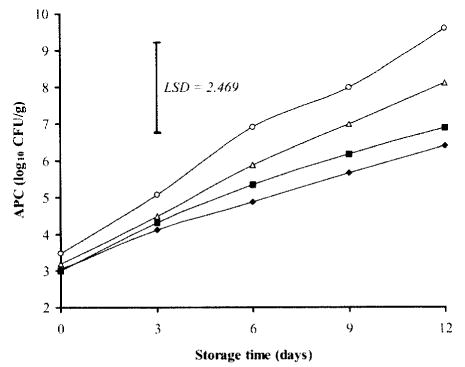
Values represent means of three replicates; LSD is defined at P < 0.05. (▪): 10% TSP; (▵): 10% NaCl; (♦): 7.5% TSP + 7.5% NaCl; (○): Control.
At storage day 0, the initial PTC of chicken breast samples ranged from 3.04 log10 CFU/g in chicken samples dipped in TSP and NaCl combination to 3.6 log10 CFU/g in control. In addition, the growth pattern of PTC was comparable to that of APC, with control also being the highest at day 12 (9.93 log10 CFU/g), followed by samples treated with NaCl (8.39 log CFU/g), while much lower counts were detected in samples treated with TSP either alone (7.27 log10, CFU/g) or in combination with NaCl (6.89 log10 CFU/g) (Fig. 2). However, the psychrotrophic population was relatively higher than APC, and, by day 12 of storage, the difference in count ranged from 0.3 log in NaCl-dipped chicken breasts to 0.5 log in samples dipped in TSP and NaCl combination, which was attributed to the refrigerated storage (2°C) condition of chicken breasts.
Fig. 2. Effects of TSP and/or NaCl dipping on psychrotrophic counts (PTC) in chicken breasts during storage at 2°C.
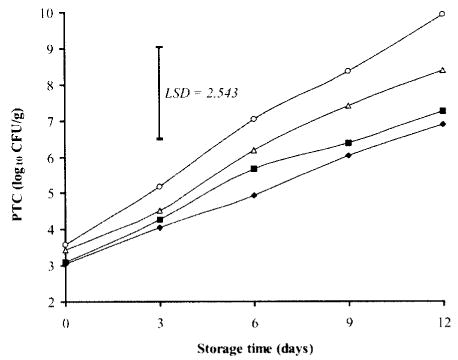
Values represent means of three replicates; LSD is defined at P < 0.05. (▪): 10% TSP; (▵): 10% NaCl; (♦): 7.5% TSP + 7.5% NaCl; (○): Control.
Significant differences (P<0.05) were detected in PTC between samples dipped in TSP either alone or in combination with NaCl, and that of control. This result is in accordance with that of Capita et al. (30), who noted a significant decrease in psychrotrophic population on the skin of chicken carcasses immersed in 8–12% solution of TSP for 15 min during refrigerated storage at 2°C.
Length of refrigerated storage (2°C) had a significant (P <0.05) effect on PTC, which tended to increase as the storage time increased. However, Egbert, et al. (31) claimed that the length of refrigerated storage (14 days at −2 to 0°C) or retail display (48 hr at 5 to 7°C) of low-fat ground beef had no effect on PTC, which remained between 7.8 and 7.9 log10 CFU/g during this period.
The initial count (log10 CFU/g) of LAB ranged from 3.17 in TSP-dipped samples to 3.37 in control meat. At storage day 12, however, no significant differences (P> 0.05) in LAB populations were observed between control and all other treatments, although it was about 1 log lower for samples dipped in TSP either alone (6.66) or in combination with NaCl (6.48) in comparison with control (7.61) (Fig. 4). This finding is in agreement with that of Ellerbroek et al. (32), who claimed that spraying or dipping of chicken meat with 10% TSP did not produce any significant effect on lactobacilli counts when compared with control.
Fig. 4. Effects of TSP and/or NaCl dipping on Enterobactericeae counts (EBC) in chicken breasts during storage at 2°C.
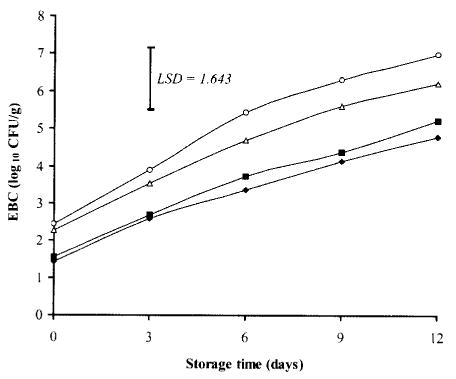
Values represent means of three replicates; LSD is defined at P < 0.05. (▪): 10% TSP; (▵): 10% NaCl; (♦): 7.5% TSP + 7.5% NaCl; (○): Control.
The growth of Entembacteriaceae was slower than those of APC, PTC, and LAB (Fig. 4). The initial EEC of chicken breast samples dipped in TSP solution either alone or in combination with NaCl was about 1 log lower than that of control (1.54 and 1.43 log10 CFU/g, respectively versus 2.45 log10 CFU/g for control). Whyte et al. (33) reported a significantly higher reduction of 1.86 log10 CFU/g for Enterobacteriaceae counts in broiler neck skin samples dipped in 10% TSP solution for 15 s. In addition, Ellerbroek et al. (32) claimed that spraying or dipping of chicken meat with 10% TSP significantly reduced the Enterobacteriaceae count.
By storage day 12, both TSP-dipped samples and samples dipped in TSP and NaCl combination exhibited significantly (P < 0.05) lower EBC of 5.21 and 4.78 log10 CFU/g, respectively in comparison with control which reached a higher level of 6.98 log10 CFU/g.
Changes in MYC (log10 CFU/g) of chicken breasts during storage at 2°C (Fig. 5) revealed that the initial count ranged from 3.41 in control to 3.57 in NaCl-dipped samples. By day 12, however, all treatments, as well as control, exhibited MYC ranging between 4.38 and 5.12, which indicates no significant effect of storage on mold and yeast populations. This finding is in agreement with that of Egbert et al. (31), who reported that the length of refrigerated storage of low-fat ground beef had no significant effect on mold and yeast populations. The present study also revealed that dipping of chicken breasts in TSP or NaCl solutions either alone or in combination did not show any significant effects on MYC after 12 days of storage when compared with control. Ismail et al. (34) suggested that yeasts might play a more prominent role than previously recognized in the spoilage of fresh and processed poultry stored at refrigeration temperature. Molds are very rarely a problem, whereas yeasts play an important role in the alteration of flavor characteristics of meat products (35).
Fig. 5. Effects of TSP and/or NaCl dipping on mold and yeast counts (MYC) in chicken breasts during storage at 2°C.
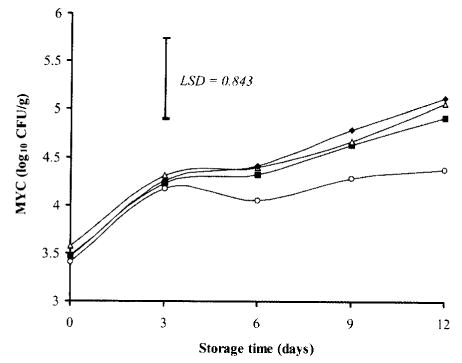
Values represent means of three replicates; LSD is defined at P < 0.05. (▪): 10% TSP; (▵): 10% NaCl; (♦): 7.5% TSP + 7.5% NaCl; (○): Control.
It conclusion, dipping of chicken breasts in TSP either alone or in combination with NaCl was effective against the proliferation of aerobic microorganisms, psychrotrophic bacteria, and Enterobacteriaceae. Therefore these treatments could be utilized successfully to improve the microbial safety, and extend the shelf life of chicken breasts during refrigerated storage.
Fig. 3. Effects of TSP and/or NaCl dipping on growth of lactic acid bacteria (LAB) in chicken breasts during storage at 2°C.
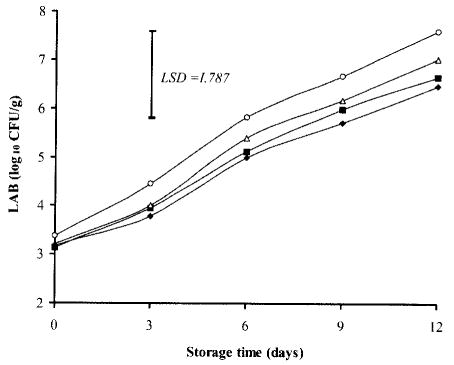
Values represent means of three replicates; LSD is defined at P < 0.05. (▪): 10% TSP; (▵): 10% NaCl; (♦): 7.5% TSP + 7.5% NaCl; (○): Control.
Contributor Information
Khalid Ibrahim Sallam, Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt.
Kunihiko Samejima, Department of Food Science, Faculty of Dairy Science, Rakuno Gakuen University, Ebetsu, Hokkaido 069–8501, Japan.
References
- 1.Synder OP Hospitality Institute of Technology and Management. Food Safety Through Quality Assured Retail Food Systems. St. Paul; MN, USA: 1998. Menu management and purchasing. Chapter 18; pp. 11–13. [Google Scholar]
- 2.Bean NH, Griffin PM, Goulding JS, Ivey CB. Foodborne disease outbreaks, 5-year summary, 1983–1987. J Food Prot. 1990;53:711–728. doi: 10.4315/0362-028X-53.8.711. [DOI] [PubMed] [Google Scholar]
- 3.Hafez HM. Poultry meat and food safety: pre- and post-harvest approaches to reduce foodborne pathogens. World's Poult Sci J. 1999;55:269–280. [Google Scholar]
- 4.Stern NJ, Line JE. Comparison of three methods for recovery of Campylobacter spp. from broiler carcasses. J Food Prot. 1992;55:663–666. doi: 10.4315/0362-028X-55.9.663. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Ge B, De Villena J, Sudler R, Yeh E, Zhao S, White DG, Wagner D, Meng J. Prevalence of Campylobacter spp. Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, DC, area. Appl Environ Microbiol. 2001;67:5431–5436. doi: 10.1128/AEM.67.12.5431-5436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izat AL, Colberg M, Adams MH, Reiber MA, Waldroup PW. Production and processing studies to reduce the incidence of Salmonellae on commercial broilers. J Food Prot. 1989;52:670–673. doi: 10.4315/0362-028X-52.9.670. [DOI] [PubMed] [Google Scholar]
- 7.Jiménez SM, Salsi MS, Tiburzi RC, Rafaghelli RC, Pirovani ME. Combined use of acetic acid treatment and modified atmosphere packaging for extending the shelf-life of chilled chicken breast portions. J Appl Microbiol. 1999;87:339–344. doi: 10.1046/j.1365-2672.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- 8.Smulders FJM, Greer GG. Integrating microbial decontamination with organic acids in HACCP programmes for muscle foods: prospects and controversies. Int J Food Microbiol. 1998;44:149–169. doi: 10.1016/s0168-1605(98)00123-8. [DOI] [PubMed] [Google Scholar]
- 9.Vasavada M, Carpenter CE, Cornforth DP, Ghorpade V. Sodium levulinate and sodium lactate effects on microbial growth and stability of fresh pork and turkey sausages. J Muscle Foods. 2003;14:119–129. [Google Scholar]
- 10.Williams SK, Phillips K. Sodium lactate affects sensory and objective characteristics of tray-packed broiler chicken breast meat. Poult Sci. 1998;77:765–769. doi: 10.1093/ps/77.5.765. [DOI] [PubMed] [Google Scholar]
- 11.Hwang CA, Beuchat LR. Efficacy of selected chemicals for killing pathogenic and spoilage microorganisms on chicken skin. J Food Prot. 1995;58:19–23. doi: 10.4315/0362-028X-58.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Kim CR, Kim KH. Physicochemical quality and gram negative bacteria in refrigerated chicken legs treated with trisodium phosphate and acetic acid. Food Sci Biotechnol. 2000;9:218–221. [Google Scholar]
- 13.Kim CR, Marshall DL. Microbiological, colour and sensory changes of refrigerated chicken legs treated with selected phosphates. Food Res Int. 1999;32:209–215. [Google Scholar]
- 14.Yoon KS, Oscar TP. Survival of Salmonella typhimurium on sterile ground chicken breast patties after washing with salt and phosphates during refrigerated and frozen storage. J Food Sci. 2002;67:772–775. [Google Scholar]
- 15.Federal Register. Use of trisodium phosphate on raw, chilled poultry carcasses. Fed Regist. 1994;59:551–554. [Google Scholar]
- 16.Capita R, Alonso-Calleja C, Garcia-Fernandez MC, Moreno B. Review: Trisodium phosphate (TSP) treatment for decontamination of poultry. Food Sci Technol Int. 2002;8:11–24. [Google Scholar]
- 17.Giese J. Salmonella reduction process receives approval. Food Technol. 1993;47:110–110. [Google Scholar]
- 18.Rodríguez-de-Ledesma AM, Riemann HP, Farver TB. Short-time treatment with alkali and/or hot water to remove common pathogenic and spoilage bacteria from chicken wing skin. J Food Prot. 1996;59:746–750. doi: 10.4315/0362-028X-59.7.746. [DOI] [PubMed] [Google Scholar]
- 19.Sampathkumar B, Khachatourians GG, Korber DR. High pH during trisodium phosphate treatment causes membrane damage and destruction of Salmonella enterica serovar enteritidis. Appl Environ Microbiol. 2003;69:122–129. doi: 10.1128/AEM.69.1.122-129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capita R, Alonso-Calleja C, Sierra M, Moreno B, Garcia-Fernandez MC. Effect of trisodium phosphate solutions washing on the sensory evaluation of poultry meat. Meat Sci. 2000;55:471–474. doi: 10.1016/s0309-1740(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 21.Hathcox AK, Hwang CA, Resurreccion AVA, Beuchat LR. Consumer evaluation of raw and fried chicken after washing in trisodium phosphate or lactic acid/sodium benzoate solutions. J Food Sci. 1995;60:604–605. 610. [Google Scholar]
- 22.Cousin MA, Jay JM, Vasavada PC. Psychrotrophic microorganisms. In: Vanderzand C, Splittstoesser DP, editors. Compendium of Methods for the Microbiological Examination of Food. 3rd ed. American Public Health Association; Washington, DC, MD, USA: 1992. pp. 153–168. [Google Scholar]
- 23.Koburger JA, Marth EH. Yeast and molds. In: Speck ML, editor. Compendium of Methods for the Microbiological Examination of Foods. American Public Health Association; Washington, DC, MD, USA: 1984. p. 200. [Google Scholar]
- 24.SAS Institute, Inc. SAS User's Guide. Statistical Analysis System Institute; Gary, NC, USA: 1990. [Google Scholar]
- 25.Jay JM. Spoilage of fresh and processed meats, poultry, and seafoods. Chapter 9. In: Jay JM, editor. Modern Food Microbiology. Van Nostrand Reinhold; NY, USA: 1992. pp. 199–233. [Google Scholar]
- 26.Yang Z, Li Y, Slavik M. Use of antimicrobial spray applied with an inside-outside birdwasher to reduce bacterial contamination on prechilled chicken carcasses. J Food Prot. 1998;61:829–832. doi: 10.4315/0362-028x-61.7.829. [DOI] [PubMed] [Google Scholar]
- 27.Lillard HS. Effect of trisodium phosphate on salmonellae attached to chicken skin. J Food Prot. 1994;57:465–469. doi: 10.4315/0362-028X-57.6.465. [DOI] [PubMed] [Google Scholar]
- 28.Lin KW, Chuang CH. Effectiveness of dipping with phosphate, lactate and acetic acid solutions on the quality and shelf-life of pork loin chop. J Food Sci. 2001;66:494–499. [Google Scholar]
- 29.ICMSF. Microorganisms in Foods 2. Sampling for microbiological analysis: Principles and specific applications. 2nd ed. University of Toronto Press; Toronto, Canada: 1986. International Commission on Microbiological Specification for Foods. [Google Scholar]
- 30.Capita R, Alonso-Calleja C, Garcia-Arias MT, Moreno B, Garcia-Fernandez MC. Effect of trisodium phosphate on mesophilic and psychrotrophic bacterial flora attached to the skin of chicken carcasses during refrigerated storage. Food Sci Technol Int. 2000;6:345–350. [Google Scholar]
- 31.Egbert WR, Huffman DL, Chen CM, Jones WR. Microbial and oxidative changes in low-fat ground beef during simulated retail distribution. J Food Sci. 1992;57:1269–1274. 1293–1293. [Google Scholar]
- 32.Ellerbroek L, Okolocha EM, Weise E. Decontamination of poultry meat with trisodium phosphate and lactic acid. Fleischwirtschaft. 1997;77:1092–1094. [Google Scholar]
- 33.Whyte P, Collins JD, McGill K, Monahan C, O'Mahony H. Quantitative investigation of the effects of chemical decontamination procedures on the microbiological status of broiler carcasses during processing. J Food Prot. 2001;64:179–183. doi: 10.4315/0362-028x-64.2.179. [DOI] [PubMed] [Google Scholar]
- 34.Ismail SA, Deak T, Abd El-Rahman H, Yassien MA, Beuchat LR. Presence and changes in populations of yeasts on raw and processed poultry products stored at refrigeration temperature. Int J Food Microbiol. 2000;62:113–121. doi: 10.1016/s0168-1605(00)00414-1. [DOI] [PubMed] [Google Scholar]
- 35.Margalith PZ. Meat, microorganisms and flavor. In: Margalith PZ, editor. Flavor Microbiology. Charles C Thomas; Springfield, IL, USA: 1981. pp. 119–139. [Google Scholar]


