Abstract
The Drosophila auditory system is presented as a powerful new genetic model system for understanding the molecular aspects of development and physiology of hearing organs. The fly’s ear resides in the antenna, with Johnston’s organ serving as the mechanoreceptor. New approaches using electrophysiology and laser vibrometry have provided useful tools to apply to the study of mutations that disrupt hearing. The fundamental developmental processes that generate the peripheral nervous system are fairly well understood, although specific variations of these processes for chordotonal organs (CHO) and especially for Johnston’s organ require more scrutiny. In contrast, even the fundamental physiologic workings of mechanosensitive systems are still poorly understood, but rapid recent progress is beginning to shed light. The identification and analysis of mutations that affect auditory function are summarized here, and prospects for the role of the Drosophila auditory system in understanding both insect and vertebrate hearing are discussed.
Keywords: chordotonal organ, Johnston’s organ, sensory cilia, axoneme, mechanotransduction
INTRODUCTION
Research on the molecular mechanisms underlying auditory function has rapidly accelerated in the last decade. Much recent progress has come in the form of identifying genes responsible for hereditary deafness in humans and in vertebrate model systems. In parallel, our understanding of molecular mechanisms of touch in invertebrate model organisms such as Caenorhabditis elegans and Drosophila melanogaster have also advanced remarkably. Here we will present recent work on the auditory system of D. melanogaster, which we believe is an excellent model system by which to understand how auditory mechanosensation works. Despite the obvious morphologic differences in mechanical operations of the ears in flies compared to vertebrates, it is becoming clearer that the sensory cells may have common ancient developmental genetic origins. We shall discuss the shared developmental components of hearing such as the atonal gene and its vertebrate homologs. If the development of these auditory receptors have a common origin, then the molecular mechanisms involved in their function are likely to be at least partially conserved as well. We will later discuss the extent to which this is known to be true.
Drosophila as a Genetic Model Organism for Hearing Research
The criteria for a good genetic model organism to study molecular mechanisms are that (1) it should have stereotypic behavioral or electrophysiologic responses evoked by acoustic stimuli, (2) it should be easy to manipulate genetically to identify mutants and characterize the relevant genes, and (3) it should show conservation of molecular and cellular mechanisms with human hearing. We believe that Drosophila meets this suite of criteria well.
We have developed a reliable electrophysiologic assay for recording sound-evoked potentials directly from the antenna, allowing direct insight into studying audition in Drosophila (Eberl et al., 2000). The availability of sophisticated genetic manipulations in flies, in addition to their simplified nervous system and acoustic and mechanosensory transducing structures, present a compelling opportunity to dissect the roles of genes required for hearing. Furthermore, Drosophila may provide an ideal medium in which to elucidate the roles of genes responsible for human syndromic and nonsyndromic deafness. Although the basic developmental and molecular mechanisms of sense organs are evolutionarily conserved in mammals and flies and, often, the Drosophila homologs of various human genes exhibit similar morphologic and physiologic defects, the evolutionary distance between flies and humans is vast. This disadvantage, we believe, is more than compensated for by the strength of the genetic tools available in Drosophila and the powers of manipulation they confer not only in identifying components but also in elucidating their biologic functions. In fact, the differences may be as illuminating as the similarities, as has been found for eye development and physiology (Ranganathan et al., 1995; Gehring and Ikeo, 1999).
How the Antenna Works as an Ear: Courtship, Hearing, and Acoustic Physics
The antenna of Drosophila is an asymmetric, flagellar structure composed of three segments [a1 (antennal segment) or scape, a2 or pedicelus, and a3 or funiculus] and a feathery arista extending from the distal-most segment (Fig. 1). The arista resonates in the presence of the species-specific courtship song and twists a3 relative to a2. The feather-like arista (antennal segments 4, 5, and 6) extends anterolaterally from a3 and slightly downward (Fig. 1). The arista is innervated with three sensilla; these are not physiologically involved in hearing, but rather likely in thermosensation (Foelix et al., 1989). For hearing, the arista is a component of the mechanical operation of the antenna.
Figure 1.
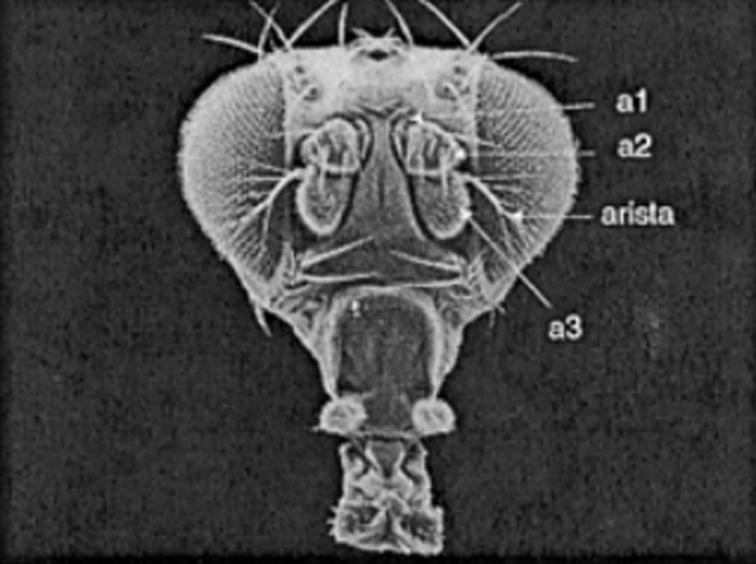
Scanning electron micrograph of a Drosophila head, with the three large proximal segments and arista of one antenna labeled. The arista is set in motion by near-field sound and rotates a3 relative to a2. a2 houses the mechanoreceptive JO that transduces the vibrations. The prelabeled image was generated by F. Rudy Turner, Indiana University, and is used here with his permission. It was downloaded from Flybase (http://flybase.bio.indiana.edu).
The only known acoustic stimulus to which Drosophila responds is the courtship song, produced by the courting male. The D. melanogaster species-specific “love song” is composed of pulse and sine components. The sine song is thought to “woo” females prior to courtship (von Schilcher, 1976a; Tauber and Eberl, 2001) and is, on average, a 160-Hz sinusoidal sound wave, although there is considerable variation between males (Wheeler et al., 1988). The pulse song is composed of trains of pulses with characteristic 30-to 45-ms interpulse intervals between 5-to 10-ms pulses. The interpulse interval oscillates rhythmically with a period of 50–65 s. Interestingly, this has been described as the most relevant song feature in increasing female receptivity and stimulating both partners in the courtship to expedite copulation (e.g., Kyriacou and Hall, 1982; Alt et al., 1998; Ritchie et al., 1999). Sound intensity is expressed in a simple relationship of the product of pressure and particle velocity. The male stays less than 5 mm away from the female during courtship, less than the wavelength of the sound being produced (Bennet-Clark, 1971). At this distance, the acoustic energy is almost entirely in particle velocity rather than in pressure. When the male is positioned within one wavelength of the female, energy dissipation is low and the near-field amplitude is 80–95 dB (Bennet-Clark, 1971). In Drosophila, therefore, it is advantageous to use a displacement receptor like the arista rather than a pressure receptor (Bennet-Clark, 1971). Interestingly, the male also detects, and responds to the courtship song (von Schilcher, 1976b; Crossley et al., 1995). We have exploited this feature in the design of a mutagenesis screen for deaf mutants (Eberl et al., 1997, discussed later). The roles of courtship stimuli for male courtship are poorly understood; they may be auto-stimulatory or they may be important in competitive situations (Tauber and Eberl, 2002).
The arista and a3 together are the fly’s sound receivers that oscillate sympathetically when stimulated acoustically (Eberl et al., 2000). Göpfert and Robert (2001b, 2002) performed laser vibrometry to analyze the biomechanics of the Drosophila antenna. Measurements of oscillations taken from different locations along the arista and a3 indicate that the arista and a3 vibrate together as a stiff unit and rotate about the longitudinal axis of a3; however, a2 and the head capsule remain stationary. Thus, the major articulation is at the a2/3 joint (Fig. 2). This articulation causes stretching of the sound transducer, the Johnston’s Organ (JO). The JO is a sense organ that mediates hearing in a2 (Johnston, 1855; Eberl et al., 2000) and is a cluster of about 200 scolopidia (Fig. 2), which are the functional units of chordotonal organs (CHOs).
Figure 2.
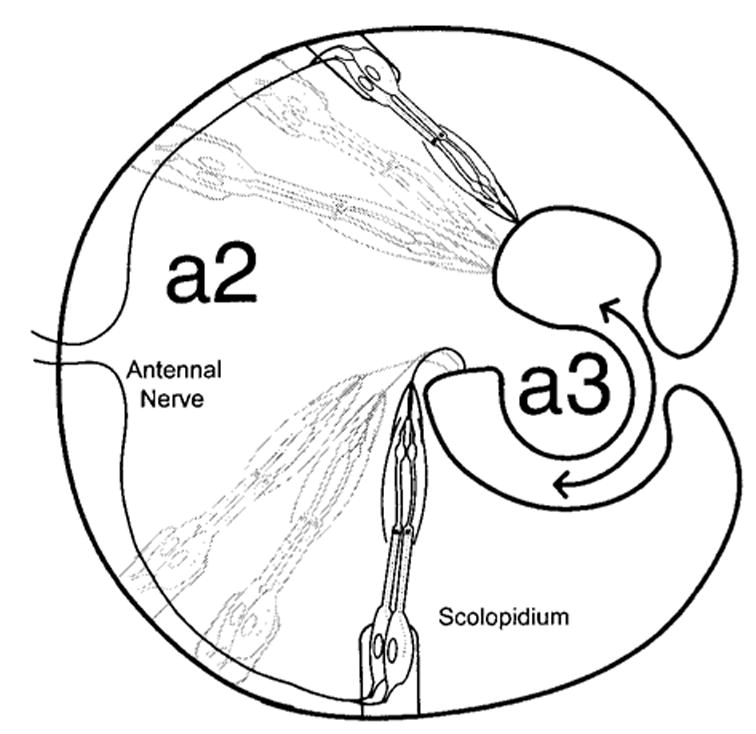
Drawing of a horizontal section through the second antennal segment (a2), showing the stalk of a3 and the arrangement of several representative scolopidia out of the 150–200 total in JO. Rotation (double-headed arrow) of a3 in one direction stretches all scolopidia, while rotation in the other direction relaxes them. Axons of the CHO neurons join the antennal nerve to enter the brain (shown for bold scolopidia only).
A useful feature of the antenna, as an asymmetric sound receiver that represents a moderately damped simple harmonic oscillator when presented with sound, is that the resonance frequency of the arista and a3 increases with the sound intensity, permitting the fly to tune a large dynamic range of sound: acute hearing at low intensities and damped sensitivity at high intensities (Göpfert and Robert, 2002). This broad tuning ensures that antennal vibrations are detectable both at frequencies below antennal resonance (when elicited at close range) and when the distance of the courting male from the female’s receiver increases (Göpfert and Robert, 2002). The arista and a3 rotate relative to a2 and vibrate visibly when presented with acoustic stimulation (Bennet-Clark, 1971; Eberl et al., 2000; Göpfert and Robert, 2002). This vibration, in turn, stimulates the mechanosensitive scolopidia of JO in a2 (Fig. 2). Indeed, the JO scolopidia are arrayed in such a way as to easily detect acoustic vibrations in the more distal antennal segments and immobilization or loss of antennal segments drastically reduces sound-evoked potentials (Eberl, Tauber, and Kernan, unpublished observations).
The nonlinearity represented by the intensity-dependent frequency response of Drosophila antennal vibrations has minimal effects on tuning sharpness and sensitivity (Göpfert and Robert, 2002). Therefore, this nonlinearity is in the stiffness of the resonator. This is in contrast to that found in the vertebrate ear (which underlies the cochlear amplifier, see Dallos, 1996; Fettiplace et al., 2001) and the mosquito antenna (Göpfert and Robert, 2001a) where the nonlinearity is introduced by negative damping. Whether the Drosophila nonlinearity in the stiffness is mediated by active processes, such as power generated by the sensory organs, or by passive processes remains to be tested.
Anatomy of the Auditory Organ
The mechanoreceptive auditory organ of the Drosophila antenna is a large CHO in a2, comprising 150–200 sensory units (Figs. 2 and 3). The homologous CHO in the mosquito had been proposed as the auditory organ by Christopher Johnston (1855); hence, this CHO is referred to as Johnston’s organ (JO). Chordotonal sensory units, called scolopidia, are classified as type I sense organs: they have monodendritic, ciliated neurons associated with accessory cells. By comparison, type II sense organs are multidendritic, nonciliated neurons with no accessory cells. CHOs are not associated with external structures, in contrast to other type I sense organs such as bristle organs and campaniform sensilla that together are called external sensory (es) organs. Rather, CHOs act as stretch receptors with attachments at two points of cuticle, often at the joints of appendages. In adult flies, each scolopidium has two or three bipolar neurons and several accessory cells. The precise composition of accessory cells in JO has not yet been well defined, but likely includes cap cells, scolopale cells, and ligament cells similar to those in larval CHOs (Brewster and Bodmer, 1995). Cap cells are responsible for apical attachments of scolopidia, although there may also be accessory epidermal cells that secrete specialized cuticular elements for attachment (see next section on developmental aspects). Larval CHO ligament cells are responsible for basal attachments; the JO equivalents must perform a similar function. The scolopale cell wraps several times around the neuronal outer dendritic segment (the cilium) in “myelin sheath-like” layers that are joined by extensive septate junctions (Carlson et al., 1997). The scolo-pale cell also seals around the inner dendritic segment of the neurons with desmosomal junctions. All these junctions allow for a sealed extracellular receptor lymph space, the scolopale space, which serves as an “endolymph-like” ionic compartment that can drive receptor potentials. Finally, the scolopale cell also elaborates a prominent series of scolopale rods composed of thick bundles of filamentous actin. It is not yet clear whether these rods are fixed structural components or contribute to adaptation by adjusting their length.
Figure 3.
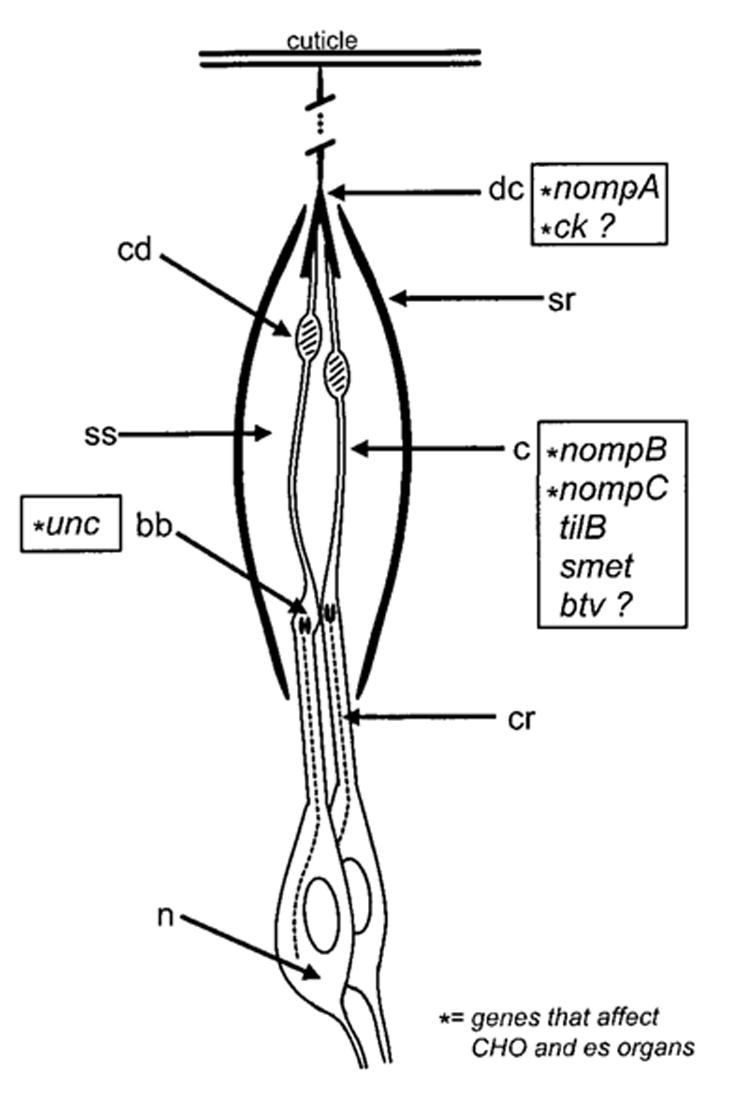
Drawing of the major elements of a JO scolopidium. Two, or in some cases, three neurons (n) are present, with a ciliary rootlet (cr) culminating in a basal body (bb) in the inner dendritic segment. From the basal body, the axoneme of the long cilium (c) is assembled, including the elaborate ciliary dilation (cd). Apically, the cilium attaches to the dendritic cap (dc), which allows connectivity to the vibrating cuticle. Around the cilium is a spindle-shaped cage of scolopale rods (sr), which are assembled by the scolopale cell (not shown) and which enclose the scolopale space (ss) containing a specialized receptor lymph. The known or putative locations of gene products or gene action are indicated by the gene symbols in boxes.
GENETICS AND DEVELOPMENT
Although the development of embryonic CHOs has been studied in exquisite detail (e.g., Brewster and Bodmer, 1995; Carlson et al., 1997; Merritt, 1997; Rusten et al., 2001), relatively few studies have focused on adult CHO development (e.g., Shanbhag et al., 1992; zur Lage and Jarman, 1999), and only a subset of these included JO (e.g., Jarman et al., 1995). In those few cases in which JO has been examined, the conclusions have usually been similar to embryonic CHO development. Thus, much of our understanding of JO development is by extrapolation from embryonic CHOs and, occasionally, from leg CHOs. The importance of this distinction is in the notion that JO is somehow a “specialized” CHO, specifically adapted for detecting vibratory stimuli. This notion is supported by the finding that the spalt genes appear to be uniquely required for JO development (see next section). Furthermore, we have identified enhancer trap transposon insertions in Drosophila, in which the reporter gene is expressed in JO but not in other CHOs (Sharma et al. 2002), suggesting that expression of some genes may be unique to JO.
In general, CHOs develop in the same way as other PNS elements. They arise from epithelia, cells of which are equipotent for CHO fates. A prepattern defined by genes that establish dorso-ventral, anterior–posterior and proximo-distal axes influences the positions at which clusters of cells acquire competence for CHO development. Competence of these proneural clusters is achieved by proneural gene expression; in the case of CHO development, atonal (ato) is the primary proneural gene. From each cluster, a single chordotonal organ precursor (COP) is selected by upregulation of the trans-membrane ligand, Delta (Dl). The Delta ligand binds to the Notch (N) receptor on the other cells in the cluster. N activation blocks proneural gene expression and the non-COPs lose their competence for chordotonal fate, via lateral inhibition. In the case of CHO clusters such as the leg CHO, COPs first recruit additional COPs in a reiterative fashion. The first-specified COPs delaminate from the epithelium and activate the rhomboid (rho) gene, which results in signaling through the epidermal growth factor receptor [EGFR; also called Drosophila EGF Receptor (DER)] on the N-activated non-COP cells of the cluster. EGFR activation mitigates N signaling and activates Dl and ato, generating additional COPs. Once COPs are specified, they undergo several asymmetric divisions to produce differentiated neuron(s) and the supporting cells of a mature scolopidium.
Proneural Genes
Proneural genes encode basic helix-loop-helix (bHLH) proteins that are transcriptional regulators. Two major PNS bHLH gene families in Drosophila are the four genes in the achaete–scute complex (AS-C) and the atonal group, which includes atonal (ato), absent multidendritic neurons and olfactory sensilla (amos), and cousin of atonal (cato). The AS-C genes specify not only neuroblasts of the central nervous system but also es organs of the PNS (reviewed by Skeath, 1999). ato is required for specification of CHOs, photoreceptor cells and a subset of olfactory sensilla (Jarman et al., 1995; Gupta and Rodrigues, 1997). amos specifies most olfactory organs (Goulding et al., 2000b). Type II PNS neurons, namely the multidendritic neurons, arise either from es organ lineages or CHO lineages (Brewster and Bodmer, 1995), with the exception of three that are specified by amos (Goulding et al., 2000b; Huang et al., 2000). amos appears not be expressed in COPs (Goulding et al., 2000b). Finally, cato is a bHLH gene required after the proneural stage for differentiation of neuron morphology in es organs and CHOs including in JO (Goulding et al., 2000a).
Mammals and Drosophila share many components of neurogenesis, patterning, specification of neural identity, and cell differentiation. The bHLH proneural gene, ato, is no exception. This molecule has been shown to specify COP cell fates from undifferentiated proneural patches (Jarman et al., 1993, 1995) and define CHO lineage identity (Jarman and Ahmed, 1998). ato directs epidermal specification of proneural patches into CHOs in the embryonic body wall and adult wings, legs, and antennae. In addition, ato has a nonproneural role in neurite arborization in three small clusters of neurons in the central brain (Hassan et al., 2000). ato mutant flies completely lack JO (Jarman et al., 1995; Eberl et al., 2000), and consequently, are completely deaf (Eberl et al., 2000). The closest mouse ato homolog, Math1, is 100% similar to ato in the basic region and 82% similar in the bHLH domain. Mouse Math1 is expressed in the auditory and vestibular sensory epithelia, and specifies the inner ear hair cells (Bermingham et al., 1999). The sensory epithelium of the vertebrate inner ear is composed of two major cell types, the hair cell and the support cell. Mice with a knock-out of Math1 lack hair cells because they are never specified. As seen with ato in Drosophila, Math1 also is expressed in other places. Math1 is expressed in Merkel cells, a type of proprioceptive mechanoreceptor, as well as in the external granule cell precursors of the cerebellum, which are involved in fine motor control and posture (Bermingham et al., 1999). In addition, Math1 is required for specification of the D1 interneurons that give rise to several precursors of the spinocerebellar and cuneocerebellar systems (Bermingham et al., 2001).
The molecular mechanisms of Math1 and ato seem similar for patterning hair cells and CHOs, respectively. Indeed, a Math1 transgene introduced into Drosophila can partially rescue ato mutant phenotypes (Ben-Arie et al., 2000). This provides support to the notion that insect CHOs and vertebrate hair cells are homologous organs (discussed by Eberl, 1999; Fritzsch et al., 2000). Nevertheless, Hassan and Bellen (2000) argue that these proteins direct different developmental pathways. As mentioned above, ato confers CHO competence and directs cell selection and lineage identity in the PNS, whereas Math1 specifies hair cells, but does not appear to mediate a classic proneural function. Specifically, Math1 expression is limited to postmitotic cells within the prosensory domain known as the zone of nonproliferating cells. These postmitotic cells give rise to hair cells (Chen et al., 2002). In Math1-deficient mice, this prosensory domain of the cochlear duct is present even though hair cell development is absent. Furthermore, a subpopulation of cells within the sensory epithelium undergoes a timed pattern of apoptosis in the absence of Math1, which resembles the pattern of hair cell formation seen in wild-type animals (Chen et al., 2002). Despite the disparity in the roles of ato and Math1 in proneural specification, the evidence that Math1 can provide some rescue to the Drosophila ato mutants, as well as the developmental similarities of sensory cell lineages between mouse and fly, provide powerful evidence that vertebrate inner ear hair cells and insect type I sense organs, in particular CHOs, are homologous and the differences reflect the evolutionary separation with different selective forces (Fritzsch et al., 2000).
Interestingly, AS-C genes and ato seem to have opposing mechanisms in the patterning of es and CHOs, respectively. Jarman and Ahmed (1998) demonstrated that ectopic expression of ato can transform es organs to CHOs, while misexpression of scute does not affect CHO formation. Furthermore, they demonstrated that ato downregulates expression of a homeobox gene, cut. cut is necessary for sense organ precursors (SOPs) to adopt an es fate and its transcriptional repression by ato prevents es organ formation and permits the SOPs to form CHOs. AS-C promotes cut activation to fully specify the es fate. In cut mutants, es SOPs are transformed into CHOs (Bodmer et al., 1987). Hence, to determine fate, ato does not activate CHO-specific genes, but rather inhibits es organ factors (Fig. 4). ato, however, does have a role in SOP clustering for CHO array formation (discussed above). Ectopic overexpression of cut in COPs results in es organ formation, indicating that cut expression must be increased to specify es fates. Vertebrate homologs of cut also can compensate for the loss of cut function in the Drosophila cut mutants (Ludlow et al., 1996). Overall, the interplay of ato and cut simplifies the complication that ato is required not only for CHOs, but also for R8 photoreceptor formation and some antennal olfactory receptors. cut is repressed in all precursor cell types, while high levels of cut are present in all precursor cells dependent on AS-C.
Figure 4.
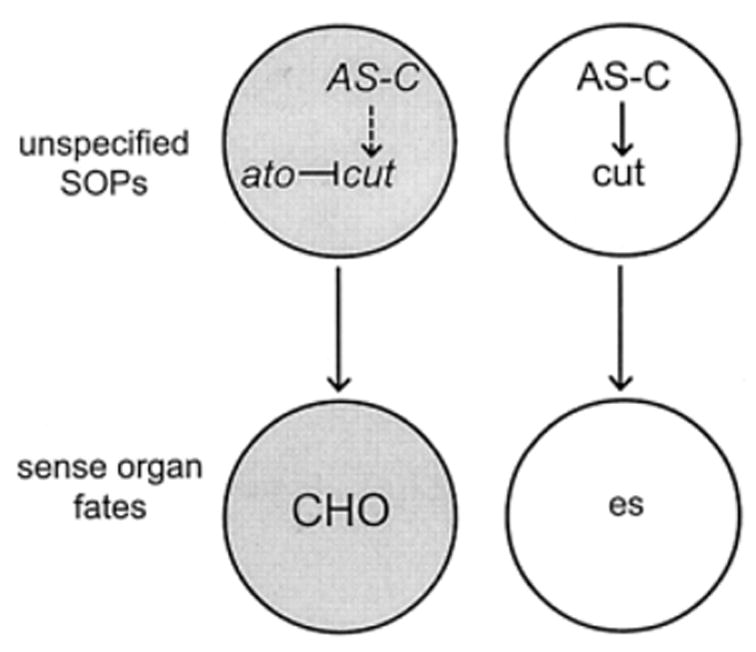
Simplified scheme for generating different sense organ types. Proneural genes in the achaete-scute Complex (AS-C) activate cut, which promotes es organ differentiation. cut may also be expressed early in chordotonal organ lineages due to some expression of AS-C genes, but cut activation is countered by expression of the proneural gene atonal to allow undifferentiated SOPs to give rise to CHO fates. It is not clear whether cut activation is mediated directly through AS-C genes or indirectly by genes downstream of AS-C to specify es organs. This caveat does not affect CHO lineage specification as atonal must still inhibit cut in these COPs.
The spalt Genes
Members of the spalt gene family encode nuclear C2H2 zinc finger proteins and act as transcription factors. The two sal genes in Drosophila, spalt (sal), and spalt-related (salr), are adjacent genes that show some shared and some separate regulation and considerable functional redundancy (Barrio et al., 1999; Kühnlein et al., 1997). The sal/salr genes are important in establishing head/trunk identity, tracheal migration, and patterning of the wing imaginal disc and nervous system (Kühnlein et al., 1994; Kühnlein and Schuh, 1996; Sturtevant et al., 1997; Rusten et al., 2001). In humans, there are three SALL homologs. SALL1 mutations result in Townes-Brock Syndrome (TBS) characterized by malformation of the anus, genitalia, and limbs, kidney defects, mental retardation, and hearing loss (Townes and Brocks, 1972; Kohlhase et al., 1998). TBS patients exhibit defects in their inner, middle, and outer ear that result in conductive and sensorineural deafness. SALL3 has recently been implicated in 18q deletion syndrome, marked by malformations in the nervous system and by hearing loss (Kohlhase et al., 1999).
We have shown that flies in which sal/salr expression is absent in the antenna are completely deaf to the courtship song because of defects in the JO (Dong, Todi, Eberl, and Panganiban, manuscript submitted). In these mutants, the specialized cuticle at the a2/a3 joint where JO is attached is missing. a2 and a3 in these flies are effectively fused, restricting rotation of the a3 relative to a2. This fusion prevents the propagation of the mechanical signal to JO; thus, sal/salr mutants have conductive hearing loss. Furthermore, most or all scolopidia of JO are absent in sal/salr mutants. Mutant scolopidia appear to be specified, but are not maintained. Interestingly, atonal and sal/salr appear to operate in parallel pathways, because both are downstream of Distalless and homothorax but neither is required for the other’s expression (Dong et al., 2002).
Surprisingly, this loss of CHO function in the antennae of sal/salr adult flies is opposite to the role of sal/salr in the embryo, where loss of sal promotes additional CHO recruitment and formation, whereas overexpression of sal results in loss of some scolopidia of the lateral pentascolopidial organ (lch5) (Elstob et al., 2001; Rusten et al., 2001). In embryos, sal is a cell fate switch between two EGFR-induced cell types, the oenocytes, and the lateral pentascolopidial precursors. sal is expressed early in the a subset of cells surrounding COPs that receive the signal from EGFR (discussed below). Thus, in sal mutant embryos, extra COPs are recruited from cells that would normally form oenocytes, while sal overexpression results in excess oenocytes at the expense of scolopidia. sal expression is found in subsequent developmental stages only in the support cells of the lch5 (lateral pentascolopidial organs); this expression is important to direct proper migration of these organs along the dorsoventral axis in the abdominal segments. Perhaps this later expression in embryonic CHOs reflects a similar role for sal/salr in late antennal CHO development where it is found in apical epidermal cells that appear to be important in scolopidial attachment to the cuticle (Dong, Todi, Eberl, and Panganiban, manuscript submitted).
Lateral Inhibition: Notch/Delta
Downstream of proneural cluster specification, only specific cells in the ectodermal patch adopt neural fates. This restriction occurs through the process of lateral inhibition, mediated by interactions of Notch (N) and Delta (Dl). Lateral inhibition is a means by which a fated cell prevents its contiguous adjacent neighbors from adopting the same fate. N signaling has been extensively studied, and is understood in great (but not complete) depth (e.g., see reviews by Artavanis-Tsakonas et al., 1999; Mumm and Kopan, 2000). Briefly, N, a single-pass transmembrane receptor with numerous EGF-like and ankyrin-like repeats on its extracellular domain, is bound by its transmembrane ligand Dl (and/or by Serrate, another N ligand) expressed on a neighboring cell. Upon binding of Dl to N, the N intracellular domain is cleaved by presenilin and moves, along with Suppressor of Hairless (Su(H)), to the nucleus, where it blocks expression of a variety of genes including proneural genes. Thus, N signaling inhibits additional rounds of neurogenesis of unspecified ectodermal cells, and Dl delivers the inhibitory signal from the primary SOP that prevents ectodermal cells from adopting a neuronal fate.
N-mediated lateral inhibition has been extensively studied in the Drosophila embryonic CNS and the embryonic and adult PNS where it is essential not only for restricting the number of SOPs for es organs and COPs for CHOs, but also for correctly executing the subsequent asymmetric cell divisions. However, these functions have not been demonstrated specifically in the development of JO; therefore, we will only mention two specific issues here. First, we expect that the general aspects of N signaling in JO indeed will be shown to be very similar to its function in other sense organs. However, minor differences in regulation may be encountered in JO. For example, adult CHOs usually have two or three neurons per scolopidium, so there must be at least one extra cell division in the CHO lineage. In addition, N signaling has been shown to be important in the specification of vertebrate auditory hair cells in the developing sensory epithelium (Lanford et al., 1999; Riley et al., 1999; Zine et al., 2000); therefore, it seems likely that these mechanisms have been largely conserved.
Reiterative Recruitment: EGFR Pathway
An important distinction between es organs and CHOs is that es organs normally develop from solitary SOPs, while CHOs usually contain tight clusters of scolopidia, each derived from a COP. Scolopidial arrays are found both in the embryonic/larval abdominal hemisegments and the adult legs and antennae. In embryos, the A1–A7 abdominal hemisegments stereotypically comprise eight CHOs: lch5, vchAB, and v′ch1. These arise at different times. ato specifies the founder COPs, namely the C1–C3 precursors of lch5, C4 for v′ch1, and C5 for vchAB (zur Lage et al., 1997). Chordotonal organ clusters are formed from proneural clusters and receive Notch-mediated lateral inhibitory signals, but differ from es organs in their ability also to recruit secondary COPs. Thus, the founder COPs, once specified, signal back to the unspecified ectoderm to recruit two additional secondary COPs to complete the lch5, and one secondary COP for vchAB. This recruitment is mediated by the EGFR signaling cascade (Okabe and Okano, 1997; zur Lage et al., 1997). Primary COPs initiate the rhomboid (rho)-mediated cascade that ultimately activates EGFR to recruit secondary SOPs.
Induction of the eight COPs per embryonic abdominal segment occurs in two steps. C1–C5 fates are adopted through ato-mediated neurogenic pathways and these cells begin to express rho and Star (S), which are thought to process spitz (spi) into a secreted form and present the ligand to EGFR. EGFR-activated cells begin to express the negative regulator, argos, and only three secondary cells, presumably those receiving the highest EGFR signal, become specified to be COPs. These secondary COPs, by virtue of their specification, prevent their immediate neighbors from adopting neural fates through lateral inhibition.
In the femoral CHOs, which contain 70–80 scolopidia, a similar but not identical mechanism of recruitment occurs (zur Lage and Jarman, 1999). The expression pattern of ato indicates that COPs develop and accumulate in the dorsal region of each leg imaginal disc from late third larval instar to early pupal stage. In femoral CHOs, cells recruited to COP fate from the unspecified but competent proneural patch are in contact with COPs, but do not respond to lateral inhibition. To show that unspecified cells were indeed receiving lateral inhibition through Notch, zur Lage and Jarman (1999) expressed in the proneural clusters either a constitutively active form of N or an Enhancer of split [E(spl)] target gene of N; in both experiments, COP recruitment was strongly suppressed. Conversely, timed loss of N function using a temperature-sensitive N allele resulted in a COP mass consistent with excess disorganized COPs below the epithelium. Thus, N signaling in the wild type is maintained at a moderate level that allows modest but not unlimited recruitment of COPs. That EGFR signaling is responsible for imposing this moderation was shown by loss of COPs upon expression of a dominant negative form of EGFR, while overexpression of EGFR signaling components, rho or pointed (pnt), increased COP numbers. Overall, zur Lage and Jarman concluded that EGFR signaling attenuates the strong N-mediated lateral inhibition and promotes COP commitment, rather than increasing proliferation of already committed COPs or preventing cell death; and, conversely, the strength of N signaling prevents over-recruitment through EGFR. Presumably similar mechanisms of reiterative recruitment occur in the formation of the JO array.
Asymmetric Division
Drosophila sense organs in the embryo, whether they are es organs or CHOs, usually comprise four cells that arise from the SOP through a series of asymmetric divisions. Brewster and Bodmer (1995) showed that the lineages are slightly different for embryonic CHOs than for es organs, in that the latter show more symmetric lineages (i.e., the SOP divides to two daughters, IIa and IIB with equivalent restriction in fate, then each of these divides again). CHOs, on the other hand, arise more as a “stem-cell” series; that is, the first division yields a cap cell and a stem cell. This stem cell then divides to form a ligament cell and another stem cell through unequal division. Finally, the neuron and scolopale cell divide from the remaining stem cell. The cap cell precursor usually divides to produce the cap cell itself and an associated ectodermal cell. The genetic control of the asymmetric divisions that form these organs in embryos and in adult es organs and olfactory sensilla has been the subject of intense study, and has been reviewed, for example, by Hawkins and Garriga (1998) and Jan and Jan (2001). The extent to which these processes can be extrapolated to JO has not yet been determined. In fact, the complete complement of cells that constitute a JO scolopidium is still unclear, and the extent of morphologic variation between JO scolopidia is not well understood. In their EM study, Uga and Kuwabara (1965) described two neurons per scolopidium, in addition to a scolopale cell, an envelope cell and a cap cell, but make no mention of a ligament cell. Clearly, there are ligaments, but whether these are formed by a single ligament cell from each scolopidium is unclear. In addition, we have found that a subset of JO scolopidia have three rather than two neurons (Todi and Eberl, unpublished observations); in these, the third neuron appears to have microtubules that are not organized into an axoneme. Such scolopidia with mixed neuron morphologies are common in other insects as well (see review by Field and Matheson, 1998). Finally, we have found that, indeed, there are accessory ectodermal cells associated with the cap cells of JO (Dong, Todi, Eberl, and Panganiban, manuscript submitted); these cells may be important in secreting or organizing specialized cuticular structures at the a2/a3 joint.
In es organ development, several distinct processes are required to achieve the asymmetric divisions that occur in the correct orientation and that differentiate the correct complement of cells. One process is the establishment of planar polarity in the precursor epithelium (in contrast to apical/basal polarity, which is important in CNS neurogenesis). The gene products of frizzled, dishevelled, and flamingo are required to establish the correct orientation of mitoses (Gho and Schweisguth, 1998; Shulman et al., 1998; Lu et al., 1999; Bellaïche et al., 2001a; Roegiers et al., 2001a, 2001b). The products of bazooka and Partner of Inscuteable (Pins) are required to transduce this polarity information to the cell division machinery, and for the asymmetric localization of the numb and Partner of numb (Pon) gene products (Bellaïche et al., 2001b; Roegiers et al., 2001a, 2001b). Numb was one of the first identified proteins that localizes asymmetrically within dividing CNS neuroblasts and dividing SOPs; this asymmetrical localization results in transmission of the numb protein to one daughter cell where it antagonizes the N signaling pathway, thereby promoting the neural fate (Rhyu et al., 1994; Spana et al., 1995; Guo et al., 1996; Spana and Doe, 1996). There is a direct binding of numb to the intracellular domain of N. This binding may block entry of N into the nucleus where it would otherwise activate the tramtrack (ttk) transcription factor (see below). Thus numb is an intrinsic cell-fate determinant, although it elicits its function by affecting cell–cell communication. Numb is required not only for the SOP division, but also for the asymmetrical division of each daughter cell in the formation of the four distinct cell types of es organs in embryo and adult, and also in embryonic CHOs (Guo et al., 1996); its role in the developing JO has not been examined.
Downstream of numb in the es lineage, each of the daughter cells of IIa and IIb is further differentiated into socket, sheath, hair cell, or neuron by various factors. Suppressor of Hairless [Su(H)] is a transcriptional activator expressed predominantly in the IIa lineage to produce the socket cell (Guo et al., 1996). Su(H) mutants produce two hair cells while in gain of function strains the IIa daughters give rise to two socket cells. Hairless, a novel basic protein that negatively regulates N and whose function is antagonistic to that of Su(H), specifies the hair fate of one daughter of IIa. In Hairless mutants, two socket cells are produced, while in hairless gain of function mutants, two hair cells are produced. tramtrak (ttk), a zinc finger protein transcriptional repressor activated by N signaling, gives rise to the sheath cell after the IIb division. ttk loss of function mutants produce two neurons in the IIb lineage (Guo et al., 1995). Finally, sanpodo affects asymmetric division in the IIb lineage and is required for the neuron fate in one of the daughter cells (Dye et al., 1998; Skeath and Doe, 1998).
A number of other factors that are important for neuroblast asymmetric divisions play less obvious roles in es organ lineage. These include inscuteable (insc), a cytoskeleton-associated scaffold protein, and miranda, an actin-binding protein recognized by insc. Both have recently been found in dividing IIb cells (Roegiers et al., 2001b); at least insc is required for proper orientation of the mitotic spindle. Prospero (pros) is a homeodomain transcription factor also found in the es organ lineage and is important both for specification of the sheath cell and for proper morphological differentiation of the neuron. pros in CNS neuroblasts relies on insc and miranda for asymmetrical localization, but in es organs has been reported discrepantly as either being asymmetrically expressed but not asymmetrically localized in the IIb cell (Manning and Doe, 1999; Reddy and Rodrigues, 1999) or indeed being asymmetrically localized (Gho et al., 1999); whether this discrepancy arises from technical differences or from variations in different es organs remains to be determined.
It is clear that many of the salient features, molecules, and mechanisms involved in asymmetric division appear once in embryonic CNS neuroblast divisions, again with some variations in the embryonic es organ SOP divisions, and yet again with even more variations in adult es organs. For a few molecules that have been tested in CHO organs, such as N and numb, there is clear functional similarity, although in some cases there are also differences. The similarities argue that considerable extrapolation is possible in understanding the molecular basis of JO development and of CHO genesis in general. The differences highlight the need to verify each process and molecular function in JO to completely understand the development of the Drosophila auditory organs and their relationship to other insect and vertebrate ears.
GENETICS AND PHYSIOLOGY
In recent years, large strides have been made towards identifying the molecular machinery that underlies JO mechanotransduction as well as the effects mutations of these components have on hearing and other mechanosenses in the fly (see Table 1). Genetic screens for hearing, proprioceptive, and mechanosensory mutants are well suited for isolating the molecular machinery involved in these processes regardless of the abundance or nature of the component. The sole prerequisite is that loss of function mutants will exhibit an easily recognizable phenotype.
Table 1.
Summary of Genes Involved in Drosophila Auditory Mechanosensation
| Gene | Auditory Behavior | SEP | TS | MRP | Adult Coordination | Predicted Protein | Vertebrate Homolog | Referencesa |
|---|---|---|---|---|---|---|---|---|
| ato | n. d. | absent | insensitive | + | slightly uncoordinated | Transcription factor | Math1 | Jarman et al., 1993 Jarman et al., 1995 Eberl et al., 2000 |
| ck | n. d. | absent | n. d. | strongly reduced | moderately uncoordinated | Myosin VIIA | MYO7A shaker-1 mariner | unpublished results |
| nompA | n. d.* | absent | insensitive | absent | uncoordinated | Type-I sensilla specific, single-pass transmembrane protein with ZP and PAN domains | Chung et al., 2001 Kernan et al., 1994 Eberl et al., 2000 | |
| nompC | n. d.* | slightly reduced | insensitive | absent | uncoordinated | transduction channel | TRP family (?) | Walker et al., 2000 Kernan et al., 1994 Eberl et al., 2000 |
| nompB | n. d.* | absent | insensitive | absent | uncoordinated | Protein composed of ten tetratricopeptide repeats | Tg7371orpk | Han and Kernan, 2001 Kernan et al., 1994 Eberl et al., 2000 |
| btvb | strongly reduced | strongly reduced | insensitive | + | slightly uncoordinated | n. d. | Eberl 1999 Eberl et al., 2000 unpublished results | |
| tilB | strongly reduced | absent | insensitive | + | slightly uncoordinated | n. d. | Kernan et al., 1994 Eberl et al., 2000 | |
| smet | n. d. | absent | insensitive | n. d. | slightly uncoordinated | n. d. | unpublished results | |
| unc | n. d.* | absent | insensitive | absent | uncoordinated | coiled-coil protein | Baker et al., 2001 Kernan et al., 1994 Eberl et al., 2000 | |
| uncl | n. d.* | absent | insensitive | absent | uncoordinated | n. d. | Baker et al., 2001 Kernan et al., 1994 Eberl et al., 2000 | |
| tko | strongly reduced | n. d. | n. d. | reduced? | n. d. | Mitochondrial ribosomal protein S12 | Mitochondrial ribosomal protein S12 | Toivonen et al., 2001 Engel and Wu, 1994 |
| sal/salr | n. d. | absent | n. d. | n. d. | n. d. | Transcription factor | SALL1 - 3 | Dong et al (submitted) |
Sound-evoked potential (SEP), Mechanoreceptor potential (MRP), Touch sensitivity (TS), wild-type phenotype (+), not determined (n. d.), n. d.
auditory behavior cannot be assayed in these mutants due to extreme uncoordination.
References for Drosophila homologs only. See text for vertebrate references.
The btv gene of Drosophila is unrelated to the recently described mouse Beethoven mutant (Kurima et al., 2002; Vreugde et al., 2002).
Kernan et al. (1994) isolated numerous mutants in an EMS mutagenesis screen for genes encoding mechanosensory transduction components because they exhibited reduced larval behavioral response to gentle touch. X-linked mutations in uncoordinated (unc), uncoordinated-like (uncl), and touch insensitive larva B (tilB) were isolated in the primary screen. An additional screen for mutants on the second chromosome that exhibited uncoordination resembling that of unc mutants resulted in several no mechanoreceptor potential (nomp) mutants and one reduced mechanoreceptor potential (remp) mutant (Kernan et al., 1994). Mutations in these nomp genes produce flies with aberrant touch-sensitivity, coordination, and acoustic reception (Kernan et al., 1994; Eberl et al., 2000). Bristle function was measured by attaching a voltage recording pipette to the K+-rich receptor lymph of an exposed mechanosensory bristle and using a piezoelectric stage to deliver a precise movement of the bristle shaft. In wild-type flies, a bristle in the resting position exhibits a positive transepithelial potential (TEP: the voltage difference between the apical and basal sides of the sensory epithelium), reflecting the K+-richness of the receptor lymph. Deflections of the bristle towards the body elicit a stereotypic strongly depolarizing current, carried by the flow of K+ ions from the receptor lymph into the neurons, presumably through the mechanotransduction channel. This transduction current results in a mechanoreceptor potential (MRP: the voltage change upon deflection). The unc, uncl, nomp, and remp mutations all reduced or abolished the MRP, while the TEP was relatively unaffected (Kernan et al., 1994). These mutants were later shown to also disrupt sound-evoked electrophysiologic responses measured from the antennal nerve (Eberl et al., 2000).
A number of mutants were isolated in an EMS mutagenesis screen for mutations on chromosome 2 that disrupt an auditory response in Drosophila adults (Eberl et al., 1997). Mutant strains were identified based on defects in the vigorous group courting behavior normally seen when males are presented with the pulse song. The previous assay for auditory function was female receptivity as measured statistically by a difference in latency of copulation, an assay prohibitive to screening efficiency. From this screen, 15 mutant lines exhibiting a loss or reduction of male chaining behavior were further characterized. Of these, the 5P1 mutant [later named beethoven (btv)] was the only one to affect JO sensory electrophysiology severely (Eberl et al., 2000). We have since found that the 5D10 mutant also has a moderate effect on JO physiology (Todi and Eberl, unpublished).
Finally, Drosophila homologs to vertebrate deafness genes have begun to show promise. Perhaps the best example of this is the crinkled gene (Todi and Eberl, unpublished observations), which encodes a myosin VIIa homolog (Chen et al., 1991; Ashburner et al., 1999). In addition, one mutant, smetana, was discovered as an unrelated mutation on a chromosome carrying another mechanosensory mutation (Eberl, unpublished observations).
Mutations analyzed from these various sources could potentially disrupt several different structures or processes involved in mechanosensation in JO. First, the acoustically induced mechanical vibrations must be propagated to the mechanosensory neurons. This is likely achieved by a tensioned system that relies on counteracting forces, namely, elasticity of the cuticle opposing tension from the JO scolopidia. Propagation of the signal requires intact structural linkages of the vibrating distal elements. Second, given that mechanical vibrations are delivered to the neurons, the mechanotransduction machinery within the neuron must be intact to allow activation of a physiologic sensory response. This must occur somewhere along the outer dendritic segment, which is bathed in the receptor lymph, although the precise location is not yet known. Transduction likely occurs by direct activation of an ion channel, which may be part of a multiprotein complex. Specialized cytoskeletal architecture is likely required not only for localization of transduction components by motor proteins, but also for anchoring the transduction complex to allow direct activation. To the extent that they are understood, we will present genes and their products in order of their effects on the above processes.
crinkled: Myosin VIIa and Apical Scolopidial Attachment
The crinkled (ck) gene of Drosophila encodes myosin VIIa (Chen et al., 1991; Ashburner et al., 1999). Vertebrate myosin VIIa is an unconventional myosin expressed primarily in sensory hair cells (Hasson, 1997). Orthologues of crinkled have been studied in humans (MYO7A, Weil et al., 1995), mouse (shaker-1, Gibson et al., 1995) and zebrafish (mariner, Ernest et al., 2000). In humans, defects in MYO7A primarily underlie Usher syndrome type 1B, characterized by sensorineural deafness, vestibular dysfunction, and retinitis pigmentosa, and specific mutations are responsible for two forms of nonsyndromic deafness, DFNB2 and DFNA11 (Weil et al., 1995, 1997; Lévy et al., 1997; Liu et al., 1997a, 1997b).
In Drosophila, we have found that strong ck mutants are completely deaf (Todi and Eberl, unpublished), as determined electrophysiologically by the absence of sound-evoked potentials in the antennal nerve. In preliminary histologic examination of ck mutant antennae, the JO scolopidia appear detached from the a2/a3 joint. This suggests that the mechanical vibrations of the arista and a3 cannot be propagated to the scolopidia. Although this defect would adequately explain the deafness phenotype, it is quite possible that the myosin motor encoded by ck is required not only for apical scolopidial attachments or their maintenance, but also for physiologic function of the scolopidia. Such a dualism appears to be true for vertebrate myosin VIIa as well (Self et al., 1998; Ernest et al., 2000; Gillespie, 2002) because mutants not only show gross structural defects in the stereocilia, suggesting a morphologic maintenance role (Hasson, 1997), but also show defects in gating properties of the transduction channel (Kros et al., 2002), suggesting an additional more intimate role in transduction.
Bristle shafts in ck adults are also shorter and appear bent when compared with the wild type. We have found that bristle MRP amplitudes are severely reduced in ck mutants (Todi and Eberl, unpublished). This is consistent with broad sharing of mechanotransduction components between bristle organs and CHOs (Eberl et al., 2000). The structural or physiological basis of ck bristle dysfunction is not yet clear.
nompA: Ciliary Attachment to Dendritic Cap
Like ck, the no mechanoreceptor potential A (nompA) mutants disrupt the physical propagation of the mechanical signal to the sensory neurons (Chung et al., 2001). However, in nompA the disruption appears as a detachment of the sensory dendrites from the dendritic cap elaborated by the support cells. Thus, the disruption is farther along the mechanical stimulus chain. Nevertheless, some apical detachment of scolopidia from the a2/a3 joint is also seen (Chung et al., 2001). nompA was recovered as a complementation group of two mutant alleles by Kernan et al. (1994) associated with severe adult uncoordination and a third allele was identified later (Chung et al., 2001). Mutations in nompA affect both es and CHOs as evidenced by the lack of bristle MRPs (Kernan et al., 1994) and lack of sound-evoked potentials from JO (Eberl et al., 2000), both of which are rescued with a nompA+ transgene (Chung et al., 2001). Chung et al. have cloned nompA; it encodes a type I sensilla-specific, single-pass transmembrane protein with extracellular domains that include a Zona Pellucida (ZP) domain and five plasminogen N-terminal (PAN) modules. In CHO, the nompA protein is a component of the dendritic cap produced by the scolopale cell, but is not expressed in the neuron. The ZP domain is necessary and sufficient for incorporation into the cap matrix, while the divergent PAN domains may permit the cap to bind to a diverse array of attachment sites (Tordai et al., 1999; Chung et al., 2001). In es organs, the shaft (trichogen) and socket (tormogen) cells secrete K+-rich endolymph that generates the TEP (Thurm and Küppers, 1980; Kernan et al., 1994) while the sheath cell (thecogen) ensheathes the sensory cilium and produces the dendritic cap (Keil, 1997). nompA is expressed primarily in the sheath cell of es organs; in mutants the dendrite is usually detached from the dendritic cap, consistent with uncoupling of the mechanical stimulus from the mechanotransduction machinery. Clearly, nompA encodes a protein essential for organization of the cap and its proper attachment to mechanosensory cilia and to apical cuticular structures.
nompC: Transduction Channel for Bristle Organs
No mechanoreceptor potential C (nompC) mutants were also isolated by Kernan et al. (1994) because of severe uncoordination, loss of larval touch-sensitivity, and loss of MRPs in bristle organs. The nompC gene has been cloned (Walker et al., 2000); it encodes a six-transmembrane domain protein, distantly related to the TRP (transient receptor potential) family of ion channels, and at the cytoplasmic amino terminus it has a long array of 29 ankyrin repeats, which could mediate associations with a variety of cellular components, including the cytoskeleton. Under voltage clamp conditions, three nonsense alleles of nompC, all causing strong uncoordination, exhibited an almost complete loss of mechanoreceptor current (MRC: receptor current evoked by mechanical stimulation) when the bristle was deflected toward the body and a severe reduction of action potentials fired. A fourth missense allele, nompC4, with less severe uncoordination showed retention of the robust mechanically evoked response, but exhibited adaptation to mechanical perturbations much more quickly than in wild-type controls and a twofold reduction of action potentials. Therefore, nompC4 flies produce fewer action potentials due to the rapid adaptation that limits the time of receptor depolarization. These features argue strongly that nompC represents the major mechanotransduction channel in bristle organs and mimics very closely the biophysical properties of vertebrate hair cell transduction channels.
Surprisingly, nompC mutants show only a modest reduction of sound-evoked potentials recorded from the JO when presented with the pulse song (Eberl et al., 2000). In fact, of all bristle MRP mutants tested for JO response, nompC was the only one that retained much of the sound-evoked response. Thus, nompC is absolutely required for meaningful bristle organ function, it appears to play only a minor role in transducing auditory stimuli in JO. Therefore, an additional transduction channel is inferred to operate in CHOs. This additional inferred channel may be CHO-specific, or it may be responsible for the small MRC remaining in bristle organs in nompC mutants (Walker et al., 2000).
nompB: Intraflagellar Transport
nompB flies, like nompA, exhibit no bristle MRP (Kernan et al., 1994) and no sound-evoked potentials in response to the pulse song (Eberl et al., 2000). As with nomp A, nompB mutants show gaps between the tips of sensory dendrites and the external sensory structures of campaniform sensilla (Han and Kernan, 2001). Unlike nompA, it is the sensory ending of the neuron rather than the dendritic cap that is abnormal. This is clear in the long outer segments of CHOs, which are missing or malformed in nompB mutants. Molecular analysis of the nompB locus suggests that the basis of the dendritic detachment phenotype is different from that of nompA. nompB encodes a protein containing ten tetratricopeptide repeats that compose two potential protein interacting domains (Han and Kernan, 2001). It is homologous to the mouse and human Tg737/orpk gene (Pazour et al., 2000), to osm-5 (CeTg737) in C. elgans (Haycraft et al., 2001) and to IFT88 in Chlamydomonas (Pazour et al., 2000). These proteins are all associated with ciliary elongation defects, which, in some of these systems, have been shown to result from defects in intraflagellar transport (IFT). IFT is a mechanism by which proteins required for assembly and maintenance of the axoneme are trafficked along the axonemal microtubules of cilia and flagella microtubules (Cole, 1999). Thus, the mutant phenotype of nompB is consistent with the role of the putative IFT component it encodes.
btv: Intraflagellar Transport or Cell Adhesion?
The 5P1 mutant, recovered as an auditory behavior mutant (Eberl et al., 1997), was later named beethoven (btv) (Eberl et al., 2000) because it disrupts JO function but leaves bristle MRPs intact. These flies are slightly uncoordinated and sedentary, consistent with defects in all CHOs. Preliminary electron microscopy reveals ciliary defects in the outer dendritic segment of JO neurons, as well as other abnormalities in the appearance of the scolopidia (Eberl et al., 2000). Homozygous and hemizygous btv5P1 males have motile sperm and are fertile, whereas males deficient for the btv region in overlapping chromosomal deficiency combinations are sterile. We do not yet know whether the male sterility results from complete deletion of btv or from a separate genetic function, although we favor the latter interpretation.
We have mapped the btv gene to the 36DE region of chromosome 2. The sequence in the candidate region encodes two compelling candidates for the btv gene. One is a new cadherin gene (Cad-2) which is adjacent to DN-Cad (Iwai et al., 1997), and which likely arose from a tandem duplication-divergence of a single ancestral gene. The other major candidate is a dynein heavy chain (DHC36D), specifically the 1b isoform implicated in IFT in other organisms (Pazour et al., 1999; Porter et al., 1999; Signor et al., 1999; Wicks et al., 2000). A few other predicted genes of unknown function are also candidates. Nevertheless, defects in retrograde intraflagellar transport, potentially mediated by DHC36D, might explain some of the morphological abnormalities in the ciliary region. On the other hand, Cad-2 may be necessary for functional attachment of the sensory cilia to the dendritic cap, for other cell contacts required for maintaining receptor lymph, or another undefined function. Novel cadherins are also involved in human hearing. Usher syndrome 1D and nonsyndromic deafness DFNB12 in humans are caused by mutations in a novel cadherin-like gene, CDH23 (Bolz et al., 2001; Bork et al., 2001), while mutations in a protocadherin, PCDH15, are responsible for Usher syndrome type 1F (Ahmed et al., 2001). Understanding the molecular and cellular basis of btv mutant defects must await conclusive identification of the correct gene. It should be noted that the btv gene of Drosophila is unrelated to the recently described mouse Beethoven mutant (Kurima et al., 2002; Vreugde et al., 2002).
tilB and smet: Axonemal Architecture
The two touch insensitive larva B (tilB) mutations, recovered in the larval touch screen by Kernan et al. (1994), like btv, disrupt CHOs but have no effect on bristle physiology or MRP, mirroring the defect seen in btv. Unlike the nomp genes discussed above, tilB mutants only show slight motor uncoordination. tilB sound-evoked antennal nerve potentials are completely absent (Eberl et al., 2000). In addition to auditory dysfunction, these mutants are also male sterile because of sperm amotility. Ultrastructurally, tilB spermatid axonemes have defects in the outer microtubule doublet arrangement. The axonemes of wild-type spermatids are 9 × 2 + 2. Each microtu-bule doublet has inner and outer dynein arms extending from the A microtubule and a nexin linkage between AB microtubules. The dynein arms, required for microtubule sliding to effect sperm motility, are absent in tilB mutants (as perhaps is the nexin bridge). tilB maps genetically to an unsequenced gap in the genome on the X chromosome (Kernan and Eberl, unpublished observations).
Similar to tilB, smetana (smet) flies are defective for hearing and male fertility (Eberl, unpublished observations). This combination suggests that smet is also required for axonemal integrity of sperm flagella and CHOs. smet was discovered fortuitously as an additional unrelated mutation on a nompC mutant chromosome that resulted in complete loss of sound-evoked potentials (cf Eberl et al., 2000). smet has been mapped to an unsequenced gap near the histone gene cluster on chromosome 2.
unc: from Centrioles to Basal Bodies
The function of unc in Drosophila is also currently under investigation (Baker et al., 2001). In addition to their uncoordination and bristle MRP defects (Kernan et al., 1994), unc mutants are also deaf (Eberl et al., 2000) and male sterile (Baker et al., 2001). Males do not produce mature sperm, and the flagella of primary spermatids show gross defects in their axonemal structure. unc encodes a coiled-coil protein that is expressed solely in neurons of type I ciliated mechanoreceptors and in the male germline. It is localized to the centrioles of primary spermatocytes and the junction of the nucleus and flagellum in differentiating spermatids, but not in mature sperm. The inner dendritic segments of leg and JO scolopidia are normal, but the ciliated outer dendritic segments fail to connect to the dendritic cap. Clearly, unc has an important role in axonemal formation in sensory cilia and flagella, because it appears to be involved in the conversion of mitotic centrioles into ciliogenic basal bodies (Baker et al., 2001).
tko: Mitochondrial Deafness
Recently, Toivonen et al. (2001) have investigated the role of technical knockout (tko), a nuclear gene encoding mitochondrial ribosomal protein S12, in hearing. In humans, mitochondrial DNA (mtDNA) mutations are responsible for a large number of pathologic syndromes with which sensorineural deafness is often associated (reviewed by Jacobs, 1997; Fischel-Ghodsian, 1999). In some cases, the only physiologic defect in patients is hearing loss. tko flies are bang-sensitive, exhibiting a temporary paralysis from the mechanical perturbation (Ganetzky and Wu, 1982). The paralysis could be explained in part by altered sensory feedback from the mechanosensitive bristles (Engel and Wu, 1994). This sensitivity to mechanical vibration is similarly found in humans as a result of aberrant sensory signaling from mechanoreceptors in the inner ear. In addition to lengthened developmental times, tko males also show a reduced male courtship response upon presentation of the pulse song, suggesting a hearing deficiency (Toivonen et al., 2001). These phenotypes are associated with a single mis-sense mutation (L85H) of tko, and mutant larvae have reduced activity of mitochondrial redox enzymes and mitochondrial small subunit rRNA. Raising wild-type flies on doxycyclin generates a phenocopy of the tko phenotypes (Toivonen et al., 2001). It is likely that hearing is an energetically costly process and, consistent with this, reduced levels of ATP were found in tko flies (Toivonen et al., 2001). Electrophysiologic recordings will be necessary in the future to determine the anatomical location of tko behavioral deafness. Nevertheless, this mutant provides a compelling model for mitochondrial deafness that could be used to study not only the precise role of mitochondria themselves, but perhaps also their intracellular transport to relevant parts of the cell.
CONCLUSIONS
Construction of a functional auditory receptor requires proper integration of developmental and mechanical processes. Specification, asymmetric divisions and differentiation of the cells producing sense organs must proceed unperturbed. Then, the sense organs must express the diverse assortment of cellular components that establish the intercellular and intracellular environment for the sense organ to be poised for mechanosensation. Furthermore, the mechanoreceptive cells must be mechanically linked to the acoustically sensitive vibrating structures.
The development of JO, the Drosophila auditory organ in the antenna, is understood in many fundamental ways, at least vicariously. Although few studies have focused specifically on JO, we believe that the basic steps of PNS development, which are generally well understood, will also hold for JO. Thus, further studies on JO development must focus on determining the extent of the variations on a well-known theme. In some cases we have already seen that the mechanisms can differ in JO, such as in the role of sal/salr. In contrast, studies on the mechanisms of mechanotransduction operating in JO are still in the early stages of establishing the major elements in the theme itself. This endeavor is much more recent, but several important advances have been made in the identification of specific components. In both cases, i.e., development and molecular physiology, Drosophila is already beginning to serve as a useful model system for understanding hearing. The increasing evidence for evolutionary homology of fly and vertebrate auditory organs makes the fly auditory system correspondingly important, not only for the similarities in development and function, but also for the important differences.
Many future prospects remain for research in Drosophila hearing. First, it is important to understand the developmental issues that make JO like other sense organs, and those that set it apart as a specialized organ for hearing. Second, cloning more transduction components will illuminate our understanding of the nature of the mechanosensory machinery. In particular, the transduction channel that operates in JO and acts in parallel with nompC, must be identified. Third, to understand the function of each component, determining the relationship of components to each other will be crucial. Epistasis experiments, through interaction screens or localization of components in genetic backgrounds mutant for various other auditory components, would be useful experimental paradigms. Fourth, the fundamental role of ciliary action in transduction of CHOs is still not understood. However, analysis of auditory mutants with laser vibrometry may add greatly to this endeavor. Finally, for Drosophila to achieve its greatest usefulness in understanding the relationship between insect and vertebrate auditory mechanisms, continual comparison, and testing of homology with vertebrate auditory genes and mechanisms must be carried out. The Drosophila auditory system is poised to become an important test system for dissecting the function of human homologues, even human-specific auditory components.
We have benefitted immensely from discussions with Maurice Kernan, James Baker, Grace Panganiban, P.D. Si (Duc) Dong, Martin Göpfert, and many others including our fellow lab members.
Footnotes
Contract grant sponsor: Whitehall Foundation. Contract grant sponsor: National Institutes of Health; contract grant number: DC04848 (to D.F.E.).
Contract grant sponsor: the Department of Biological Sciences.
References
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Wilcox ER. Mutations of the protocadherin gene, PCDH15, cause Usher syndrome type 1F. Am J Hum Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt S, Ringo J, Talyn B, Bray W, Dowse H. The period gene controls courtship song cycles in Drosophila melanogaster. Anim Behav. 1998;56:87–97. doi: 10.1006/anbe.1998.0743. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Misra S, Roote J, Lewis SE, Blazej R, Davis T, Doyle C, Galle R, George R, Harris N, Hartzell G, Harvey D, Hong L, Houston K, Hoskins R, Johnson G, Martin C, Moshrefi A, Palazzolo M, Reese MG, Spradling A, Tsan G, Wan K, Whitelaw K, Kimmel B, Celniker S, Rubin GM. An exploration of the sequence of a 2.9-Mb region of the genome of Drosophila melanogaster. The Adh region. Genetics. 1999;153:179–219. doi: 10.1093/genetics/153.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JD, Esenwa V, Kernan M. Uncoordinated is a novel protein required for the organization and function of ciliogenic centrioles. Mol Biol Cell. 2001;12(Suppl):447a. [Google Scholar]
- Barrio R, de Celis JF, Bolshakov S, Kafatos FC. Identification of regulatory regions driving the expression of the Drosophila spalt complex at different developmental stages. Dev Biol. 1999;215:33–47. doi: 10.1006/dbio.1999.9434. [DOI] [PubMed] [Google Scholar]
- Bellaïche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol. 2001a;3:50–57. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- Bellaüche Y, Radovic A, Woods DF, Hough CD, Parmentier M-L, O’Kane CJ, Bryant PJ, Schweisguth F. The partner of inscuteable/discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell. 2001b;106:355–366. doi: 10.1016/s0092-8674(01)00444-5. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, Bellen HJ, Zoghbi HY. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark HC. Acoustics of insect song. Nature. 1971;234:255–259. [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1, an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zhoghbi HY. Pro-prioceptor pathway development is dependent on MATH1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Barbel S, Shepherd S, Jack JW, Jan LY, Jan YN. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell. 1987;51:293–307. doi: 10.1016/0092-8674(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Bolz H, von Brederlow B, Ramírez A, Bryda EC, Kutsche K, Nothwang HG, Seeliger M, del C-Salcedó Cabrera M, Caballeró Vila M, Pelaez Molina O. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet. 2001;27:108–112. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, Polomeno R, Ramesh A, Schloss M, Sri-sailpathy CRS, Wayne S, Bellman S, Desmukh D, Ahmed Z, Khan SN, Der Kaloustian VM, Li XC, Lalwani A, Riazuddin S, Bitner-Glindzicz M, Nance WE, Liu X-Z, Wistow G, Smith RJH, Griffith AJ, Wilcox ER, Friedman TB, Morell RJ. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet. 2001;68:26–37. doi: 10.1086/316954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster R, Bodmer R. Origin and specification of type II sensory neurons in Drosophila. Development. 1995;121:2923–2936. doi: 10.1242/dev.121.9.2923. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Hilgers SL, Juang J-L. First developmental signs of the scolopale (glial) cell and neuron comprising the chordotonal organ in the Drosophila embryo. Glia. 1997;19:269–274. [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002:129. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen TL, Edwards KL, Lin RC, Coates LW, Kiehart DP. Drosophila myosin heavy chain at 35BC. J Cell Biol. 1991;115:330a. [Google Scholar]
- Chung YD, Zhu J, Han Y-G, Kernan MJ. nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron. 2001;29:415–428. doi: 10.1016/s0896-6273(01)00215-x. [DOI] [PubMed] [Google Scholar]
- Cole DG. Kinesin-II, coming and going. J Cell Biol. 1999;147:463–465. doi: 10.1083/jcb.147.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley SA, Bennet-Clark HC, Evert HA. Courtship song components affect male and female Drosophila differently. Anim Behav. 1995;50:827–839. [Google Scholar]
- Dallos P. Overview: cochlear neurobiology. In: Dallos P, Popper AN, Fay RR, editors. The cochlea. New York: Springer; 1996. pp. 1–43. [Google Scholar]
- Dong PDS, Scholz Dicks J, Panganiban G. Distalless and homothorax regulate multiple targets to pattern the Drosophila antenna. Development. 2002;129:1967–1974. doi: 10.1242/dev.129.8.1967. [DOI] [PubMed] [Google Scholar]
- Dye CA, Lee J-K, Atkinson RC, Brewster R, Han P-L, Bellen HJ. The Drosophila sandpodo gene controls sibling cell fate and encodes a tropomodulin homolog, an actin/tropomyosin-associated protein. Development. 1998;125:1845–1856. doi: 10.1242/dev.125.10.1845. [DOI] [PubMed] [Google Scholar]
- Eberl DF. Feeling the vibes: chordotonal mechanisms in insect hearing. Curr Opin Neurobiol. 1999;9:389–393. doi: 10.1016/S0959-4388(99)80058-0. [DOI] [PubMed] [Google Scholar]
- Eberl DF, Duyk GM, Perrimon N. A genetic screen for mutations that disrupt an auditory response in Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94:14837–14842. doi: 10.1073/pnas.94.26.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl DF, Hardy RW, Kernan M. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstob PR, Brodu V, Gould AP. spalt-dependent switching between two cell fates that are induced by the Drosophila EGF receptor. Development. 2001;128:723–732. doi: 10.1242/dev.128.5.723. [DOI] [PubMed] [Google Scholar]
- Engel JE, Wu C-F. Altered mechanoreceptor response in Drosophila bang-sensitive mutants. J Comp Physiol A. 1994;175:267–278. doi: 10.1007/BF00192986. [DOI] [PubMed] [Google Scholar]
- Ernest S, Rauch G-J, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Ricci AJ, Hackney CM. Clues to the cochlear amplifier from the turtle ear. Trends Neurosci. 2001;24:169–175. doi: 10.1016/s0166-2236(00)01740-9. [DOI] [PubMed] [Google Scholar]
- Field LH, Matheson T. Chordotonal organs of insects. In: Evans PD, editor. Advances in insect physiology. San Diego: Academic Press; 1998. pp. 1–228. [Google Scholar]
- Fischel-Ghodsian N. Mitochondrial deafness mutations reviewed. Hum Mutat. 1999;13:261–270. doi: 10.1002/(SICI)1098-1004(1999)13:4<261::AID-HUMU1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Foelix RF, Stocker RF, Steinbrecht RA. Fine structure of a sensory organ in the arista of Drosophila melanogaster and some other dipterans. Cell Tissue Res. 1989;258:277–287. doi: 10.1007/BF00239448. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Bermingham NA. Developmental evolutionary biology of the vertebrate ear: conserving mechanoelectric transduction and developmental pathways in diverging morphologies. NeuroReport. 2000;11:R35–R44. doi: 10.1097/00001756-200011270-00013. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;9:371–379. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Gho M, Schweisguth F. Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature. 1998;393:178–181. doi: 10.1038/30265. [DOI] [PubMed] [Google Scholar]
- Gho M, Bellaïche Y, Schweisguth F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development. 1999;126:3573–3584. doi: 10.1242/dev.126.16.3573. [DOI] [PubMed] [Google Scholar]
- Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SDM. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- Gillespie PG. Myosin-VIIa and transduction channel tension. Nat Neurosci. 2002;5:3–4. doi: 10.1038/nn0102-3. [DOI] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. Active auditory mechanics in mosquitos. Proc R Soc Lond B. 2001a;268:333–339. doi: 10.1098/rspb.2000.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. Turning the key on Drosophila audition. Nature. 2001b;411:908. doi: 10.1038/35082144. [DOI] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. The mechanical basis of Drosophila audition. J Exp Biol. 2002;205:1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- Goulding SE, White NM, Jarman AP. cato encodes a basic helix-loop-helix transcription factor implicated in the correct differentiation of Drosophila sense organs. Dev Biol. 2000a;221:120–131. doi: 10.1006/dbio.2000.9677. [DOI] [PubMed] [Google Scholar]
- Goulding SE, zur Lage P, Jarman AP. amos, a proneural gene for Drosophila olfactory sense organs that is regulated by lozenge. Neuron. 2000b;25:69–78. doi: 10.1016/s0896-6273(00)80872-7. [DOI] [PubMed] [Google Scholar]
- Guo M, Bier E, Jan LY, Jan YN. tramtrack acts downstream of Numb to specify distinct daughter cell fates during asymmetric cell divisions in the Drosophila PNS. Neuron. 1995;14:913–925. doi: 10.1016/0896-6273(95)90330-5. [DOI] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:21–26. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Gupta BP, Rodrigues V. atonal is a proneural gene for a subset of olfactory sense organs in Drosophila. Genes Cells. 1997;2:225–233. doi: 10.1046/j.1365-2443.1997.d01-312.x. [DOI] [PubMed] [Google Scholar]
- Han Y-G, Kernan MJ. NOMPB, the Drosophila homolog of an intraflagellar transport (IFT) protein, is required for differentiation of ciliated sensory neurons but not for spermatogenesis. Mol Biol Cell. 2001;12(Suppl):445a. [Google Scholar]
- Hassan BA, Bellen HJ. Doing the MATH: is the mouse a good model for fly development? Genes Dev. 2000;14:1852–1865. [PubMed] [Google Scholar]
- Hassan BA, Bermingham NA, He Y, Sun Y, Jan Y-N, Zoghbi HY, Bellen HJ. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- Hasson T. Unconventional myosins, the basis for deafness in mouse and man. Am J Hum Genet. 1997;61:801–805. doi: 10.1086/514890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins N, Garriga G. Asymmetric cell division: from A to Z. Genes Dev. 1998;12:3625–3638. doi: 10.1101/gad.12.23.3625. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Swoboda P, Taulman PD, Thomas JH, Yoder BK. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms. Development. 2001;128:1493–1505. doi: 10.1242/dev.128.9.1493. [DOI] [PubMed] [Google Scholar]
- Huang M-L, Hsu C-H, Chien C-T. The proneural gene amos promotes multiple dendritic neuron formation in the Drosophila peripheral nervous system. Neuron. 2000;25:57–67. doi: 10.1016/s0896-6273(00)80871-5. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Usai T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Jacobs HT. Mitochondrial deafness. Ann Med. 1997;29:483–491. doi: 10.3109/07853899709007472. [DOI] [PubMed] [Google Scholar]
- Jan Y-N, Jan LY. Asymmetric cell division in the Drosophila nervous system. Nat Rev Neurosci. 2001;2:772–779. doi: 10.1038/35097516. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Ahmed I. The specificity of proneural genes in determining Drosophila sense organ identity. Mech Dev. 1998;76:117–125. doi: 10.1016/s0925-4773(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- Johnston C. Auditory apparatus of the Culex mosquito. Q J Microscop Sci. 1855;3(Old Series):97–102. [Google Scholar]
- Keil TA. Functional morphology of insect mechanoreceptors. Microsc Res Tech. 1997;39:506–531. doi: 10.1002/(SICI)1097-0029(19971215)39:6<506::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Hausmann S, Stojmenovic G, Dixkens C, Bink J, Schulz-Schaeffer W, Altmann M, Engel W. SALL3, a new member of the human spalt-like gene family, maps to 18q23. Genomics. 1999;62:216–222. doi: 10.1006/geno.1999.6005. [DOI] [PubMed] [Google Scholar]
- Kohlhase J, Wischerman A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18:81–83. doi: 10.1038/ng0198-81. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SDM, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Kühnlein R, Frommer G, Friedrich M, Gonzalez-Gaitian M, Weber A, Wagner-Berhholz J. spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene funciton in the head and tail region of the Drosophila embryo. EMBO J. 1994;13:168–179. doi: 10.1002/j.1460-2075.1994.tb06246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnlein RP, Schuh R. Dual function of the region- specific homeotic gene spalt during Drosophila tracheal system development. Development. 1996;122:2215–2223. doi: 10.1242/dev.122.7.2215. [DOI] [PubMed] [Google Scholar]
- Kühnlein RP, Bronner G, Taubert H, Schuh R. Regulation of Drosophila spalt gene expression. Mech Dev. 1997;66:107–118. doi: 10.1016/s0925-4773(97)00103-2. [DOI] [PubMed] [Google Scholar]
- Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, Ghosh M, Menon PSN, Deshmikh D, Oddoux C, Ostrer H, Khan S, Riazuddin S, Deininger PL, Hampton LL, Sullivan SL, Battey JF, Jr, Keats BJB, Wilcox ER, Friedman TB, Griffith AJ. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30:277–284. doi: 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. The function of courtship song rhythms in Drosophila. Anim Behav. 1982;30:794–801. doi: 10.1006/anbe.1998.0976. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lévy G, Levi-Acobas F, Blanchard S, Gerber S, Larget-Piet D, Chenal V, Liu X-Z, Newton V, Steel KP, Brown SDM, Munnich A, Kaplan J, Petit C, Weil D. Myosin VIIA gene: heterogeneity of the mutations responsible for Usher syndrome type 1B. Hum Mol Genet. 1997;6:111–116. doi: 10.1093/hmg/6.1.111. [DOI] [PubMed] [Google Scholar]
- Liu X-Z, Walsh J, Mburu P, Kendrick-Jones J, Cope MJTV, Steel KP, Brown SDM. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nat Genet. 1997a;16:188–190. doi: 10.1038/ng0697-188. [DOI] [PubMed] [Google Scholar]
- Liu X-Z, Walsh J, Tamagawa Y, Kitamura K, Nishizawa M, Steel KP, Brown SDM. Autosomal dominant non-syndromic deafness caused by a mutation in the myosin VIIA gene. Nat Genet. 1997b;17:268–269. doi: 10.1038/ng1197-268. [DOI] [PubMed] [Google Scholar]
- Lu B, Usui T, Uemura T, Jan L, Jan Y-N. Flamingo controls the planar polarity of sensory bristles and asymmetric division of sensory organ precursors in Drosophila. Curr Biol. 1999;9:1247–1250. doi: 10.1016/s0960-9822(99)80505-3. [DOI] [PubMed] [Google Scholar]
- Ludlow C, Choy R, Blochlinger K. Functional analysis of Drosophila and mammalian cut proteins in flies. Dev Biol. 1996;178:149–159. doi: 10.1006/dbio.1996.0205. [DOI] [PubMed] [Google Scholar]
- Manning L, Doe CQ. Prospero distinguishes sibling cell fate without asymmetric localization in the Drosophila adult external sense organ lineage. Development. 1999;126:2063–2071. doi: 10.1242/dev.126.10.2063. [DOI] [PubMed] [Google Scholar]
- Merritt DJ. Transformation of external sensilla to chordotonal sensilla in the cut mutant of Drosophila assessed by single-cell marking in the embryo and larva. Microsc Res Tech. 1997;39:492–505. doi: 10.1002/(SICI)1097-0029(19971215)39:6<492::AID-JEMT4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Okabe M, Okano H. Two-step induction of chordotonal organ precursors in Drosophila embryogenesis. Development. 1997;124:1045–1053. doi: 10.1242/dev.124.5.1045. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b(DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Malicki DM, Zuker CS. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. doi: 10.1146/annurev.ne.18.030195.001435. [DOI] [PubMed] [Google Scholar]
- Reddy GV, Rodrigues V. Sibling cell fate in the Drosophila adult external sense organ lineage is specified by Prospero function, which is regulated by Numb and Notch. Development. 1999;126:2083–2092. doi: 10.1242/dev.126.10.2083. [DOI] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Riley BB, Chiang M-Y, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- Ritchie MG, Halsey EJ, Gleason JM. Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou & Hall cycles in D. melanogaster. Anim Behav. 1999;58:649–657. doi: 10.1006/anbe.1999.1167. [DOI] [PubMed] [Google Scholar]
- Roegiers F, Younger-Shepherd S, Jan LY, Jan YN. Bazooka is required for localization of determinants and controlling proliferation in the sensory organ precursor cell lineage in Drosophila. Proc Natl Acad Sci USA. 2001a;98:14469–14474. doi: 10.1073/pnas.261555598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegiers F, Younger-Shepherd S, Jan LY, Jan YN. Two types of asymmetric divisions in the Drosophila sensory organ precursor cell lineage. Nat Cell Biol. 2001b;3:58–67. doi: 10.1038/35050568. [DOI] [PubMed] [Google Scholar]
- Rusten TE, Cantera R, Urban J, Technau G, Kafatos FC, Barrio R. Spalt modifies EGFR-mediated induction of chordotonal precursors in the embryonic PNS of Drosophila promoting the development of oenocytes. Development. 2001;128:711–722. doi: 10.1242/dev.128.5.711. [DOI] [PubMed] [Google Scholar]
- Self T, Mahoney M, Fleming J, Walsh J, Brown SDM, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- Shanbhag SR, Singh K, Singh RN. Ultrastructure of the femoral chordotonal organs and their novel synaptic organization in the legs of Drosophila melanogaster Meigen (Diptera: Drosophilidae) Int J Insect Morphol Embryol. 1992;21:311–322. [Google Scholar]
- Sharma Y, Cheung U, Larsen EW, Eberl DF. pPT-GAL, a convenient Ga14 P-element vector for Testing expression of the enhancer fragments in Drosophila. Genesis. 2002 doi: 10.1002/gene.10127. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J, Perrimon N, Axelrod J. Frizzled signaling and the developmental control of cell polarity. Trends Genet. 1998;14:452–458. doi: 10.1016/s0168-9525(98)01584-4. [DOI] [PubMed] [Google Scholar]
- Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particlles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol. 1999;147:519–530. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath JB. At the nexus between pattern formation and cell-type specification: the generation of individual neuroblast fates in the Drosophila embryonic central nervous system. BioEssays. 1999;21:922–931. doi: 10.1002/(SICI)1521-1878(199911)21:11<922::AID-BIES4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Doe CQ. Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development. 1998;125:1857–1865. doi: 10.1242/dev.125.10.1857. [DOI] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron. 1996;17:21–26. doi: 10.1016/s0896-6273(00)80277-9. [DOI] [PubMed] [Google Scholar]
- Spana EP, Kopczynski C, Goodman CS, Doe CQ. Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development. 1995;121:3489–3494. doi: 10.1242/dev.121.11.3489. [DOI] [PubMed] [Google Scholar]
- Sturtevant M, Biehs B, Marin E, Bier E. The spalt gene links the A/P compartment boundary to a linear adult structure in the Drosophila wing. Development. 1997;124:21–32. doi: 10.1242/dev.124.1.21. [DOI] [PubMed] [Google Scholar]
- Tauber E, Eberl DF. Song production in auditory mutants of Drosophila: the role of sensory feedback. J Comp Physiol A. 2001;187:341–348. doi: 10.1007/s003590100206. [DOI] [PubMed] [Google Scholar]
- Tauber E, Eberl DF. The effect of male competition on the courtship song of Drosophila melanogaster. J Insect Behav. 2002;15:109–120. [Google Scholar]
- Thurm U, Küppers J. Epithelial physiology of insect sensilla. In: Locke M, Smith DS, editors. Insect biology in the future. New York: Academic Press; 1980. pp. 735–763. [Google Scholar]
- Toivonen JM, O’Dell KMC, Petit N, Irvine SC, Knight GK, Lehtonen M, Longmuir M, Luoto K, Touraille S, Wang Z, Alziari S, Shah ZH, Jacobs HT. technical knockout, a Drosophila model of mitochondrial deafness. Genetics. 2001;159:241–254. doi: 10.1093/genetics/159.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordai H, Banyai L, Patthy L. The PAN module: the N-terminal domains of plasminogen and hepatocyte growth factor are homologous with the apple domains of the prekallikrein family and with a novel domain found in numerous nematode proteins. FEBS Lett. 1999;461:63–67. doi: 10.1016/s0014-5793(99)01416-7. [DOI] [PubMed] [Google Scholar]
- Townes PL, Brocks ER. Hereditary syndrome of imperforate anus with hand, foot, and ear anomalies. J Pediatr. 1972;81:321–326. doi: 10.1016/s0022-3476(72)80302-0. [DOI] [PubMed] [Google Scholar]
- Uga S, Kuwabara M. On the fine structure of the chordotonal sensillum in antenna of Drosophila melanogaster. J Electron Microsc. 1965;14:173–181. [Google Scholar]
- von Schilcher F. The function of pulse song and sine song in the courtship of Drosophila melanogaster. Anim Behav. 1976a;24:622–625. [Google Scholar]
- von Schilcher F. The role of auditory stimuli in the courtship of Drosophila melanogaster. Anim Behav. 1976b;24:18–26. [Google Scholar]
- Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, Kurima K, Wilcox ER, Friedman TB, Griffith AJ, Balling R, Hrabé de Angelis M, Avraham KB. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet. 2002;30:257–258. doi: 10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, Kelley PM, Kimberling WJ, Wagenaar M, Levi-Acobas F, Larget-Piet D, Munnich A, Steel KP, Brown SDM, Petit C. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Weil D, Küssel P, Blanchard S, Lévy G, Levi-Acobas F, Drira M, Ayadi H, Petit C. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet. 1997;16:191–193. doi: 10.1038/ng0697-191. [DOI] [PubMed] [Google Scholar]
- Wheeler DA, Fields WL, Hall JC. Spectral analysis of Drosophila courtship songs: D. melanogaster, D. simulans, and their interspecific hybrid. Behav Genet. 1988;18:675–703. doi: 10.1007/BF01066850. [DOI] [PubMed] [Google Scholar]
- Wicks SR, de Vries CJ, van Luenen HGAM, Plasterk RHA. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol. 2000;221:295–307. doi: 10.1006/dbio.2000.9686. [DOI] [PubMed] [Google Scholar]
- Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127:3373–3383. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- zur Lage P, Jarman AP. Antagonism of EGFR and Notch signalling in the reiterative recruitment of Drosophila adult chordotonal sense organ precursors. Development. 1999;126:3149–3157. doi: 10.1242/dev.126.14.3149. [DOI] [PubMed] [Google Scholar]
- zur Lage P, Jan YN, Jarman AP. Requirement for EGF receptor signalling in neural recruitment during formation of Drosophila chordotonal sense organ clusters. Curr Biol. 1997;7:166–175. doi: 10.1016/s0960-9822(97)70087-3. [DOI] [PubMed] [Google Scholar]


