Abstract
Background
We demonstrated that a Chinese herbal formula, which we refer to as RCM-101, developed from a traditional Chinese medicine formula, reduced nasal and non-nasal symptoms of seasonal allergic rhinitis (SAR). The present study in primary and cultured cells was undertaken to investigate the effects of RCM-101 on the production/release of inflammatory mediators known to be involved in SAR.
Methods
Compound 48/80-induced histamine release was studied in rat peritoneal mast cells. Production of leukotriene B4 induced by the calcium ionophore A23187 was studied in porcine neutrophils using an HPLC assay and lipopolysaccharide-stimulated prostaglandin E2 production was studied in murine macrophage (Raw 264.7) cells by immune-enzyme assay. Expression of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) was determined in Raw 264.7 cells, using western blotting techniques.
Results
RCM-101 (1–100 μg/mL) produced concentration-dependent inhibition of compound 48/80-induced histamine release from rat peritoneal mast cells and of lipopolysaccharide-stimulated prostaglandin E2 release from Raw 264.7 cells. Over the range 1 – 10 μg/mL, it inhibited A23187-induced leukotriene B4 production in porcine neutrophils. In addition, RCM-101 (100 μg/mL) inhibited the expression of COX-2 protein but did not affect that of COX-1.
Conclusion
The findings indicate that RCM-101 inhibits the release and/or synthesis of histamine, leukotriene B4 and prostaglandin E2 in cultured cells. These interactions of RCM-101 with multiple inflammatory mediators are likely to be related to its ability to reduce symptoms of allergic rhinitis.
Background
Allergic rhinitis, in particular seasonal allergic rhinitis (SAR) or hay fever, is a common allergic condition [1]. World-wide, SAR afflicts 10 – 40% of individuals [2], with approximately 20% affected in the United States, 13% in Western Europe [3] and 16.1% in Australia [4]. SAR is an immune response to a wide variety of pollens from grasses, weeds and trees. It involves the interaction of allergens with specific immunoglobulin E (IgE) antibodies bound to high affinity Fcε receptors on the surface of mast cells and basophils in the nasal mucosa [5]. This interaction causes degranulation of these cells, releasing a number of inflammatory mediators which are responsible for a cascade of symptoms. Histamine, tryptase, prostaglandin and bradykinin are responsible for the immediate allergic response of sneezing, nasal itch and rhinorrhoea [5]. The late phase response, usually 4 – 6 hours after the immediate response, involves a large increase of eosinophils, basophils and other leukocytes at the inflammatory sites, in response to chemoattractants. In the late phase response, it is likely that histamine and leukotrienes are released from basophils rather than from mast cells because there is no corresponding increase in tryptase which originates from mast cells [5].
The conventional management of SAR is usually symptomatic, with histamine H1 receptor antagonists, sympathomimetic amine vasoconstrictors and corticosteroids. However, these treatments frequently have certain undesirable side effects and, often do not provide complete symptom relief [6]. Except corticosteroids, which have more significant side-effects, conventional treatments usually target a single inflammatory mediator, which probably explains their limited effectiveness [7].
Complementary/alternative therapies are becoming increasingly used in Western countries for the treatment of allergic diseases, with growing perceptions that such treatments are effective and that they are associated with fewer and less severe side effects [8]. Certain Chinese herbal formulae have been reported to be beneficial for the treatment of asthma and allergic rhinitis, including SAR, with some results showing that their effectiveness is comparable to prednisolone [8]. Recently, we conducted a randomized placebo-controlled clinical trial on a Chinese herbal formula which was developed from a traditional Chinese medicine formula for the treatment of symptoms associated with rhinitis. The formula was optimized on the basis of Chinese medicine syndrome theory for the treatment of SAR. We demonstrated that, after eight weeks of treatment, the herbal medicine formula, which we refer to as RCM-101, was effective in reducing the nasal and non-nasal symptoms of SAR [9].
In a previous investigation of the possible mechanism(s) of the anti-inflammatory/anti-allergic activity of RCM-101 in SAR, we found that the herbal formula inhibited histamine release from isolated guinea-pig tracheal preparations and the production of nitric oxide and prostaglandin E2 by cultured macrophages [10]. In the present study, as an extended investigation into the pharmacological activities of RCM-101 in reducing the symptoms of SAR, we have investigated its effects on histamine release, leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) production, and the expression of two enzymes involved in inflammatory processes, namely cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2).
Methods
All experimental procedures involving animals were approved by RMIT University Animal Ethics Committee and were conducted in compliance with the Australian National Health and Medical Research Council guidelines.
Histamine, LTB4 and PGE2 are three key inflammatory mediators in allergic conditions such as SAR. To investigate the effects of RCM-101 on the synthesis/release of these mediators, we used three well characterized cell-based models, namely rat peritoneal mast cells for histamine [11], porcine neutrophils for LTB4 [12] and murine macrophage cells (Raw 164.7) for PGE2 [13,14].
Preparation and extraction of RCM-101
RCM-101 is a herbal formula with 18 herbal ingredients, modified from a traditional Chinese medicine formula. Each herb for the formula was supplied in a granulated form produced under Good Manufacturing Practices by Min Tong Pharmaceutical Company (Taichong, Taiwan) which holds certification from the Australian Therapeutic Goods Administration (TGA-GMP No: 1888). Authenticated, quality-certified raw herbs were first tested to ensure that they were free of heavy metals. They were then washed, dried and extracted in boiling water for 1 – 1.5 hour. The aqueous extract was separated by filtration (100 mesh) and the water content was reduced to 60% by heating (50 – 60°C) under reduced pressure (50 – 70 mmHg) for 2 – 5 hours. The concentrated extract of each herb was combined with starch as an excipient and the product was dried and ground into fine granules. For each preparation, 1 g of granulated product was equivalent to 5 g of the raw herb. The granulated herbal preparations were sterilised and sealed in plastic bottles. In our laboratory, the granulated preparations of the herbs were combined in the proportions given in Table 1 to produce the herbal formula. All herbal ingredients of RCM-101 are approved in the Australian Register of Therapeutic Goods as active raw herbs for use in medicines.
Table 1.
Herbal constituents of RCM-101 (% of granulated herbs by weight*)
| Scientific name | Botanical name | Chinese name | % |
| Flos Magnoliae | Magnolia liliflora (Desr.) | Xin Yi | 3.81 |
| Frutus Schisandrae Chinensis | Schisandra Chinensis (Turcz.) | Wu Wei Zi | 2.25 |
| Frutus Terminaliae Chebulae | Terminalia chebula Retz. | He Zi | 13.87 |
| Frutus Xanthii Sibirici | Xanthii Sibirici Patr. Ex Widd. | Cang Er Zi | 7.11 |
| Herba Asari | Asarum sieboldii Miq. | Xi Xin | 3.81 |
| Herba Menthae Haplocalysis | Mentha haplocalyx Briq. | Bo He | 4.68 |
| Herba Schizonepetae Tenuifoliae | Schizonepeta Tenuifolia Briq. | Jing Jie | 14.21 |
| Pericappium Citri Reticulatae | Citrus reticulata Blanco | Chen Pi | 9.36 |
| Radix Angelicae Sinensis | Angelica sinensis (Oliv.) Diels | Dang Gui | 4.68 |
| Radix Astragali Membranaceus | Astragalus membranaceus (Fisch.) Bge | Huang Qi | 4.68 |
| Radix Bupleuri | Bupleurum chinense D.C | Chai Hu | 3.81 |
| Radix Codonopsitis pilosulae | Codonopsis pilosula (Franch.) Nannf. | Dang Shen | 2.25 |
| Radix Glycyrrhizae Uralensis | Glycyrrhiza uralensis (Fisch.) | Gan Cao | 4.68 |
| Radix Saposhnikoviae Divaricata | Saposhnikovia divaricata (Turcz.) | Fang Feng | 4.51 |
| Rhizoma Atractylodis Macrocephalae | Atractylodes macrocephala Koidz | Bai Zhu | 4.68 |
| Rhizoma Cimicifugae | Cimicifuga foetida L. | Sheng Ma | 4.68 |
| Rhizoma Ligustici Chuanxiong | Ligusticum chuanxiong (Hort.) | Chuan Xiong | 4.68 |
| Semen Plantaginis | Plantago asiatica L. Wild. | Che Qian Zi | 2.25 |
* 1 g of each granulated herb is equivalent to 5 g of the raw herb (dry weight).
The herbal formula was extracted with ethanol (120 mg/mL) at room temperature with continuous agitation for 4 hours. The ethanol extract was collected by centrifugation (5000 rpm for 10 minutes) and vacuum filtration. The extract was dried using a rotary evaporator (Büchi Rotavapor, Brinkman Company, Westbury, NY, USA) and stored below -20°C. It was diluted to the required concentrations on the day of use.
Reagents
Compound 48/80, histamine hydrochloride, O-phthalaldehide, spermidine hydrochloride, bovine serum albumin (BSA), phosphate buffer saline, heparin, disodium ethylenediaminetetraacetic acid (EDTA), lipopolysaccharide (LPS) E.Coli, calcium ionophore A23187, Hanks' balanced salt solution, RPMI 1640 medium, fetal bovine serum, phenylmethylsulfonyl fluoride, gentamycin, leupeptin, pepstatin A and nordihydroguaiaretic acid (NDGA) were obtained from Sigma Chemical Company (St Louis, MO, USA). Monoclonal mouse anti-rat cyclooxygenase 2 antibodies and mouse macrophage lysate were obtained from Transduction Laboratories (Lexington, KY, USA). Goat anti-rabbit horseradish peroxidase conjugated immunoglobulin G was obtained from Dako Corporation (CA, USA). The Coomassie blue protein assay kit was purchased from Bio-Rad (USA). Polyclonal rabbit anti-mouse cyclooxygenase-1 antibody, immune-enzyme analysis PGE2 kit, arachidonic acid, prostaglandin B2, LTB4, 6-trans LTB4, 6 trans-12 epi LTB4, 5-hydroxyeicosatetraenoic acid (5-HETE) and 15-hydroxyeicosatetraenoic acid (15-HETE) were obtained from Cayman Chemical Company (Ann Arbor, MI, USA). HPLC-grade methanol was supplied by Selby-Biolab (Clayton, Victoria, Australia). All other analytical reagents were obtained from Merck Pty Ltd (Kilsyth, Victoria, Australia).
Histamine release from rat peritoneal mast cells
Rat peritoneal mast cells were collected in Tyrode buffer as previously described [11]. Briefly, rats (Sprague-Dawley, 200 – 300 g) of either sex were killed and 10 mL of Tyrode buffer (NaCl, 137 mM; KCl, 2.7 mM; HEPES, 10 mM; MgCl2, 1 mM; CaCl2, 1.0 mM; NaH2PO4, 0.41 mM), containing 0.3% BSA and 5 units/mL heparin was injected into the peritoneal cavity. The abdomen was gently massaged for about 90 seconds, and then carefully opened and the cell-containing peritoneal fluid collected with a transfer pipette. The cell-containing fluid was centrifuged at 4°C at 800 rpm for 5 minutes. The cells were collected, washed in 10 mL of Tyrode buffer and centrifuged again. This procedure was repeated twice [11]. The cells were then suspended in the concentration of 1 × 106 cells/mL in 10 mM HEPES-Tyrode buffer (pH 7.4) containing 0.1% BSA.
The 200 mL peritoneal cell suspensions were incubated with various concentrations of RCM-101 for 10 minutes at 37°C and then exposed to compound 48/80 for 10 minutes. Aliquots of 100 μL of rat peritoneal mast cells in Tyrode buffer were combined with 100 μL aliquots of RCM-101 extract in Tyrode buffer such that 5 × 105 cells/mL were incubated with RCM-101 at concentrations of 1, 10 and 100 μg/mL for 10 minutes immediately prior to stimulation of the cells with compound 48/80. The cell suspensions were then centrifuged at 4°C at 4800 rpm and the supernatant collected. As an internal standard, 10 μL of spermidine (1 mg/mL) was added to 200 mL aliquots of the supernatant, followed by 20 μL of 30% perchloric acid (HCIO4). The mixture was then filtered and 100 μL was transferred into HPLC vials for histamine determination [11]. The Ca2+ chelating agent, EDTA, (100 μM) was used as a positive control.
The HPLC system (Shimadzu, Kyoto, Japan), which included a fluorescent detector (Shimadzu RF10XL), C-10ATvp pumps, SIL-10ADvp auto-injector and STRODS-II reversed phase column, equipped with post-column derivatisation was set up as previously described [11]. Samples of the peritoneal cell supernatant/internal standard solution were injected into the HPLC system using an autosampler. Histamine and spermidine were detected with excitation and emission wavelengths of 360 nm and 440 nm, respectively. Four-point standard curves for histamine were prepared, ranging from 50 – 2500 ng/mL in 10 mM HEPES-Tyrode buffer.
Leukotriene B4 production
Synthesis of LTB4 was induced in neutrophils as previously described [15], with slight modification. Porcine blood was collected from a local abattoir. Neutrophils were isolated using a Percoll gradient and suspended in Hanks' buffer, containing 5 mM HEPES. Suspended neutrophils (2.8 × 106 cells/mL) were incubated (37°C) with RCM-101 extract, NDGA (as a positive control, 0.1, 1 or 10 μg/mL) or vehicle (ethanol), for 5 minutes before the addition of arachidonic acid (2.5 μM) substrate. Porcine neutrophils were suspended in Hanks' buffer in concentration of 2.8 × 106 cells/mL. RCM-101 (0.1, 1, 100 μg/mL) was added 10 minutes before the calcium ionophore A23187 (2.5 μM). After 5 minute incubation, production of LTB4 was initiated by the addition of the calcium ionophore A23187 (2.5 μM) and 5 minutes later the reaction was terminated by adjusting the pH to 3 with citric acid. PGB2 (45 ng) and 15-HETE (83 ng) were then added as internal standards. The reaction mixture was extracted with 5 mL of chloroform/methanol (7:3 v/v) and dried under vacuum. The residue was dissolved in 120 μL of HPLC mobile phase (methanol-water-acetic acid, 76/34/0.08, v/v/v, pH 3.0) and leukotriene metabolites were assayed using a Waters HPLC system equipped with an auto sampler, a multi-solvent delivery system and a Waters 996 Photodiode Array Detector. Standard curves were prepared by the addition of LTB4 (10 – 200 ng) and 5-HETE (500 – 800 ng) to neutrophil suspensions. Data were analysed using Water Millenium Software, Version 3.2, results being expressed as percentage of the vehicle control which was taken as 100%.
Prostaglandin E2 production
Murine macrophages (Raw 264.7 cells, American Type Culture Collection, Rockville, MD, USA) were grown in RPMI 1640 medium, supplemented with 10% heat-inactivated FBS, 100 μg/mL gentamycin, 1.5 g/L sodium bicarbonate and 10 mM HEPES, at 37°C, in an atmosphere containing 5% CO2. Cells were sub-cultured once a week by harvesting them with trypsin/EDTA and seeding them in 75 cm2 flasks. Once confluent, murine macrophages were suspended in serum-free RPMI medium at concentration of 2 × 105 cells/mL, and the cells were seeded in 24-well plates (1 × 105 cells/well), in serum-free RPMI medium. Cells were then treated with RCM-101 (1, 10, or 100 μg/mL) or vehicle 10 minutes before the addition of LPS (1 μg/mL). The supernatant and the cells were separated. PGE2 was assayed in the supernatant using an immune-enzyme analysis kit. The assay depends on competition between PGE2 and PGE2acetylcholinesterase conjugate (PGE2-tracer) for a limited amount of monoclonal PGE2antibody. The assays were carried out according to the manufacturer's protocol, in triplicate. PGE2 release was calculated using software supplied by the kit manufacturer.
Determination of COX-1 and COX-2 protein expression in Raw 264.7 cells
Cultured Raw 264.7 cells prepared as described above for determination of PGE2 production, with and without incubation with RCM-101, were washed twice with ice-cold phosphate buffer saline then lysed with 100 μL/well of lysis buffer (50 mM Tris base, pH 7.6, 2 mM MgCl2, 1 mM EGTA, 1% TritonX, 1 mM phenyl PMSF, 1 mM pepstatin, 1 mM aprotinin, 1 mM leupeptin) for 5 minutes. The cells and the supernatant were collected and centrifuged for 5 minutes at 14000 rpm. The cell debris was discarded and the supernatant was assayed for protein concentration using Coomassie Protein Assay Kit (Bio-Rad Laboratories Pty Ltd, California, USA) and the UV-visible spectrophotometer (Cintra 5, GBC Scientific Equipment Pty Ltd, Illinois, USA)
COX-1 and COX-2 protein was measured by Western blotting as previously described [16] with a slight modification. Aliquots of 20 μg of total protein were loaded to each lane of 7.5% SDS-polyacrylamide gels. The proteins were then electrically transferred to nitrocellulose membranes which were incubated overnight with a polyclonal anti-rabbit COX-1 antibody or a monoclonal mouse anti-rat COX-2 antibody (diluted 1:500 and 1:2500 respectively) in 5% non-fat milk in Tris-buffered saline (Tris base 25 mM, glycine 19 mM, methanol 20%). On the next day, membranes were washed with Tris-buffered saline (Tris base 20 mM, NaCl 137 mM, Tween-20 0.1%, pH 7.5) for 40 minutes with constant agitation, during which time the buffer was changed every 5 minutes. The membranes were then incubated with swine anti-rabbit or goat anti-mouse secondary conjugated to horseradish peroxidase diluted 1:5000 with blocking buffer (Tris base 20 mM, NaCl 137 mM, Tween-20 0.1%, pH 7.5 and 5% non-fat milk). The results were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Statistical analysis
Data are expressed as means ± standard deviation (SD). The statistical significance of differences between means was determined by unpaired, two-tailed Student's t-test or, for more than two groups, by first testing for global differences by one- or two-way analysis of variance (ANOVA) and then testing for differences between predetermined pairs of means by Dunnet's test. The differences with probability levels less than 0.05 (P < 0.05) were considered to be statistically significant.
Results
Inhibition of compound 48/80-induced histamine release from rat peritoneal mast cells
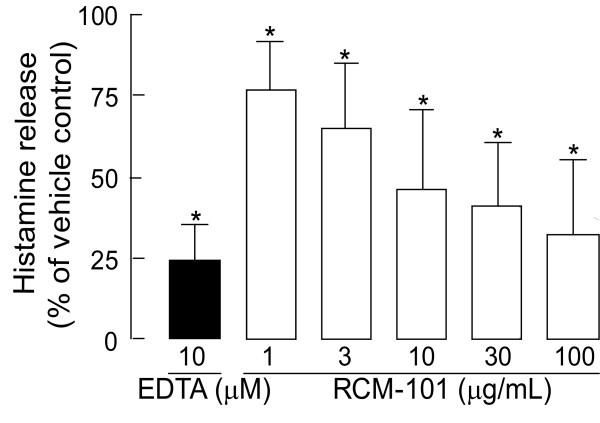
The amount of histamine released from rat unstimulated peritoneal mast cell preparations was 46.3 ± 37.1 ng/mL (n = 14). When the cells were stimulated with compound 48/80 (1 μg/mL) histamine release increased markedly to 638.3 ± 308 ng/mL (n = 14). As shown in Figure 1, compound 48/80-induced histamine release was inhibited by RCM-101 (1 – 100 μg/mL) in a concentration dependent manner. Compound 48/80-induced histamine release was also reduced by 10μM EDTA (Figure 1).
Figure 1.
Inhibition of compound 48/80-stimulated histamine release from rat peritoneal mast cells by RCM-101. EDTA was used as a positive control. Data are plotted as means ± SD, (n = 8). *P < 0.05, One-way ANOVA and Dunnet's test
Inhibition of leukotriene B4 production in porcine neutrophils
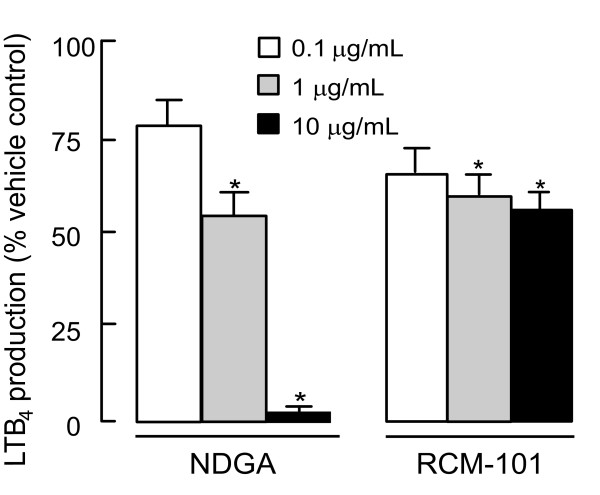
In the absence of calcium ionophore A23187 incubation, the production of LTB4 by porcine neutrophils was 9.58 ± 4.6 ng/mL. In vehicle (ethanol) control experiments, A23187 incubation increased LTB4 production to 167.77 ± 70.4 ng/mL (n = 8).
As shown in Figure 2, LTB4 production in A23187-incubated neutrophils was inhibited by RCM-101 at concentrations of 1 and 10 μg/mL. NDGA (1 and 10 μg/mL) also inhibited A23187-induced LTB4 production.
Figure 2.
Inhibition of LTB4 formation in porcine neutrophils by RCM-101 and nordihydroguaiaretic acid (NDGA). Data are plotted as means ± SD, (n = 8 in each case). *P < 0.05, One-way ANOVA and Dunnet's test
Inhibition of prostaglandin E2 production in LPS-stimulated Raw 264.7 cells
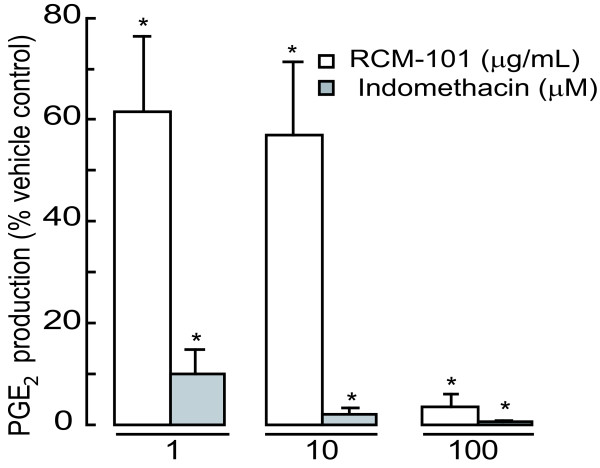
Unstimulated Raw 267.4 cells incubated in serum-free RPMI medium for 24 hours produced a baseline concentration of PGE2 of 52 ± 24.4 pg/mL (n = 6). Incubating the cells with LPS (1 μg/mL) increased the PGE2 level to 3874 ± 818.13 pg/mL (n = 6). This induced production of PGE2 was reduced in a concentration-dependent manner by RCM-101 (1 – 100 μg/mL), when present during incubation with LPS. Indomethacin (1, 10 and 100 μM), when present during LPS incubation, completely blocked PGE2 production. The data are shown in Figure 3.
Figure 3.
Inhibition of PGE2 production in LPS-stimulated Raw 264.7 cells by RCM-101 and indomethacin. Data are plotted as means ± SD of percentage control PGE2 production (n = 6 in each case). *P < 0.05, One-way ANOVA and Dunnet's test
Effects of RCM-101 on COX-1 and COX-2 protein expression in LPS-stimulated Raw 267.4 cells
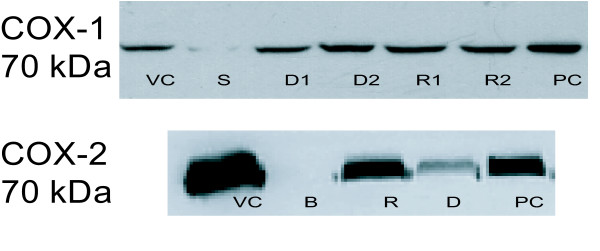
As shown in Figure 4, immunoreactivity bands corresponding to COX-1 and COX-2 (70 kDa) were detected by Western blot analysis of the supernatant of lysed Raw 264.7 cells. Densiometeric analysis of the marker chemiluminescence indicated that expression of COX-1 protein was unaffected by RCM-101 (10 and 100 μg/mL). Similarly, dexamethasone (10 and 100 μM) also did not alter COX-1 protein expression. In contrast, the expression of COX-2 protein was significantly (P < 0.05, one-way ANOVA, Dunnet's test) reduced by 100 μg/mL RCM-101 and also by 100 μM dexamethasone. Figure 4 shows examples of the visualised bands on nitrocellulose membranes corresponding to COX-1 and COX-2 proteins.
Figure 4.
Western blot assay of COX-1 and COX-2 expression by macrophage (Raw 264.7. 20) cells after LPS stimulation. For each lane of SDS polyacrylamide gels, 20 μg of protein was loaded. COX-1 and COX-2. The proteins were detected on nitrocellulose membranes using specific antibodies and visualized by enhanced chemiluminescence. (A) COX-1: VC = vehicle control; S = separation lane (not loaded with protein); D1 = dexamethasone (100 μM); D2 = dexamethasone (10 μM); R1 = RCM-101 (100 μg/mL); R2 = RCM-101 (10 μg/mL); PC = positive control (COX-1 lysate). (B) COX-2: VC = vehicle control; B = cells not stimulated with LPS; R = RCM-101 (100 μg/mL); D = dexamethasone (100 μM); PC = positive control (COX-2 lysate).
Discussion
This study was undertaken to extend our previous investigation of possible pharmacological mechanisms for the effects of the herbal formula RCM-101 in reducing SAR symptoms [10]. The main findings of the present study are that RCM-101 inhibits compound 48/80-induced release of histamine from isolated rat peritoneal mast cells and inhibits the production of LTB4 by porcine neutrophils and of PGE2 by Raw 264.7 cells. Histamine, PGE2 and LTB4 are well known mediators of inflammatory/allergic responses. Taken together with our previous findings in isolated tissues from rats and guinea-pigs, it seems that RCM-101, a herbal formula with 18 constituent Chinese herbs, has activity directed to inhibition of the synthesis or release of multiple key inflammatory mediators.
Mast cell-derived mediators, particularly histamine are considered to be responsible for the acute (early stage) allergic symptoms of SAR [17]. These mediators act on the smooth muscle cells of small blood vessels, blood platelets, mucous glands and sensory nerve endings to produce or contribute to symptoms such as nasal congestion, nasal and throat itching, sneezing and hypersecretion of mucus [18]. The release of mast cell-derived histamine is inhibited by RCM-101. While the inhibition mechanisms are not clear, RCM-101 has been shown to contain several herbal ingredients that inhibit the release or action of histamine. For example, Rhizoma Cimicifugae was reported to exert a potent inhibitory action on histamine-mediated contractions in guinea pig ileum [19] and Flos Magnoliae inhibits mast cell-mediated allergic reactions by preventing mast cell degranulation and IgE-mediated histamine release [20]. Herba Schizonepetae was also reported to reduce compound 48/80-induced histamine release [21]. Moreover, the Chinese herbal formula Xiao Chai Hu Tang, which contains several of the herbal ingredients of RCM-101, has also been shown to inhibit histamine release from rat peritoneal mast cells [11].
Limited information is available about the chemical constituents of the herbs in RCM-101 responsible for inhibition of the release or action of histamine or their action mechanisms. However, glycyrrhetinic acid, which is present in Radix Glycyrrhizae, a herbal component of RCM-101, was shown to inhibit the release of histamine by targeting protein kinase C-β (nPKC β) [22]. Conjugated linoleic acid, identified in Rhizoma Chuanxiong, another herbal component of the formula, is known to inhibit immediate anaphylaxis, histamine release and the synthesis of arachidonic acid metabolites [23].
Prostaglandins and leukotrienes were found to be involved in the pathophysiology of SAR [24] and LTB4 is released by infiltrating neutrophils during the immediate phase of allergic responses [25]. The present study found that RCM-101 inhibits the production/release of LTB4 induced by the calcium ionophore A23187 in porcine neutrophils. Previous studies showed that extracts of the herb Radix Glycyrrhizae inhibit A23187-induced release of arachidonic acid from cell membranes by inhibiting phospolipaseA2 and that they also inhibit 5-lipoxygenase, acting together to suppress the production of LTC4 and LTB4 [26]. Glycyrrhetinic acid and caffeic acid, present in Radix Glycyrrhizae, Herba Menthae, Rhizoma Ligusticum Chuanxiong and Rhizoma Cimicifugae [27], both were shown to inhibit arachidonic metabolite formation [28,29]. These findings suggest a possible action of RCM-101 on SAR through the inhibition of the release of LTB4.
PGE2 is released in both the early phase (from mast cells) and late phase (from basophils and eosinophils) responses of SAR [17]. We found that LPS-induced production of PGE2 by murine macrophages was inhibited by RCM-101. The findings are consistent with previous studies on individual herbal components of RCM-101. There is also evidence indicating that Radix Glycyrrhizae inhibits PGE2 production in rats tissues and Rhizoma Cimicifugae blocks LPS-induced production of PGE2 [19]. In addition, both topical and oral administration glycyrrhetinic acid was reported to prevent ear oedema and to inhibit PGE2 and LTC4 formation induced by arachidonic acid in mice [30]. These findings suggest a possible action of RCM-101 on SAR through the inhibition of PGE2 production.
The inhibition of prostaglandin production by RCM-101 is most likely due to inhibition of COX-2 protein expression, because we observed that COX-2 protein expression was markedly reduced by RCM-101 whereas the expression of COX-1 protein was unaffected. It is known that COX-2 is responsible for prostaglandin production in Raw 264.7 cells [14]. Previous studies also observed that Rhizoma Cimicifugae and Radix Glycyrrhizae inhibited COX-2 activity [26].
Conclusion
The results obtained in this study indicate that RCM-101 has inhibitory actions on multiple inflammatory mediators, including the release of histamine from mast cells, and production of LTB4 and PGE2 by neutrophils and Raw 264.7 cells, respectively. In addition, RCM-101 also selectively inhibits the expression of the inducible enzyme COX-2. These actions of RCM-101 may contribute to its efficacy in SAR. The exact mechanisms of these actions and the contributions by individual herbal ingredients of RCM-101 require further investigation.
Abbreviations
5-HETE: 5-hydroxyeicosatetraenoic acid
15-HETE: 15-hydroxyeicosatetraenoic acid
ANOVA: Analysis of variance
BSA: Bovine serum albumin
COX-1: Cyclooxygenase-1
COX-2: Cyclooxygenase-2
EDTA: Disodium ethylenediaminetetraacetic acid
EGTA: Ethylene glycol tetraacetic acid
HPLC: High performance liquid chromatography
IgE: Immunoglobulin E
LPS: Lipopolysaccharide
LTB4: Leukotriene B4
LTC4: Leukotriene C4
NDGA: Nordihydroguaiaretic acid
PGE2: Prostaglandin E2
PMSF: Phenylmethanesulphonylfluoride
SAR: Seasonal allergic rhinitis
SD: Standard deviation
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
GBL conducted the experiments, contributed to the interpretation of the findings and preparation of the manuscript. CCLX contributed to the design of the study, the interpretation of the findings and preparation of the manuscript. DFS contributed to the interpretation of the findings and critically revised the manuscript. FCKT contributed to the design of the study and preparation of the manuscript. SM conducted some of the experiments, contributed to the interpretation of the findings and preparation of the manuscript. CGL contributed to the design and conduct of the study, the interpretation of the findings and preparation of the manuscript. All authors approved the final manuscript.
Acknowledgments
Acknowledgements
The authors would like to thank Prof Theodore Macrides, The Natural Products Group, School of Medical Sciences, RMIT University for his useful discussion. We also acknowledge RMIT University for providing a Postgraduate Research Scholarship for GBL.
Contributor Information
George B Lenon, Email: george.lenon@rmit.edu.au.
Charlie CL Xue, Email: charlie.xue@rmit.edu.au.
David F Story, Email: david.story@rmit.edu.au.
Frank CK Thien, Email: frank.thien@med.monash.edu.au.
Sarah McPhee, Email: smcphee@wdg.com.au.
Chun G Li, Email: chun.guang.li@rmit.edu.au.
References
- Bousquet J, Vignola AM, Demoly P. Links between rhinitis and asthma. Allergy. 2003;58:691–706. doi: 10.1034/j.1398-9995.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Bachert C, Bousquet J, Canonica GW, Durham SR, Klimek L, Mullol J, Van Cauwenberge PB, Van Hammee G. Levocetirizine improves quality of life and reduces costs in long-term management of persistent allergic rhinitis. J Allergy Clin Immunol. 2004;114:838–844. doi: 10.1016/j.jaci.2004.05.070. [DOI] [PubMed] [Google Scholar]
- Senti G, Johansen P, Martinez Gomez J, Prinz Varicka BM, Kundig TM. Efficacy and safety of allergen-specific immunotherapy in rhinitis, rhinoconjunctivitis, and bee/wasp venom allergies. Int Rev Immunol. 2005;24:519–531. doi: 10.1080/08830180500370944. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics . National Health Survey: Summary of Results, 2004-05, Cat no 4364.0. Canberra , ABS; 2006. [Google Scholar]
- Baraniuk JN. Pathogenesis of allergic rhinitis. J Allergy Clin Immunol. 1997;99:S763–72. doi: 10.1016/S0091-6749(97)70125-8. [DOI] [PubMed] [Google Scholar]
- Davies RJ, Bagnall AC, McCabe RN, Calderon MA, Wang JH. Antihistamines: topical vs oral administration. Clin Exp Allergy. 1996;26:11–17. doi: 10.1111/j.1365-2222.1996.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Salib RJ, Howarth PH. Safety and tolerability profiles of intranasal antihistamines and intranasal corticosteroids in the treatment of allergic rhinitis. Drug Saf. 2003;26:863–893. doi: 10.2165/00002018-200326120-00003. [DOI] [PubMed] [Google Scholar]
- Passalacqua G, Bousquet PJ, Carlsen KH, Kemp J, Lockey RF, Niggemann B, Pawankar R, Price D, Bousquet J. ARIA update: I--Systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol. 2006;117:1054–1062. doi: 10.1016/j.jaci.2005.12.1308. [DOI] [PubMed] [Google Scholar]
- Xue CC, Thien FC, Zhang JJ, Da Costa C, Li CG. Treatment for seasonal allergic rhinitis by Chinese herbal medicine: a randomized placebo controlled trial. Altern Ther Health Med. 2003;9:80–87. [PubMed] [Google Scholar]
- Lenon GB, Li CG, Xue CCL, Thien FCK, Story DF. Inhibition of Release of Vasoactive and Inflammatory Mediators in Airway and Vascular Tissues and Macrophages By a Chinese Herbal Medicine Formula for Allergic Rhinitis . Evid Based Complement Alternat Med. 2006. doi: 10.1093/ecam/ne1083. [DOI] [PMC free article] [PubMed]
- Ikarashi Y, Yuzurihara M, Sakakibara I, Takahashi A, Ishimaru H, Maruyama Y. Effects of an oriental herbal medicine, "Saiboku-to", and its constituent herbs on Compound 48/80-induced histamine release from peritoneal mast cells in rats. Phytomedicine. 2001;8:8–15. doi: 10.1078/0944-7113-00012. [DOI] [PubMed] [Google Scholar]
- Provost P, Borgeat P, Merhi Y. Platelets, neutrophils, and vasoconstriction after arterial injury by angioplasty in pigs: effects of MK-886, a leukotriene biosynthesis inhibitor. Br J Pharmacol. 1998;123:251–258. doi: 10.1038/sj.bjp.0701611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BY, Lee JH, Koo TH, Kim HS, Hong YS, Ro JS, Lee KS, Lee JJ. Furanoligularenone, an Eremophilane from Ligularia fischeri, Inhibits the LPS-Induced Production of Nitric Oxide and Prostaglandin E2 in Macrophage RAW264.7 Cells. Planta Med. 2002;68:101–105. doi: 10.1055/s-2002-20250. [DOI] [PubMed] [Google Scholar]
- Shin KM, Kim IT, Park YM, Ha J, Choi JW, Park HJ, Lee YS, Lee KT. Anti-inflammatory effect of caffeic acid methyl ester and its mode of action through the inhibition of prostaglandin E2, nitric oxide and tumor necrosis factor-alpha production. Biochem Pharmacol. 2004;68:2327–2336. doi: 10.1016/j.bcp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Betts WH, Hurst NP, Murphy GA, Cleland LG. Auranofin stimulates LTA hydrolase and inhibits 5-lipoxygenase/LTA synthase activity of isolated human neutrophils. Biochem Pharmacol. 1990;39:1233–1237. doi: 10.1016/0006-2952(90)90268-P. [DOI] [PubMed] [Google Scholar]
- Seo WG, Pae HO, Oh GS, Chai KY, Kwon TO, Yun YG, Kim NY, Chung HT. Inhibitory effects of methanol extract of Cyperus rotundus rhizomes on nitric oxide and superoxide productions by murine macrophage cell line, RAW 264.7 cells. J Ethnopharmacol. 2001;76:59–64. doi: 10.1016/S0378-8741(01)00221-5. [DOI] [PubMed] [Google Scholar]
- Naclerio RM. Allergic rhinitis. N Engl J Med. 1991;325:860–869. doi: 10.1056/NEJM199109193251206. [DOI] [PubMed] [Google Scholar]
- Kuby J. Immunology. 2nd. New York , W.H. Freemen and company; 1994. [Google Scholar]
- Kim SJ, Kim MS. Inhibitory effects of cimicifugae rhizoma extracts on histamine, bradykinin and COX-2 mediated inflammatory actions. Phytother Res. 2000;14:596–600. doi: 10.1002/1099-1573(200012)14:8<596::AID-PTR731>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Kim HM, Yi JM, Lim KS. Magnoliae flos inhibits mast cell-dependent immediate-type allergic reactions. Pharmacol Res. 1999;39:107–111. doi: 10.1006/phrs.1998.0414. [DOI] [PubMed] [Google Scholar]
- Fung D, Lau CB. Schizonepeta tenuifolia: chemistry, pharmacology, and clinical applications. J Clin Pharmacol. 2002;42:30–36. doi: 10.1177/0091270002042001003. [DOI] [PubMed] [Google Scholar]
- Lee YM, Hirota S, Jippo-Kanemoto T, Kim HR, Shin TY, Yeom Y, Lee KK, Kitamura Y, Nomura S, Kim HM. Inhibition of histamine synthesis by glycyrrhetinic acid in mast cells cocultured with Swiss 3T3 fibroblasts. Int Arch Allergy Immunol. 1996;110:272–277. doi: 10.1159/000237298. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Oku H, Suitani A, Yamamoto Y. Effects of conjugated linoleic acid on anaphylaxis and allergic pruritus. Biol Pharm Bull. 2002;25:1655–1657. doi: 10.1248/bpb.25.1655. [DOI] [PubMed] [Google Scholar]
- Azevedo M, Castel-Branco MG, Oliveira JF, Ramos E, Delgado L, Almeida J. Double-blind comparison of levocabastine eye drops with sodium cromoglycate and placebo in the treatment of seasonal allergic conjunctivitis. Clin Exp Allergy. 1991;21:689–694. doi: 10.1111/j.1365-2222.1991.tb03197.x. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Vignola AM, Campbell AM, Michel FB. Pathophysiology of allergic rhinitis. Int Arch Allergy Immunol. 1996;110:207–218. doi: 10.1159/000237289. [DOI] [PubMed] [Google Scholar]
- Hamasaki Y, Kobayashi I, Hayasaki R, Zaitu M, Muro E, Yamamoto S, Ichimaru T, Miyazaki S. The Chinese herbal medicine, shinpi-to, inhibits IgE-mediated leukotriene synthesis in rat basophilic leukemia-2H3 cells. J Ethnopharmacol. 1997;56:123–131. doi: 10.1016/S0378-8741(97)01520-1. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yan X, Xie G. In: Traditional Chinese Medicine: Molecular Structures, Natural Sources and Application. Milne GWA, editor. Hampshire , Ashgate; 2003. [Google Scholar]
- Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim Biophys Acta. 1984;792:92–97. [PubMed] [Google Scholar]
- Inoue H, Saito H, Koshihara Y, Murota S. Inhibitory effect of glycyrrhetinic acid derivatives on lipoxygenase and prostaglandin synthetase. Chem Pharm Bull (Tokyo) 1986;34:897–901. doi: 10.1248/cpb.34.897. [DOI] [PubMed] [Google Scholar]
- Inoue H, Mori T, Shibata S, Koshihara Y. Inhibitory effect of glycyrrhetinic acid derivatives on arachidonic acid-induced mouse ear oedema. J Pharm Pharmacol. 1988;40:272–277. doi: 10.1111/j.2042-7158.1988.tb05242.x. [DOI] [PubMed] [Google Scholar]