Abstract
We tested whether GABAA receptor (R) subunit mRNA levels are homeostatically influenced by short-term exposure to GABA in two adjacent regions of the posterior hypothalamus. mRNA levels for seven GABAAR subunits and GABA-synthesizing enzyme (GAD) were quantified in the perifornical (PF) and dorsomedial (DM) hypothalamus following superfusion of slices for 90 min with a drug-free medium, GABA uptake blocker with or without GABAAR antagonist, gabazine, or GABAAR agonist with tetrodotoxin. Increasing endogenous GABA decreased mRNAs for all seven GABAAR subunits in the PF, and for three also in the DM, region; gabazine antagonized these effects in the PF region only and increased GAD-65 mRNA. Stimulation of GABAARs in the presence of tetrodotoxin decreased mRNA for one GABAAR subunit (β1). We conclude that, in the PF region where GABA facilitates sleep, increased GABA release may limit GABAAR-mediated inhibition, whereas in the DM region, GABA-induced changes are mainly mediated by non-GABAA receptors.
Keywords: GABAA receptors, gabazine, GAD, hypothalamus, muscimol, RT-PCR, sleep homeostasis, synaptic plasticity
INTRODUCTION
GABAA receptors (GABAARs) control excitability of neurons by opening Cl channnels, thereby causing a rapid hyperpolarization. Functional GABAARs are pentamers assembled from at least 20 subunits [1]. Inhibition mediated by GABAARs can be modulated by exogenous and endogenous compounds through alterations in the receptors' sensitivity, trafficking and assembly. Such a plasticity of GABAergic inhibition occurs in cortical and hippocampal neurons, as well as neuronal cell cultures, following treatments lasting several days [2-7], and has been discussed in the context of homeostatic mechanisms that regulate synaptic strength in relation to the magnitudes of synaptic excitation and inhibition [8]. However, it is not clear whether such a plasticity also contributes to short-term regulation of GABAARs in relation to the control of normal physiologic functions. Here, we focused on the potential for GABAAR regulation at the mRNA level in the posterior hypothalamus, where manipulations with GABAergic transmission have powerful effects on sleep and wakefulness.
The perifornical (PF) region of the posterior hypothalamus has dense GABAergic innervation [9,10]. GABAAR-mediated inhibition exerted in this region may impact several homeostatically regulated systems, including those for feeding, metabolism and motor activity, but its role in the control of sleep is especially well documented. Endogenous GABA levels increase in the PF region during slow-wave sleep (SWS) [11], local microinjections of GABAAR agonists facilitate sleep [12], and GABAAR antagonists promote wakefulness [13,14]. Importantly, the sleep-promoting effects of GABA in the posterior hypothalamus appear to be strengthened following anterior hypothalamic lesions that eliminate an important source of GABAergic input to the posterior hypothalamus and cause insomnia [12]. These findings prompted us to hypothesize that the potency of GABAergic inhibition in the PF region may increase following a period of reduced stimulation of GABAARs. Such a mechanism could contribute to the homeostatic regulation of sleep by facilitating sleep after a period of wakefulness and by reducing sleep propensity ("sleepiness") following a period of rest, thus acting over a time-scale compatible with the normal sleep-wake cycle [15]. To begin addressing this hypothesis, we developed an in vitro model that allows one to quantify the local effects of GABA on mRNAs relevant for GABAergic transmission. We determined that a 90 min-long increase in GABAergic inhibition within the posterior hypothalamus decreases mRNA levels for GABAAR subunits in a regionally selective and GABAAR-dependent manner. A preliminary report has been published [16].
EXPERIMENTAL PROCEDURES
Animals
Eighteen adult male Sprague-Dawley rats (300-370 g) were used. The procedures for animal handling followed the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
In vitro exposure of hypothalamic slices to GABAergic drugs
Rats were deeply anaesthetized with isoflurane (3.5%) and decapitated. Transverse slices, 400 μm thick, were cut, and one was selected from the region just caudal to the decussation of the optic tract and divided along the midline. Each half-slice was initially placed in a separate perfusion chamber (0.4 ml) and superfused (1ml/min) for 1 h at room temperature with O2-saturated artificial cerebrospinal fluid (ACSF, see [17] for composition; the same medium was previously used to record from hypothalamic neurons [18]).
Gabazine (SR-95531), a selective GABAAR antagonist; muscimol, a selective agonist of GABAARs, and NO-711, a selective blocker of type 1 GABA transporter (uptake blocker) [19], were purchased from Sigma-Aldrich (Saint Louis, MO), and tetrodotoxin (TTX) from Tocris (Ellisville, MO). All drugs were freshly dissolved in ACSF. The concentration of gabazine (20 μM) was sufficient to fully and selectively block and functionally antagonize GABAARs in hypothalamic slices in vitro [20,21].
After stabilization in ACSF, to increase endogenous GABA levels, one half-slice was superfused with ACSF containing 20 μM NO-711 only and the other with 20 :M gabazine and NO-711, both for 90 min at 34°C. In another series, one half-slice was superfused with 10 :M muscimol and 1 μm TTX, and the other with TTX only. TTX was used to suppress synaptic interactions among cells in the slice, with 1 μm being sufficient to block synaptic transmission in hypothalamic slices [18]. To determine mRNA levels under baseline conditions, separate half-slices were superfused with ACSF for 90 min at 34°C.
All exposures were concluded with 10 min superfusion with ACSF at 34°C, and then two 700 μm circular punches were cut from each half-slice, one from the perifornical (PF) region and the other from the dorsomedial/paraventricular (DM) region (see Fig. 1A and [17] for anatomical definitions of PF and DM punches). To verify punch locations, slices were fixed in formalin, cut into 25 μm sections, mounted and stained with Neutral red.
Figure 1.
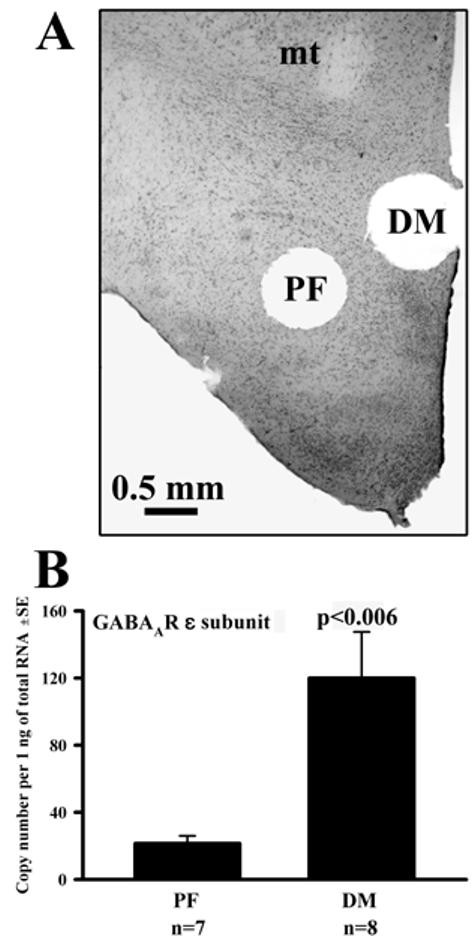
(A) Example of tissue punches extracted from the perifornical (PF) and dorsomedial (DM) regions of a hypothalamic half-slice (mt, mammillothalamic tract). (B) Basal levels of the mRNA for ε subunit of GABAA receptor were higher in the DM than PF region.
Quantitative RT-PCR, quality control and data analysis
RNA extraction, its quantification, and subsequent quantitative RT-PCRs were performed as described previously [17]. Total RNA was extracted from each punch, and one half of each sample reverse-transcribed (PowerScript reverse transcriptase, BD Biosciences-Clontech, Palo Alto, CA). PCRs were conducted and calibrated using LightCycler® system (Roche Diagnostics, Indianapolis, IN), with primer sets for GABAAR subunits and GAD isoforms designed using Vector NTI software (Invitrogen Bioinformatics, Carlsbad, CA) (see Supplementary Material for primer sequences and reaction conditions). Individual transcripts were quantified as cDNA copy numbers per 1 ng (or 1 pg) of total RNA extracted from the sample.
To control for amplification of genomic DNA, 34 DNase-treated but not reverse-transcribed RNA samples were submitted to PCRs with different primer sets. None of those reactions was positive. Differences in mRNA levels were examined using one-way ANOVA with Bonferroni's correction (Analyse-It Software, UK). To minimize the effect of variability in slice conditions, paired tests were used to assess the difference in transcript levels between two halves of the same slice simultaneously subjected to different treatments.
RESULTS AND DISCUSSION
Regional differences in mRNA levels
To assess the sensitivity and regional selectivity of our methodology, we verified that we could detect one known regional difference in basal mRNA levels between the PF and DM region. Consistent with prior in situ hybridization study [22], in slices superfused with ACSF, the mRNA levels for ε subunit of GABAAR were significantly higher in the DM than the PF region (120±28 (SE) copies/ng of total RNA vs. 21±5, p<0.006; Fig. 1B). The basal mRNA levels for the remaining six GABAAR subunits studied did not significantly differ between the two regions.
Effects of endogenous GABA and GABAAR antagonist on GABAAR subunit and GAD mRNA
We quantified mRNA levels for seven GABAAR subunits that are expressed in the posterior hypothalamus (α1, α2, α3, β1, β2, β3 and ε) [22-24] in two anatomically adjacent but functionally different hypothalamic regions following three in vitro treatments: superfusion of slices with plain ACSF, GABA uptake blocker (NO-711), or the latter combined with the GABAAR antagonist, gabazine.
When compared to incubation in ACSF, NO-711 reduced the mRNA levels of all seven studied GABAAR subunits in the PF region and for three also in the DM region (α1, α2 and ε). Figure 2 shows the changes in mRNA levels for those GABAAR subunits that were significantly reduced by NO-711 in the PF region only, whereas Fig. 3 shows those GABAAR subunit mRNAs that were significantly reduced by NO-711 in both regions (and in contrast to those illustrated in Fig. 2, were not sensitive to gabazine - see below). In the PF region, the effect of NO-711 varied from a ∼2-fold decrease for the α3 and β2 subunits (Fig. 2A and C) to ∼24-fold decrease for the α1 subunit (Fig. 3A).
Figure 2.
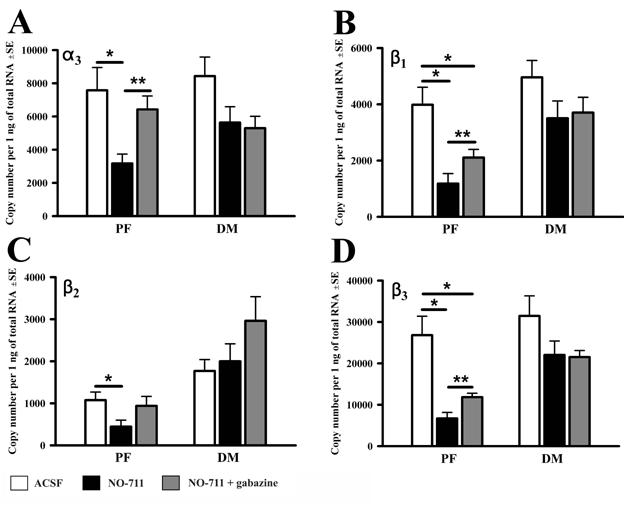
mRNA levels of those GABAA receptor subunits that were significantly reduced by the GABA uptake blocker (NO-711) in the PF region only. For these subunits, NO-711 and gabazine exerted opposite effects in the PF region, whereas their effects in the DM region were not significant. The data were obtained from 8 half-slices superfused with neutral medium (ACSF), and 6 pairs of half-slices of which one was superfused with NO-711 and the other with NO-711+gabazine. * p<0.05, one-way ANOVA; **p<0.05, paired t-test.
Figure 3.
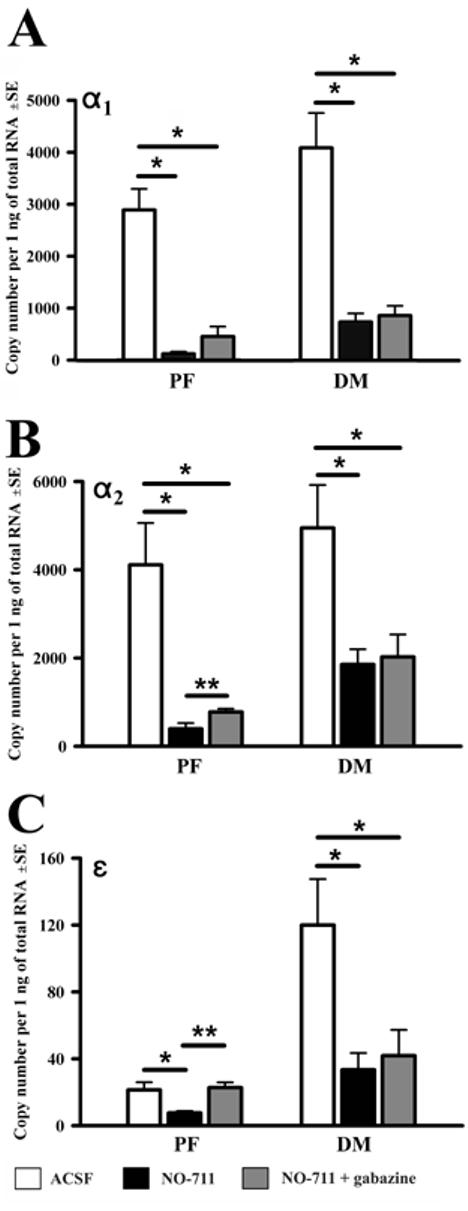
mRNA levels for those GABAA receptor subunits that were significantly reduced by the GABA uptake blocker (NO-711) in both the PF and DM region (but with the effects of gabazine being significant in the PF region only). The data were obtained from the same half-slices as in Fig. 2. * p<0.05, one-way ANOVA; **p<0.05, paired t-test.
Unique to the PF region was that incubation with gabazine in the presence of NO-711 entirely abolished, or at least significantly attenuated, the decreases resulting from superfusion with NO-711 only. The effect was significant for five subunits (α2,α3, β1,β3 and ε) and the remaining two (α1 and β2) exhibited the same trend (Figs. 2 and 3). The mRNAs for the α2, α3, β1, β3 and ε subunit were ∼2-fold higher following combined exposure to gabazine and NO-711 than to NO-711 only. The level of α2 subunit mRNA was 400±130 (SE) copies/ng of total RNA in NO-711 only and 780±70 (p<0.01) in gabazine with NO-711. The corresponding levels for the remaining four significantly affected subunits were: 3,200±560 vs. 6,400±800 (p<0.02) for the α3, 1,200±400 vs. 2,100±300 (p<0.04) for β1, 6,700±1,400 vs. 11,900±900 (p<0.04) for β3, and 8±1 vs. 23±3 (p<0.01) for ε subunit. The mRNA levels for three subunits, α3, β2 and ε, measured following combined exposure to NO-711 and gabazine were not significantly different from those in ACSF, suggesting that the entire effect of NO-711 on these mRNAs was mediated by GABAARs. In a stark contrast to the PF region, in the DM region, gabazine had no effect when compared to NO-711 only (Figs. 2 and 3), suggesting that, in this region, the effects of the GABA reuptake blocker were not mediated by GABAARs.
These results show that increased local GABA levels reduce mRNA levels for all seven posterior hypothalamic GABAAR subunits studied. With the regard to our hypothesis, this finding supports the concept that increased GABAAR stimulation may lead to a reduced GABAAR expression, thus limiting the potential for GABAergic inhibition mediated by these receptors. Importantly our exposures were relatively short (90 min), and thus compatible with the timing of physiologic changes in GABA levels. Comparable periods of sleep or lack thereof cause measurable behavioral and electrophysiological changes [15].
The sharp difference in the effects of gabazine between the PF and DM region is one of the most remarkable findings of this study. Only three out of the seven studied subunits were affected by the GABA uptake blocker in the DM region, and none was significantly affected by gabazine even though the basal levels of all but one subunit were not different between the two regions. This potentially important regional selectivity may be related to the cellular composition and functions of the two regions, and our ability to detect the difference to our use of exposures lasting less than 2 hrs, rather than days.
The lack of gabazine effects in the DM region suggests that this region has a relatively large pool of non-GABAA type GABA receptors. Indeed, although GABABRs are present in both regions [25], at least the type 1 GABABR-like immunoreactivity is higher in the DM than PF region [26]. Thus, actions mediated by GABABRs may be considered among the mechanisms underlying the gabazine-insensitive effect of NO-711 in the DM region. The regional differences in the effects of GABA and gabazine are probably also related to differences in the functions and cellular compositions of the two regions. The PF region contains two neuronal groups, orexin (ORX) and melanin-concentrating hormone (MCH), involved in the control of sleep and wakefulness [27-29]. The DM region, comprising the hypothalamic dorsomedial and paraventricular nuclei, may also contribute to the circadian control of sleep [30], but is primarily involved in neuroendocrine and autonomic homeostasis, including the responses to stress [31]. In the PF region, GABA, MCH and ORX neurons have α2 or α3 subunits [24], ORX and MCH cells have subunits [21], and may also express β2 and/or β3 subunits [25]. So, some of the mRNA changes that we found could occur in ORX, MCH and/or GABAergic PF neurons. Nevertheless, studies of GABAARs at the single cell level are needed to further elucidate the basis for the regional differences found in this study.
Our results pose the question as to whether the changes observed at the mRNA level translate into corresponding changes in functional GABAARs and magnitude of resulting inhibition. While this remains to be determined, current concepts of up- and downregulation of GABAergic inhibition at the level of a single synapse suggest that regulation of receptor numbers is the main mechanism [4,8]. In cultured hippocampal slices, the expression of GABAAR subunit mRNAs and their proteins increased with the level of neuronal activity [32], and a positive correlation between GABAAR subunit mRNA and the corresponding protein was observed [33]. Thus, the relatively large and involving many subunits mRNA changes that we found are likely to lead to the corresponding changes in the numbers of functional GABAARs.
Effects of endogenous GABA on GAD mRNA
Regulation of GABAergic transmission by GABA may also involve changes in GABA synthesis. However, compared to ACSF, the effects of NO-711 on mRNA levels for GAD-65 or GAD-67 were not significant in either region (Fig. 4A and B). In contrast, the combined exposure to gabazine and NO-711 resulted in a significant increase in both regions of the GAD-65 mRNA when compared to its levels in ACSF (61±14 (SE) copies/pg of total RNA vs. 26±5 (p<0.04) for the PF, and 116±31 vs. 53±4 (p<0.05) for the DM region (Fig. 4A). Thus, the mRNA for the GAD isoform proposed to be readily responsive to the need for GABA as a transmitter [34] was affected in a manner whereby increased cellular activity could lead to increased inhibition that then could limit that activity [8]. However, unlike the effects of gabazine on GABAAR subunits, those on GAD-65 mRNA had no regional selectivity.
Figure 4.
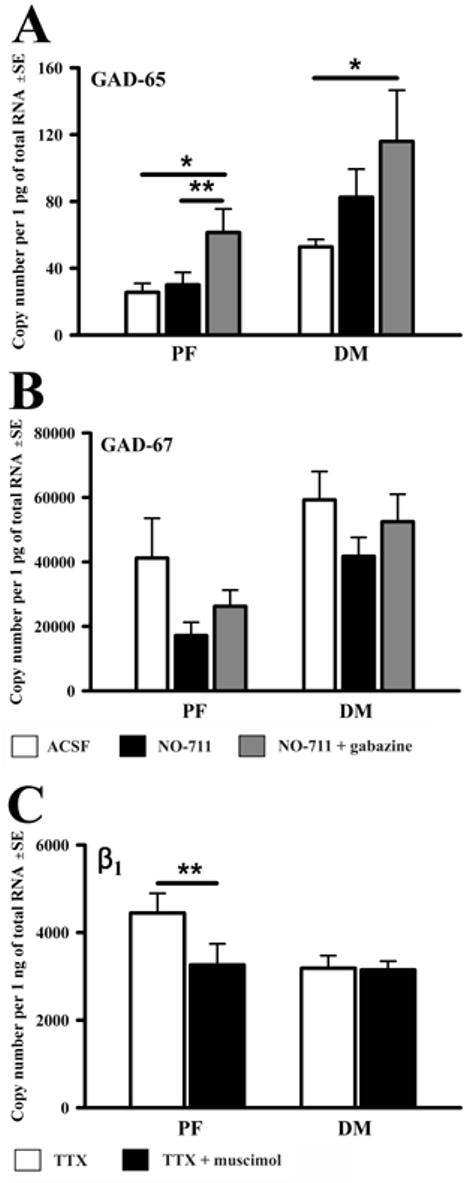
Effects of the GABA uptake blocker (NO-711) with or without gabazine on GAD-65(A) and GAD-67 (B) mRNA levels, and the effect of the GABAAR agonist, muscimol, in the presence of tetrodotoxin (TTX) on the GABAAR β1 subunit mRNA (C) in the PF and DM regions. The data in (A and B) are from the same half-slices as in Fig. 2. The data in (C) are from six PF and five DM pairs of punches extracted from half-slices that were simultaneously superfused with TTX only or TTX+muscimol. * p<0.05, one-way ANOVA; **p<0.05, paired t-test.
Effects of GABAAR agonist, muscimol, with synaptic interactions suppressed with TTX
The effects observed in our experiments could be secondary to changes in the level of local cell activity [6-8]. To test whether the changes described in the preceding sections could be mediated by stimulation of GABAAR in the absence of cellular activity and local synaptic interactions, we measured mRNA levels in half-slices of which one was superfused with muscimol and TTX and the other with TTX only. Most transcripts had reduced mRNA levels in TTX compared to ACSF (data not shown) and were not further reduced by the combined exposure to muscimol and TTX. However, mRNA for one GABAAR subunit (β1), and in the PF region only, was significant reduced in muscimol with TTX when compared to TTX only (3,300±480 (SE) copies/ng of total RNA vs. 4,500±450, p<0.05) (Fig. 4C).
The absence of the effects of muscimol on most GABAAR subunits and GAD in TTX, suggests that most of the changes observed in response to NO-711 and gabazine were related to the level of cellular activity. However, the decrease of the β1 subunit mRNA following GABAAR stimulation in TTX shows that GABA may negatively regulate the expression of GABAARs in relation to the use of the receptor. Notably, this effect is autologous because it occurred in synaptically isolated neurons. Similarly, stimulation of GABAARs altered transcription in cells of the mammalian circadian clock in the absence of cellular activity [35], and downregulated a promoter of the human β1 subunit of GABAAR [36].
Potential physiologic role
Our results demonstrate that mRNA levels of multiple GABAAR subunits, and hence potentially also synthesis of new GABAARs, decrease with increased magnitude of GABAergic stimulation. The effect occurs after relatively short-lasting exposures and has different underlying mechanisms in the two compared hypothalamic regions.
Neurons in the PF region are directly involved in critical functions such as feeding, motivation and motor activity that are closely associated with sleep and vigilance [27,28]. This places the regulation of sleep in the center of interest with the regard to mRNA changes that we found in the PF region. In our separate in vivo study [37], 6 hrs of sleep deprivation increased the β1, β3 and ε subunit mRNA levels in the PF region only similarly to the effects of gabazine in the present study. Also of interest with the regard to our hypothesis is the finding that, in humans, a mutation in the gene for the β3subunit is associated with insomnia [38]. Since appropriate mRNA changes occur mainly in the PF region, it will be of interest to identify the mechanisms that regulate the magnitude of GABAAR-mediated inhibition in neurochemically distinct PF neurons.
Supplementary Material
ACKNOWLEDGEMENTS
The study was supported by the American Sleep Medicine Foundation award 26-CA-04 and NIH grant HL-071097. The authors thank Jennifer L. Branconi, Tyana Singletary, Janet Lee and Michael Loguidice for technical assistance.
REFERENCES
- [1].Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Cur. Op. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- [2].Roca DJ, Rozenberg I, Farrant M, Farb DH. Chronic agonist exposure induces down-regulation and allosteric uncoupling of the gamma-aminobutyric acid/benzodiazepine receptor complex. Mol. Pharmacol. 1990;37:37–43. [PubMed] [Google Scholar]
- [3].Montpied P, Ginns EI, Martin BM, Roca D, Farb DH, Paul SM. Gamma-aminobutyric acid (GABA) induces a receptor-mediated reduction in GABAA receptor alpha subunit messenger RNAs in embryonic chick neurons in culture. J. Biol. Chem. 1991;266:6011–6014. [PubMed] [Google Scholar]
- [4].Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- [5].Lyons HR, Gibbs TT, Farb DH. Turnover and down-regulation of GABAA receptor α1, β2S, and γ1 subunit mRNAs by neurons in culture. J. Neurochem. 2000;74:1041–1048. doi: 10.1046/j.1471-4159.2000.0741041.x. [DOI] [PubMed] [Google Scholar]
- [6].Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABAA receptors clustered at neocortical synapses. J. Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marty S, Wehrle R, Fritschy J-M, Sotelo C. Quantitative effects produced by modifications of neuronal activity on the size of GABAA receptor clusters in hippocampal slice cultures. Eur. J. Neurosci. 2004;20:427–440. doi: 10.1111/j.1460-9568.2004.03491.x. [DOI] [PubMed] [Google Scholar]
- [8].Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- [9].Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- [10].Kitahama K, Sallanon M, Okamura H, Geffard M, Jouvet M. Cells presenting GABA immunoreactivity in the hypothalamus of the cat. C. R. Acad. Sci. Paris. 1989;308:507–511. [PubMed] [Google Scholar]
- [11].Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am. J. Physiol. 1996;271:R1707–R1712. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- [13].Kubin L, Mann G, Bloch L, Ross R, Morrison AR. Antagonism of hypothalamic perifornical GABAA receptors reduces sleep. Soc. Neurosci. Abstr. 2002;28 224.9. [Google Scholar]
- [14].Alam MN, Kumar S, Bashir T, Suntsova N, Methiparra MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin-but not melanin-concentrating hormone-immunoreactive neurons during sleep in rats. J. Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tobler I, Borbély AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav. Brain Res. 1990;36:73–78. doi: 10.1016/0166-4328(90)90161-7. [DOI] [PubMed] [Google Scholar]
- [16].Volgin DV, Kubin L. Endogenous activation of GABAA receptors in posterior hypothalamic slices in vitro decreases GABAA receptor subunit mRNA expression. Sleep. 2005;28(Suppl):A34. [Google Scholar]
- [17].Volgin DV, Kubin L. Chronic intermittent hypoxia alters hypothalamic transcription of genes involved in metabolic regulation. Auton. Neurosci. 2006;126:93–99. doi: 10.1016/j.autneu.2006.03.013. [DOI] [PubMed] [Google Scholar]
- [18].Yamanaka A, Murak Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem. Biophys. Res. Comm. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- [19].Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J. Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jones MV, Sahara Y, Dzubay JA, Westbrook GL. Defining affinity with the GABAA receptor. J. Neurosci. 1998;18:8590–8604. doi: 10.1523/JNEUROSCI.18-21-08590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kononenko NI, Dudek E. Mechanism of irregular firing of suprachiasmatic nucleus neurons in rat hypothalamic slices. J. Neurophysiol. 2004;91:267–273. doi: 10.1152/jn.00314.2003. [DOI] [PubMed] [Google Scholar]
- [22].Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABAA receptor epsilon subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967:285–289. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- [23].Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- [24].Bäckberg M, Ultenius C, Fritschy J-M, Meister B. Cellular localization of GABAA receptor α subunit immunoreactivity in the rat hypothalamus: relationship with neurons containing orexigenic or anorexigenic peptides. J. Neuroendocrinol. 2004;16:589–604. doi: 10.1111/j.1365-2826.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- [25].Henny P, Modirrousta M, Bayer L, Muhlethaler M, Jones BE. Ionotropic and metabotropic GABA receptors on orexin/hypocretin neurons. Sleep. 2005;28(Suppl):A28. [Google Scholar]
- [26].Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABAB receptors in the rat central nervous system. J. Comp. Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [27].Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu. Rev. Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- [28].Kilduff TS. Hypocretin/orexin: maintenance of wakefulness and a multiplicity of other roles. Sleep Med. Rev. 2005;9:227–230. doi: 10.1016/j.smrv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- [29].Goutagny R, Luppi P-H, Salvert D, Gervasoni D, Fort P. GABAergic control of hypothalamic melanin-concentrating hormone-containing neurons across the sleep-wake cycle. NeuroReport. 2005;16:1069–1072. doi: 10.1097/00001756-200507130-00008. [DOI] [PubMed] [Google Scholar]
- [30].Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J. Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: 1998 update. Proc. Soc. Exp. Biol. Med. 1998;218:284–306. doi: 10.3181/00379727-218-44296. [DOI] [PubMed] [Google Scholar]
- [32].Holopainen IE, Lauren HB. Neuronal activity regulates GABAA receptor subunit expression in organotypic hippocampal slice cultures. Neuroscience. 2003;118:967–974. doi: 10.1016/s0306-4522(03)00046-0. [DOI] [PubMed] [Google Scholar]
- [33].Saha S, Sieghart W, Fritschy J-M, McWilliam PN, Batten TFC. γ-Aminobutyric acid receptor (GABAA) subunits in rat nucleus tractus solitarii (NTS) revealed by polymerase chain reaction (PCR) and immunohistochemistry. Mol. Cell. Neurosci. 2001;17:241–257. doi: 10.1006/mcne.2000.0919. [DOI] [PubMed] [Google Scholar]
- [34].Soghomonian J-J, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- [35].Bergeron HE, Danielson B, Biggs KR, Prosser RA. TTX blocks baclofen-induced phase shifts of the mammalian circadian pacemaker in vitro. Brain Res. 1999;841:193–196. doi: 10.1016/s0006-8993(99)01791-6. [DOI] [PubMed] [Google Scholar]
- [36].Russek SJ, Bandyopadhyay S, Farb DH. An initiator element mediates autologous downregulation of the human type A γ-aminobutyric acid receptor β1 subunit gene. Proc. Natl. Acad. Sci. USA. 2000;97:8600–8605. doi: 10.1073/pnas.97.15.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Volgin DV, Kubin L. GABAA receptor subunit mRNAs are differentially regulated in the hypothalamic perifornical region in association with sleep loss and circadian time. Sleep. 2003;26(Suppl):A38. [Google Scholar]
- [38].Buhr A, Bianchi MT, Baur R, Courtet P, Pignay V, Boulenger JP, Gallati S, Hinkle DJ, MacDonald RL, Sigel E. Functional characterization of the new human GABAA receptor mutation beta3 (R192H) Hum. Genet. 2002;111:154–160. doi: 10.1007/s00439-002-0766-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


