Abstract
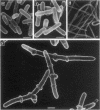
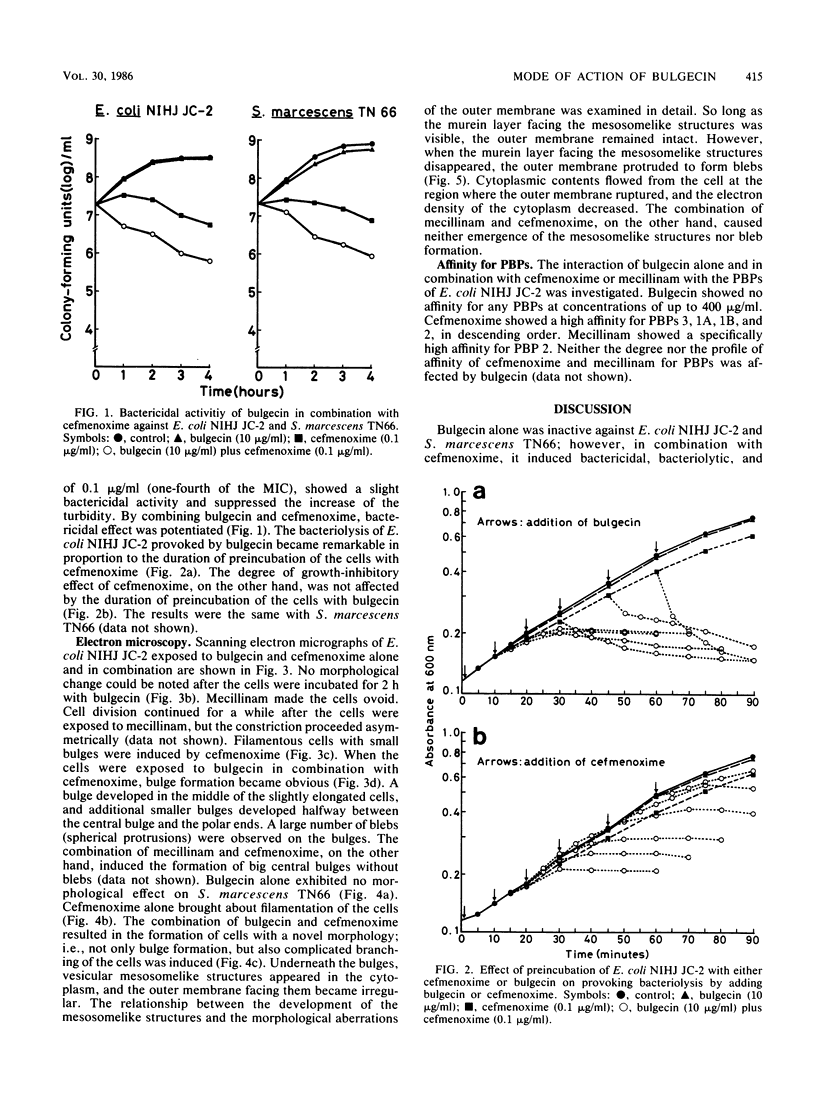
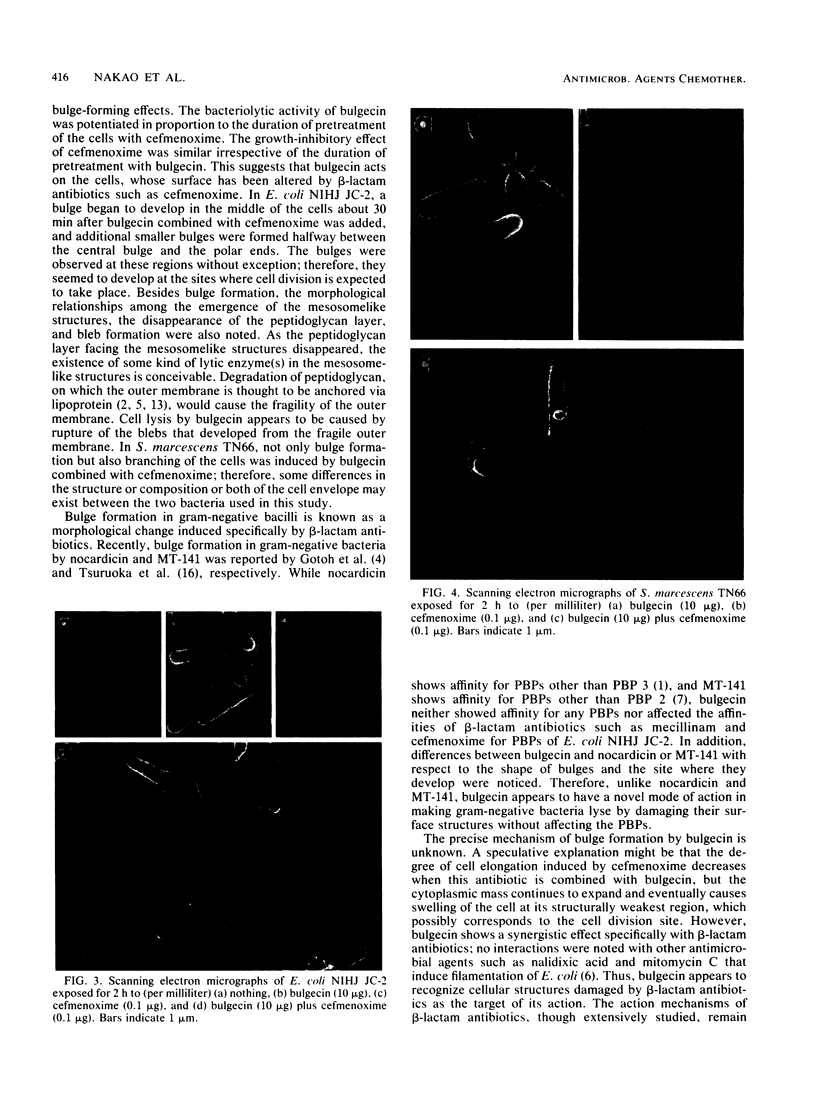
The mode of action of bulgecin was investigated by examining its bactericidal and bacteriolytic activities, its effect on bacterial morphology, and its interaction with penicillin-binding proteins (PBPs). Bulgecin alone did not show any antibacterial activity against Escherichia coli and Serratia marcescens, but in concert with cefmenoxime, it induced potent growth-inhibitory and bactericidal activities. Electron microscopic examination of E. coli cells exposed to bulgecin combined with cefmenoxime revealed that a bulge developed in the middle of the cell, and additional smaller bulges were formed halfway between the central bulge and the polar ends. At the site of bulge development, vesicular mesosomelike structures appeared in the cytoplasm, the peptidoglycan layer facing them became faint, and the outer membrane protruded to form blebs. These morphological changes were quite different from those caused by the mecillinam-cefmenoxime combination that produces big bulges in E. coli. When S. marcescens was exposed to the combination of bulgecin and cefmenoxime, not only bulge formation, but also branching of the cells was observed. Bulgecin neither showed affinity for any PBPs of E. coli nor affected the binding of cefmenoxime or mecillinam to the PBPs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenguer J., De Pedro M. A., Vázquez D. V. Interaction of nocardicin A with the penicillin-binding proteins of Escherichia coli in intact cells and in purified cell envelopes. Eur J Biochem. 1982 Aug;126(1):155–159. doi: 10.1111/j.1432-1033.1982.tb06760.x. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Fuglesang J. E., Bergan T., Bielecki T., Naterstad A., Namork E. Inhibitory activity and bactericidal kinetics of mecillinam/ampicillin combinations against Enterobacteriaceae, Pseudomonas and Acinetobacter. Infection. 1981;9(6):290–295. doi: 10.1007/BF01640994. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada A., Kintaka K., Nakao M., Shinagawa S. Bulgecin, a bacterial metabolite which in concert with beta-lactam antibiotics causes bulge formation. J Antibiot (Tokyo) 1982 Oct;35(10):1400–1403. doi: 10.7164/antibiotics.35.1400. [DOI] [PubMed] [Google Scholar]
- Inouye S., Tsuruoka T., Goi H., Iwamatsu K., Miyauchi K., Ishii T., Tamura A., Kazuno Y., Matsuhashi M. Structure-activity relationships on the terminal D-amino acid moiety of a novel cephamycin MT-141. J Antibiot (Tokyo) 1984 Nov;37(11):1403–1413. doi: 10.7164/antibiotics.37.1403. [DOI] [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- Ishino F., Tamaki S., Spratt B. G., Matsuhashi M. A mecillinam-sensitive peptidoglycan crosslinking reaction in Escherichia coli. Biochem Biophys Res Commun. 1982 Dec 15;109(3):689–696. doi: 10.1016/0006-291x(82)91995-7. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Imada A., Yoneda M. SCE-963, a new potent cephalosporin with high affinity for penicillin-binding proteins 1 and 3 of Escherichia coli. Antimicrob Agents Chemother. 1979 Jan;15(1):20–27. doi: 10.1128/aac.15.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki M. Synergistic effect of cephalexin with mecillinam. J Antibiot (Tokyo) 1981 Jun;34(6):739–752. doi: 10.7164/antibiotics.34.739. [DOI] [PubMed] [Google Scholar]
- Shinagawa S., Maki M., Kintaka K., Imada A., Asai M. Isolation and characterization of bulgecins, new bacterial metabolites with bulge-inducing activity. J Antibiot (Tokyo) 1985 Jan;38(1):17–23. doi: 10.7164/antibiotics.38.17. [DOI] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kida M., Nakao M., Iwahi T., Nishi T., Noji Y., Takeuchi M., Nozaki Y. Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56–65. doi: 10.1128/aac.19.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka T., Yamada Y., Goi H., Miyauchi K., Miyata A., Inouye S., Ishino F., Hirata A., Matsuhashi M. The bacteriolytic action of MT-141, a new cephamycin antibiotic, on gram-negative bacteria. J Antimicrob Chemother. 1985 Feb;15(2):159–171. doi: 10.1093/jac/15.2.159. [DOI] [PubMed] [Google Scholar]