Abstract
Cochlear sensory cells and neurons receive efferent feedback from the olivocochlear (OC) system. The myelinated medial component of the OC system, and its effects on outer hair cells (OHCs), has been implicated in protection from acoustic injury. The unmyelinated lateral (L)OC fibers target ipsilateral cochlear nerve dendrites, and pharmacological studies suggest the LOC's dopaminergic component may protect these dendrites from excitotoxic effects of acoustic overexposure. Here, we explore LOC function in vivo via selective stereotaxic destruction of LOC cell bodies in mouse. Lesion success in removing the LOC, and sparing the MOC, was assessed by histological analysis of brainstem sections and cochlear whole-mounts. Auditory brainstem responses (ABR), a neural-based metric, and distortion product otoacoustic emissions (DPOAEs), an OHC-based metric, were measured in control and surgical mice. In cases where the LOC was at least partially destroyed, there were increases in suprathreshold neural responses that were frequency- and level-independent, and not attributable to OHC-based effects. These interaural response asymmetries were not found in controls, or in cases where the lesion missed the LOC. In LOC-lesion cases, after exposure to a traumatic stimulus, temporary threshold shifts were greater in the ipsilateral ear, but only when measured in the neural response; OHC-based measurements were always bilaterally symmetric, suggesting OHC vulnerability was unaffected. Interaural asymmetries in threshold shift were not found in either unlesioned controls or in cases which missed the LOC. These findings suggest that the LOC modulates cochlear nerve excitability and protects the cochlea from neural damage in acute acoustic injury.
Keywords: Cochlea, Excitotoxicity, Noise-induced hearing loss, Feedback control
Introduction
Vertebrate hair cell systems, including the cochlea, vestibular organs and lateral line, are modulated by efferent feedback (for review, see Guinan 1996). In the mammalian cochlea, efferent feedback is provided by the olivocochlear (OC) bundle, which comprises two components (Figure 1A,B). Myelinated medial (M)OC neurons originate in contralateral and ipsilateral peri-olivary nuclei and innervate outer hair cells (OHCs). Unmyelinated lateral (L)OC neurons originate in and around the ipsilateral lateral superior olive (LSO) and innervate primarily afferent dendrites of the cochlear nerve near their synapses with inner hair cells (IHCs).
Figure 1.
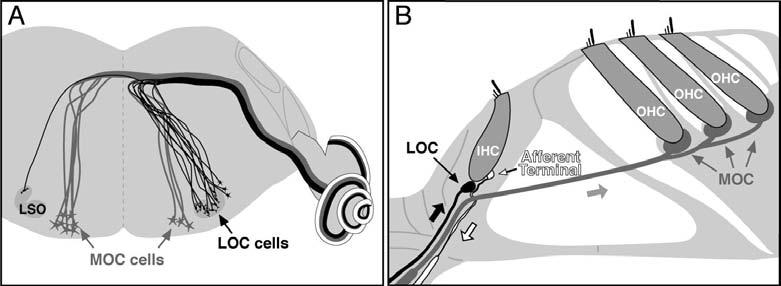
Anatomical schematics illustrate the central origins (A) and peripheral projections (B) of the medial and lateral components of the olivocochlear (OC) efferent system. Bold arrows in B indicate direction of action potential transmission. Estimates of MOC and LOC distributions patterns in mouse, i.e. 75% contralateral and 99% ipsilateral (A) are from Campbell and Henson (1988).
MOC terminals on OHCs are cholinergic, and when activated, suppress cochlear responses by decreasing the normal contribution of OHCs to amplification of cochlear mechanical vibrations (for review, see Guinan 1996). LOC terminals are more complex cytochemically, with immunohistochemical evidence for cholinergic, GABAergic, dopaminergic and peptidergic transmission (for review see Eybalin 1993). Morphological studies suggest two LOC subgroups based on cell-body location and extent of peripheral ramification (Brown 1987; Warr et al. 1997). Immunohistochemistry suggests a corresponding cytochemical dichotomy between a cholinergic/GABAergic and a dopaminergic subgroup (Darrow et al. 2006a). Consistent with the observation that LOC activation, via inferior colliculus stimulation, can evoke either slow enhancement or suppression of auditory nerve response (Groff and Liberman 2003), it can be hypothesized that the former effect stems from the cholinergic subgroup, and the latter effect from the dopaminergic subgroup.
Many studies have implicated OC feedback in protecting the cochlea from acoustic injury: electrical stimulation of the OC bundle reduces temporary threshold shifts from acoustic overexposure (Rajan 1988; Reiter and Liberman 1995), and chronic section of the OC bundle renders the ear more vulnerable to permanent acoustic injury (Handrock and Zeisberg 1982; Kujawa and Liberman 1997). Although OC contributions to protection are well documented, ambiguity remains as to the relative contributions of LOC vs. MOC systems, given that: 1) LOC and MOC axons run together in the OC bundle, thus both electrical stimulation and surgical section experiments could theoretically involve both systems, and 2) both LOC and MOC systems have cholinergic components, thus the pharmacological blockers used may not be selective.
The most definitive evidence for a protective effect of the cholinergic MOC system is the observation that mice with over-expression of 9 cholinergic receptors in OHCs have enhanced resistance to permanent and temporary acoustic injury (Maison et al. 2002). Although no direct evidence exists for an LOC protective role, it has been suggested (e.g. Ruel et al. 2001) that one LOC function is controlling what appears to be glutamate excitotoxicity in afferent nerve terminals in the acute stages of acoustic injury (Liberman and Mulroy 1982; Robertson 1983; d'Aldin et al. 1995; Puel et al. 1998): cochleas perfused with dopamine antagonists show vacuolization of cochlear terminals which mimic that seen after acoustic overexposure (Ruel et al. 2001), and infusion of a dopamine agonist prior to noise exposure reduced the extent of threshold shift and the amount of neural swelling (d'Aldin et al., 1995).
To directly assess the contribution of the LOC system to protection, we stereotaxically lesioned LOC neurons (Le Prell et al. 2003) in adult mice and compared noise-induced threshold shifts following binaural noise exposure in the ears ipsilateral and contralateral to the lesion. The integrity of the MOC pathway was verified both morphologically (in the brainstem and cochlea) and functionally (with bilateral measurement of MOC-mediated suppression of cochlear responses). In animals with unilateral LOC lesions, the ear ipsilateral to the lesion showed larger threshold shifts than the contralateral ear, but only when measured via the cochlear neural responses (ABR Wave 1); threshold shifts measured by OHC based responses (otoacoustic emissions) remained bilaterally symmetric. Results are consistent with an LOC-mediated protection of the cochlear nerve dendrites during acoustic overexposure.
Materials and Methods
Stereotaxic Surgery
All procedures were approved by the IACUC of the Massachusetts Eye and Ear Infirmary. Experimental animals were CBA/CaJ mice of either sex aged 6-8 weeks, weighing between 25-30g. Following anesthesia with xylazine (20 mg/kg i.p.) and ketamine (100 mg/kg i.p.), the mouse was held in a Kopf small-animal stereotaxic apparatus by snout clamp and ear bars. The skin overlaying the skull was slit and retracted to reveal the bregma and lambdoidal sutures. Rongeurs were used to make an opening in the skull over the right lambdoidal suture. Using coordinates modified from Franklin and Paxinos (1997), a micropipette filled with a 10 mM solution of the cytotoxin melittin (in saline) was lowered into the brain at a position 0.49 mm caudal and 0.12 mm lateral to the bregma. When a depth of 0.69 mm was reached, 2 μl of solution was injected via a 10 μl syringe (Hamilton) coupled to the micropipette. Immediately after injection, the scalp was sutured, and the animal placed in a padded cage with heat lights for ∼1 hr post surgery. A total of 18 mice underwent surgery. Animals in which the injection missed the target (see below) served as surgical controls, and 8 mice without surgery were included as an additional control.
ABR and DPOAE Measurements
Both ABRs and DPOAEs were recorded at 2 and 4 wks post-surgery, and again 6 hrs and 1 wk post-acoustic overexposure. For ABRs and DPOAE recordings, mice were anesthetized with ketamine and xylazine (same dose as for surgery), and needle electrodes were inserted at the vertex and pinna. The ABR was evoked with 5-ms tone pips (0.5-ms rise–fall, with a cos2 envelope, at 35/sec). The response was amplified (10,000x), filtered (0.1–3 kHz), and averaged with a digital I-O board in a PC-based data-acquisition system. Acoustic stimuli were delivered through a closed acoustic coupler housing two electrostatic drivers as sound sources (TDT EC-1, Tucker Davis Technologies) and one electret microphone (Knowles) at the end of a probe tube to measure sound pressures in situ. Sound level was raised in 5-dB steps from 0 to 80 dB SPL. At each level, 1,024 responses were averaged (with stimulus polarity alternated) after ‘artifact rejection’. Threshold was determined by visual inspection. DPOAEs at 2f1-f2 were recorded in response to primary tones: f1 and f2, with f2/f1 = 1.2 and f2 level 10 dB < f1 level. FFTs were computed and averaged over 5 waveform traces, and 2f1-f2 DPOAE amplitude and surrounding noise floor were extracted. Iso-response contours were interpolated from plots of amplitude vs. sound level, performed in 5 dB steps of primary level. “Threshold” is defined as the primary level (f2) required to produce a DPOAE at 0 dB SPL.
Acoustic Overexposure
Animals were acoustically over-exposed at 5 wks post-surgery. The animals were exposed free-field, awake and unrestrained, in a small reverberant chamber. Acoustic trauma consisted of a 15-minute exposure to an 8-16 kHz octave band noise presented at 94 dB SPL. Exposure level was measured at four positions inside the cage and varied by < 0.5 dB.
Medial Olivocochlear (MOC) Assay
MOC assays were performed on 6 control animals (no surgery and no acoustic exposure) and a randomly chosen subset (n=12) of the experimental animals (at least 1 wk after the exposure to noise). Animals were anesthetized as for ABR and DPOAE testing, and a posterior craniotomy and partial cerebellar aspiration exposed the floor of the IVth ventricle. Shocks (monophasic 150 μsec pulses at 200/sec) were applied through silver wires at the midline. Shock threshold for facial twitch was determined, paralysis induced with α-d-tubocurarine (1.25 mg/kg i.p.), and the animal connected to a respirator. Shock levels were raised 6 dB above twitch threshold. The MOC suppression effects on DPOAEs were then assessed in both cochleae. f2 level was set to produce a DPOAE ∼10 dB > noise floor. Repeated measures of baseline DPOAE amplitude (n=12) were made prior to a series of 17 contiguous periods in which DPOAE amplitudes were repeatedly measured during a continuous 70-sec shock train to the OC bundle. The shock epoch was followed by a series of DPOAE amplitude measurements (n=36) to observe the extinction of the MOC effect.
Histological Preparation
After final testing (∼7 wks post-surgery), animals were perfused intracardially with 10% formalin. The brainstems were extracted, post-fixed overnight, cryoprotected in sucrose, frozen and cut on a sliding microtome at 80 μm in the transverse plane. Slide-mounted sections were stained for acetylcholinesterase (AChE) activity to allow for visualization of the cholinergic OC cells in the brainstem (Osen and Roth 1969), as well as to verify that the microelectrode pipette did not sever the OC bundle. Cochleas on both sides were extracted, post-fixed overnight, decalcified in EDTA for ∼48 hrs, dissected into half-turn segments and double immunostained for cholinergic and dopaminergic markers: rabbit anti-VAT (vesicular acetylcholine transporter; Sigma catalog #V5387, at 1:1000) and sheep anti-TH (tyrosine hydroxylase; from Calbiochem, catalog #657014, at 1:500), respectively. Primaries were followed by a species-appropriate fluorophore-coupled secondary at 1:1000 (VAT - Alexafluor 568, TH - Alexafluor 488).
Morphometric Analysis
Brainstems
The location, size and success of the brainstem lesion were quantified by comparing the area of the lateral superior olive (LSO) on both sides of the brainstem. A drawing tube was used to trace the outline of surviving LSO cells in all sections from its caudal to rostral extreme. Tracings were digitized, and the areas of both medial and lateral limbs were determined by computerized planimetry.
Cochleas
Cochlear location was converted to frequency (Muller et al. 2005), and 10 log-spaced frequency loci were identified in each case. At each locus in each case, an observer blind to the physiology and brainstem analyses separately rated the innervation densities of VAT- and TH-positive terminals in both the inner and outer hair cell areas. A 3-point scale was used for VAT-positive terminals in the OHC area: the observer's task was to estimate the fraction of OHCs with at least one VAT-positive terminal: 3 = 100-67%, 2 = 66-34%, and 1 = 33-0%. VATand TH-positive terminals in the IHC area were evaluated with a 4-point scale: 3 = profuse, 2 = moderate, 1= sparse and 0 = none. Each immunostain was separately referenced to its own maximum values: i.e. TH-positive terminals are much rarer than VAT terminals, but maximum density for each would receive a rating “3”. To normalize the data, the cochlear spiral dimension was divided into 10 equal frequency bins; within each bin, ratings from all contralateral ears were averaged for each area (IHC vs OHC) and for each immunostain (TH vs VAT). Then, all values, whether from contra or ipsi ears, were referenced to the maximum mean value across all frequency bins for that area and for that immunostain. Further details can be found in the caption of Figure 3.
Figure 3.
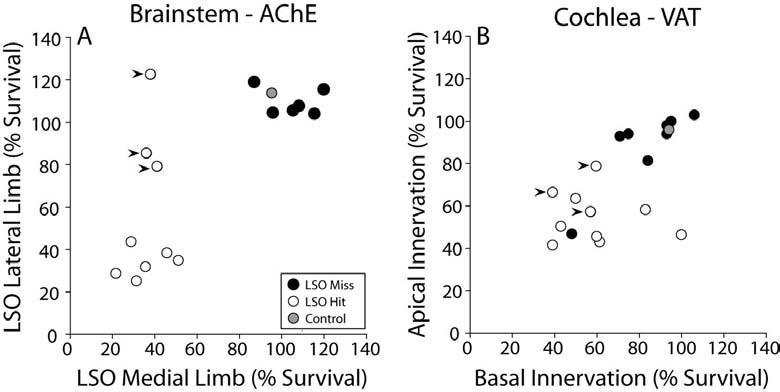
Analysis of lesion success based on (A) the fractional survival of the LSO, as seen in AChE-stained brainstem sections, and (B) the fractional survival of cholinergic terminals in the IHC area, as seen in immunostained cochlear whole mounts. Filled arrowheads in B are the three cases in A (filled arrowheads) where the lesion affected the medial limb only. A: Fractional survival is the total surviving area (as depicted in Fig. 2A,B) of each LSO limb from its rostral to caudal extent (the LSO spans ∼480 μm in the rostro-caudal plane), normalized to the mean area of control sides. B: Fractional survival of cholinergic (VAT-positive) terminals in the IHC area is calculated by dividing the cochlear spiral into two bins (apical vs. basal to the midpoint), and averaging the semi-quantitative estimates of fractional survival in each bin for each case. See Methods for further details.
Results
A. Histological assessment of completeness and selectivity of the LOC lesion
Brainstems and cochlear whole mounts were evaluated to assess the completeness and selectivity of the lesions, i.e. the extent to which they were successful in eliminating the LOC system and sparing the MOC system and the stapedius muscle reflex.
1. Brainstems
When the cytotoxin (melittin) successfully targeted the LSO, there was a clear loss of cholinergic neurons from the LSO area and a clear disruption of the LSO neuropil. The paired micrographs in Figures 2A and 2B compare surviving portions of the LSO from opposite sides of the brainstem in one injected case, i.e. regions where cholinergic cell bodies remained. Because the LSO is tonotopically organized (Kelly et al. 1998), we separately assessed the areas of the lateral (low-frequency) and medial (high-frequency) limbs. The fractional survival of medial vs. lateral limbs (Fig. 3A) suggests a clear distinction between LSO Hit and LSO Miss cases. The data also show that in a subset of cases (n=3), there was greater success in destroying the medial limb than the lateral limb (arrowheads in Fig. 3A).
Figure 2.
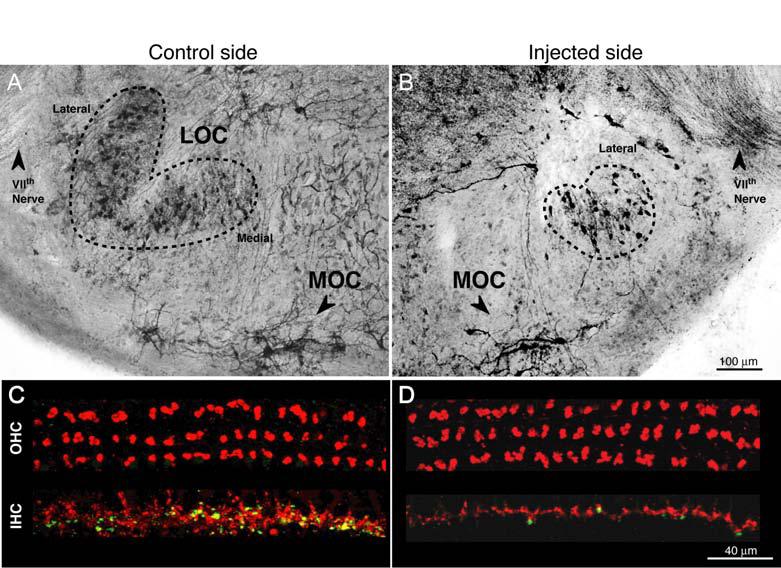
Histological verification of LOC lesions, as seen in AChE-stained brainstem sections (A,B) or in cochlear whole mounts double-immunostained for a cholinergic marker (red) and a dopaminergic marker (green). (C,D). A,B: Brainstems are from opposite sides of one LOC Hit case. Dashed lines indicate the outline of the surviving LSO in each section: on the control side, lateral and medial limbs are indicated. The AChE-positive fibers of the VIIth nerve, visible in each section, were used to identify comparable rostro-caudal locations on the two sides. Scale in B also applies to A. C,D: images are from the 22.6 kHz region of opposite sides of a LOC Hit case (different from the one shown in A and B). Scale in D also applies to C.
When estimating the extent of lesion success on the LOC cell bodies, it is also important to assess, in both the Hit and Miss cases, which others structures were damaged. In particular the extent of MOC involvement is key to interpreting the results. As schematized in Figure 4, the cholinergic MOC cells form an elongate cluster, the caudal end of which is ventro-medial to the LSO, and the rostral end which extends well beyond the rostral tip of the LSO (Campbell and Henson 1988; Brown 1993). In each LSO Hit case (Fig. 4A, n=10), the injection destroyed part of the LSO; in only one case (arrowhead in Sections 11 and 13) did the lesion appear to significantly impinge on a portion of the MOC cell cluster, near its rostral extent. In half of the LSO Miss cases (n=4), there was no sign of injection, suggesting that the pipet tip may have clogged. In the remaining LSO Miss cases (n=4), the lesion was always rostral to the LSO (Fig. 4B). In one LSO Miss case, the lesion impinged on MOC cell bodies, rostral to the LSO (Fig. 4B: arrowheads in Sections 11 and 13).
Figure 4.
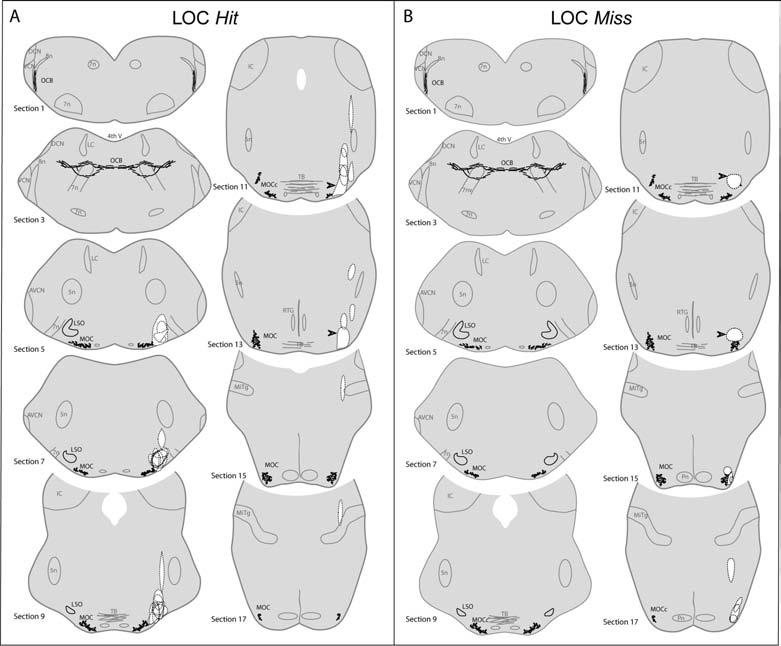
Lesion locations in all LOC Hit cases (A) and LOC Miss cases (B). In each case, the lesion is outlined (dashed lines, white centers) and superimposed on “atlas” sections (derived from an AChE-stained control mouse). Alternate 80 μm sections are shown: Section 1 is the most caudal, and Section 17 the most rostral to include the OC system. One case from each group with partial damage to MOC system is indicated by arrowheads in Sections 11 and 13. Abbreviations are: 4th V,4th ventricle; 5n, trigeminal nerve or nucleus; 7n, facial nerve or nucleus; 8n, cochlear nerve; AVCN, anteroventral cochlear nucleus; DCN, dorsal cochlear nucleus; IC, inferior colliculus; LC, locus coeruleus; LSO, lateral superior olive; MiTg, Microcellular tegmental nucleus; MOC, medial olivocochlear cells; OCB, olivocochlear bundle; Pn, pontine nuclei; RTG, reticulotegmental nucleus; TB, trapezoid body.
In addition to the MOC system, the innervation of the stapedius muscle also needs to be assessed, given that the stapedius reflex has also been implicated in cochlear protection (Henderson et al. 1994; Ryan et al. 1994). Retrograde labeling of stapedius motorneurons in rat (Shibayama et al. 1990) show a majority population of neurons in the ipsilateral brainstem ventro-medial to the facial nucleus, and a minority population (∼6%) scattered more rostrally near the facial nerve exit from the brainstem. Assuming that stapedius motorneurons are similarly located in mouse, the lesion in some hit cases may have included some stapedius motorneurons near the facial nerve exit (Fig. 4A: Sections 5 and 7).
2. Cochleas
Consistent with cochlear projections and laterality of LOC and MOC systems (Fig. 1A), successful and selective LSO Hit cases showed: 1) reduction of OC terminals in the IHC area, and not the OHC area, of the ipsilateral ear , and 2) no change in OC terminal density of the IHC and OHC areas in the contralateral ear (see Figs. 2C,D). Since the LOC innervation of the IHC area in mice comprises both cholinergic and dopaminergic terminals (Darrow et al. 2006a), each cochlea was double-stained with VAT and TH, respectively. Innervation densities in the IHC and OHC areas were semi-quantitatively evaluated by an observer blind to both brainstem histology and cochlear physiology. As shown in Figure 5, in cases classified as LSO Hit based on the brainstem histology (Fig. 3A), there was, on average, ∼50% reduction in the density of both cholinergic and dopaminergic terminals in the ipsilateral IHC area (Figs. 5A and B, respectively), without obvious reductions in the OHC area (Fig. 5C). There was no significant change of ipsilateral innervation of IHC or OHC areas in LSO Miss cases; nor was there any loss of IHC innervation contralaterally in either surgical group (Figs. 5A-C). In the contralateral OHC area, a modest, but clear-cut loss of efferent innervation was noted in two cases (Fig. 5D).
Figure 5.
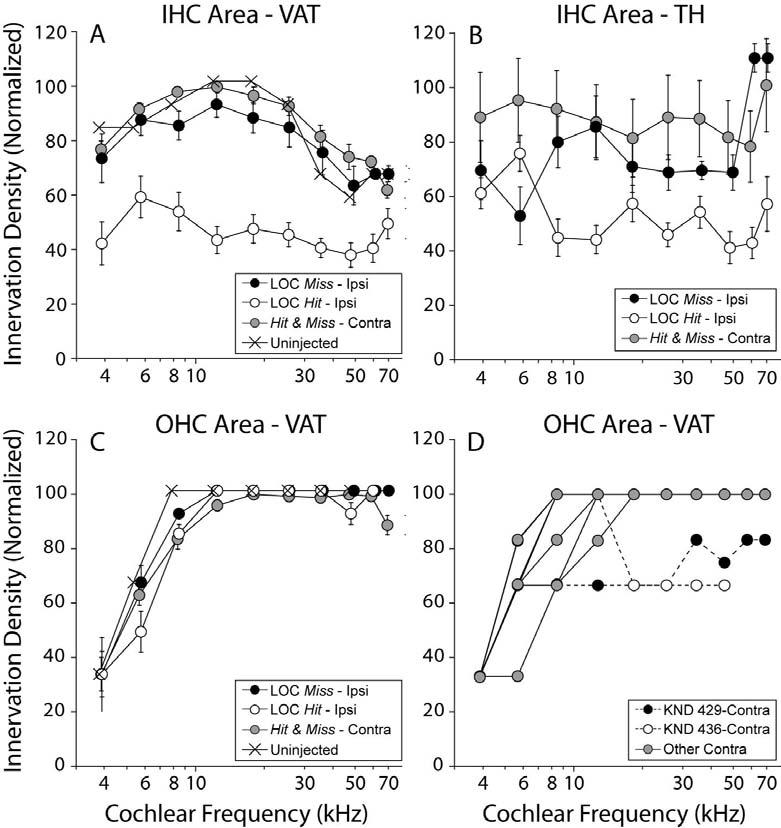
Semi-quantitative analysis of cholinergic (VAT: A,C,D) and dopaminergic (TH: B) markers in the IHC (A,B) and OHC (C,D) areas. In each cochlea, innervation density was estimated at 10 locations along the cochlear spiral (apical and basal halves of each of 5 dissected pieces). In A-C, the mean (±SEM) innervation density in ipsilateral ears from LOC Hit (open circles; n = 10) and LOC Miss (dark-filled circles; n = 8) are compared to all contralateral ears (grey-filled circles; n = 19) and uninjected controls (n=2). The key in A also applies to B and C. In D, individual contralateral ears from C are shown to indicate the two cases (dashed lines) with decreased terminal density in the OHC area. See Methods for description of analysis procedures.
B. Correlating Cochleas and Brainstems
Considered individually, each case classified as LSO Hit based on the brainstem sections (Fig. 3A) showed a reduction of OC terminals in its ipsilateral cochlea (Fig. 3B); these 9 cases are unambiguously classified as “LOC Hits”. In one injected case, the ipsilateral cochlea showed obvious loss of OC terminals, however the cholinergic cells in the LSO appeared intact. It is possible that this exceptional case arose because the pipette or the toxin destroyed OC axons without affecting the cell bodies. Nevertheless, this case is considered ambiguous and has been removed from further consideration. There was no obvious correlation between the apical-basal gradient of cochlear de-efferentation and the destruction of medial vs. lateral limb of the LSO: in the three brainstems with a “medial limb only” lesion (arrowheads in Fig 3A), there was a decrease in LOC terminals in both the apex and base of the cochlea (arrowheads in Fig. 4B indicate the same three cases). This discrepancy may also reflect the fact that some of the LOC cell bodies survived, though their peripheral projections, or other interneurons in the local neural circuitry driving them, had degenerated.
On a case-by case-basis, there was a good correlation between the brainstem and cochlear data with respect to MOC involvement. As shown in Figure 5D, the cochlear analysis identified only two cases as having a decrease in efferent terminals in the OHC area of the contralateral ear; Correspondingly, these two cases, one LOC Hit and one LOC Miss, are the only cases for which the brainstem analysis suggested that the lesion had impinged on MOC cell bodies (Fig. 4). The contralateral effect of the loss is expected based on the largely contralateral projection of the MOC system (Fig. 1A).
C. Functional Integrity of the MOC System
In addition to assessing the morphological integrity of the MOC system, we assessed MOC function by electrically stimulating the OC bundle at the midline and while recording suppression of distortion product otoacoustic emissions (DPOAEs) in both ears. DPOAEs are distortions produced and amplified by normal healthy OHCs, which are propagated as mechanical vibrations back through the middle ear to the ear canal, where they can be measured in the sound pressure waveform. Because activation of the MOC feedback system essentially turns down the gain of the OHC electromechanical amplifier (Murugasu and Russell 1996; Cooper and Guinan 2003), the suppression of DPOAEs provides a rapid and reliable assay of MOC activation level. The maximum effect of MOC stimulation on DPOAE amplitude is for primary tones near 22.6 kHz (Maison et al. 2002, 2003), thus we show data only for 22.6 kHz (Fig. 6), however, results at other test frequencies were similar.
Figure 6.
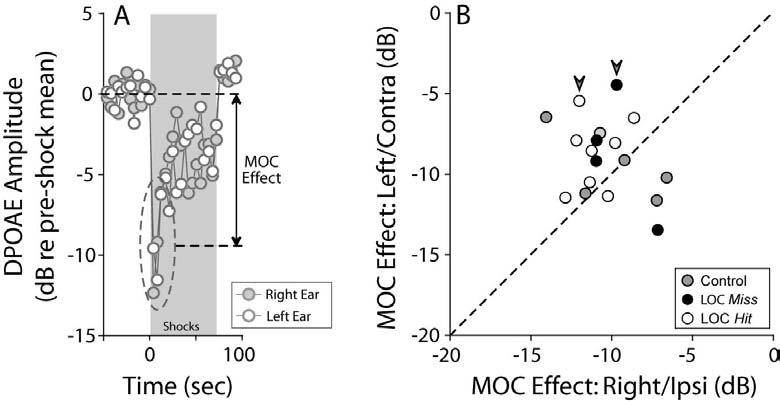
Bilateral suppression of DPOAEs, elicited via midline electrical stimulation of MOC fibers, suggests that MOC function is minimally affected by the lesions. A: One run of the MOC assay in a control case demonstrates symmetrical DPOAE suppression in right and left ears. “MOC effect” is defined as the dB difference between mean DPOAE amplitude in the first three measures after shock onset, compared to the pre-shock baseline. B: MOC effects in right and left ears of control, LOC Hit and LOC Miss cases. Large arrowheads indicate the cases with histological evidence of MOC lesions (Figures 3, 4, and 5D). For data shown, f2 was 22.6 kHz.
The bilateral symmetry of MOC effect size did not differ significantly in control vs. injected cases (Fig. 6), suggesting that: 1) MOC function is relatively symmetric in animals with normal OC innervation, and 2) our lesions did not significantly perturb MOC function. Interestingly, the two injected cases for which there was histological evidence of MOC involvement (Figs. 4, 5D) showed MOC effects roughly half as large in the contralateral vs. the ipsilateral ear (arrowheads in Fig. 6B). The paired reduction of contralateral MOC effects and contralateral OHC terminals agrees with the known projection patterns of the MOC system (Fig. 1).
D. Effect of LOC lesion on cochlear threshold and supra-threshold responses
Cochlear thresholds, as measured by either auditory brainstem responses (ABRs) or DPOAEs, were unaffected by the LOC lesions: mean values were not significantly different between ears or lesion-success groups (Fig. 7A-D) or compared to non-surgical control mice (data not shown). However, significant binaural asymmetries of suprathreshold neural responses (ABR; Figs. 8A,B) were observed in LOC Hit cases, without corresponding changes in DPOAE (Fig. 8C,D). The ABR represents the summed activity of auditory neurons along the ascending afferent pathway, and the earliest wave, Wave 1, represents the activity of the cochlear nerve. The DPOAEs reflect events “upstream” of the ABR, in the sense that the OHCs' contribution to cochlear amplification is a necessary but not sufficient component of a normal ABR response. The latter also relies on the integrity of synaptic transmission between the IHCs and the cochlear nerve, and of the cochlear nerve fibers themselves. Thus, selective changes in ABR amplitudes without accompanying shifts in DPOAE responses are consistent with expected LOC-based effects on neural activity only.
Figure 7.
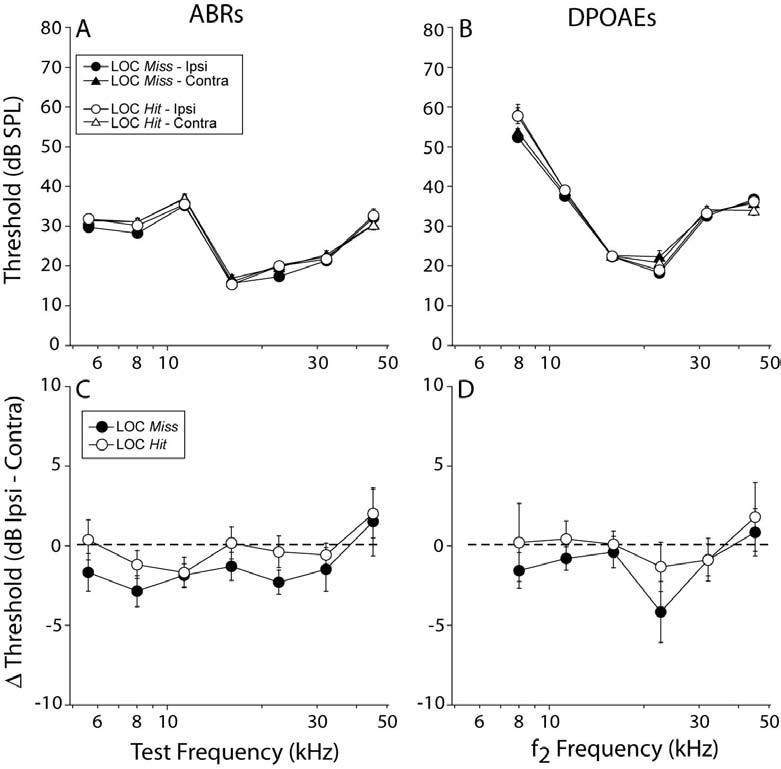
Mean cochlear thresholds (±SEM), as measured by ABR (A) and DPOAE (B) were not affected by a successful LOC lesion, nor were interaural threshold differences as measured by either ABR (C) or DPOAE (D). Keys in A and C also apply to B and D, respectively.
Figure 8.
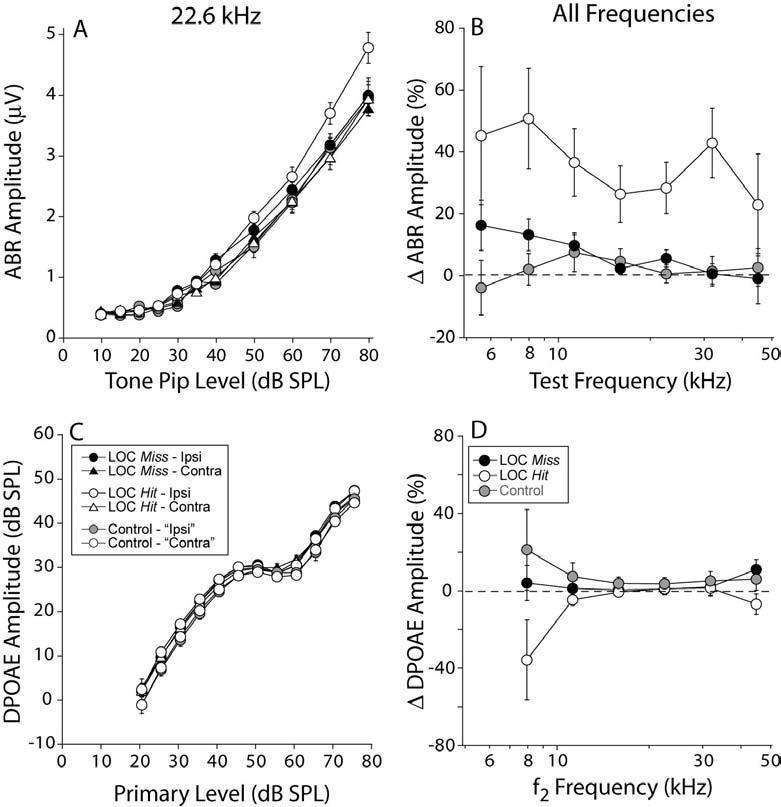
ABR amplitudes (A,B) are enhanced in the ipsilateral LOC Hit ears and not in LOC Miss ears, whereas DPOAE amplitudes (C,D) are unaffected in all groups. A,C: Mean (±SEM) amplitude vs. level functions for ABR and DPOAE, respectively, for responses at 22.6 kHz: key in panel C applies to A. B,D: mean interaural discrepancies in ABR and DPOAE amplitudes, respectively. Key in D applies to B. See text for further details.
In LOC Hit cases, mean ABR amplitudes were enhanced in the ipsilateral ear (Fig. 8A): the difference between the two ears was statistically significant in the Hit cases (e.g. at 22.6 kHz: p = 0.019, F = 6.007, by two way ANOVA) and were not in the Miss cases (e.g. at 22.6 kHz: p = 0.387, F= 0.762, by two way ANOVA)1. This enhancement of ABR amplitude was roughly a constant percentage as tone level increased (data not shown). Thus, to summarize changes across frequency, the mean interaural amplitude difference (expressed as a percentage) across the higher sound levels (50-80 dB SPL) was computed for each frequency, for each case, and then averaged across cases within each group. When viewed in this way, ABR enhancements are seen across all test frequencies (Fig. 8B), and not in DPOAEs (Fig. 8D). In those cases where the brainstem lesion appeared to spare the lateral (low-frequency) limb (Fig. 3A arrowheads) there was no obvious difference in ABR enhancements between low and high frequency regions, thus mirroring the lack of a base-apex gradient in the cochlear efferent innervation in these same cases.
E. Effect of LOC lesion on vulnerability to acoustic injury
Dendritic vacuolization in the IHC area, the morphological sign of glutamate excitotoxicity, is only seen in the acute stages (<24 hours) of acoustic injury (Liberman and Mulroy 1982; Robertson 1983; Puel et al. 1998); thus, we designed an exposure stimulus (94 dB at 8-16 kHz for 15 min) to create a moderate (∼35 dB) threshold shift when tested 6 hrs post-exposure (Fig. 9A,B), and to recover when tested at 1 wk post-exposure (Fig. 9E,F).
Figure 9.
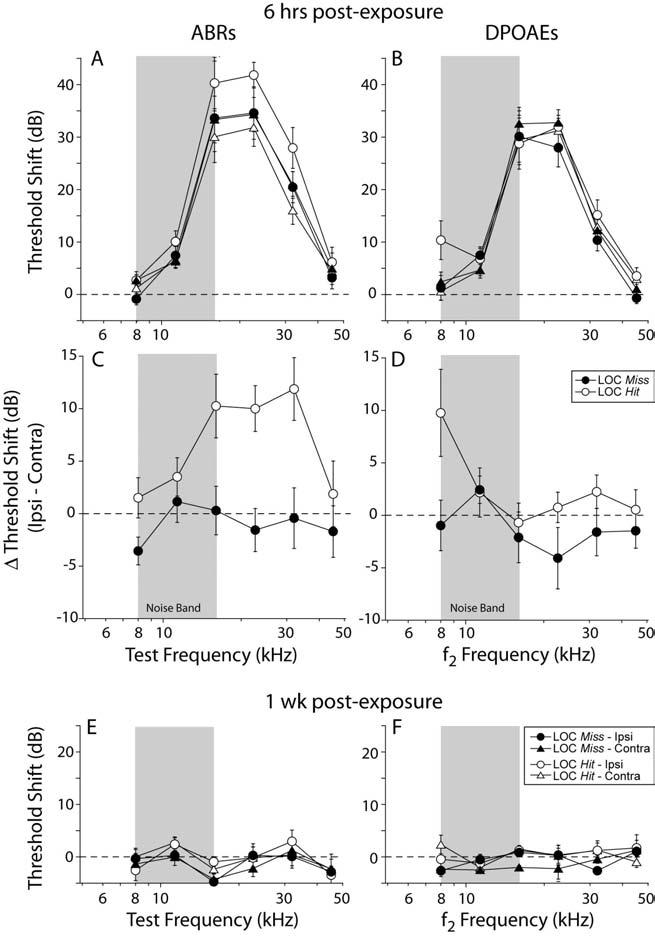
Mean ABR threshold shifts in LOC Hit mice, 6 hrs after acoustic overexposure, were 10-15 dB higher in the ipsilateral ear; this asymmetry was not present in LOC Miss cases (A,C) or in the mean DPOAE data from either Hit or Miss groups (B,D). A,B: threshold shift is defined as the difference from mean pre-exposure values for the same group. C,D: interaural threshold-shift difference defined as thresholds in ipsilateral minus contralateral ears of each case. At 1 wk post exposure, mean ABR (E) and DPOAE (F) threshold shifts returned to pre-exposure levels. Key in F, also applies to A,B and E. Key in D also applies to C. Error bars in all panels indicate ±SEMs. Grey box indicates noise exposure bandwidth.
Following exposure, threshold shifts in the LOC Miss cases were bilaterally symmetrical at 6 hrs post exposure, whether measured by ABRs (Fig. 9A,C) or DPOAEs (Fig 9B,D). Furthermore, threshold shifts in the LOC Miss cases were of similar magnitude when measured by ABRs (Fig. 9A) or DPOAEs (Fig. 9B), suggesting that the functionally important changes are occurring at, or “upstream” of, the OHCs.
In contrast, in the LOC Hit cases, the ABR threshold shifts were significantly larger in the ipsilateral ear when compared to either the contralateral ear or either of the LOC Miss ears (Fig. 9A,C). Differences between the two ears of LOC Hit mice were highly significant (p = 0.001, F = 17.385, by two way ANOVA). Although it appears that the mean threshold shifts of the contralateral ears in the LOC Hit group were slightly lower then those from the LOC Miss cases, the differences were not statistically significant (p < .525, F= 0.426, by two way ANOVA). Importantly, the interaural asymmetries in ABR threshold shift were not mirrored in the DPOAE data (Fig. 9B,D), indicating the additional vulnerability arising from the loss of the LOC system involves additional damage to IHCs or neural elements, not to OHC function. The interaural symmetry of the DPOAE-based threshold shifts, and the lack of differences between Hit and Miss cases, also argues strongly against the possibility that differences in stapedius reflex strength underlies the differences in vulnerability.
Discussion
A. Peripheral effects of the LOC system in modulating neural excitability
Peripheral effects of the LOC system have been studied by electrical stimulation, pharmacological or genetic manipulation of transmitter/receptor combinations, and surgical lesion (see below). In these studies, LOC effects are characterized by level-independent, and frequency independent changes in cochlear neural evoked potentials without corresponding alterations in OHC-based responses such as cochlear microphonics or DPOAEs (e.g. Groff and Liberman, 2003; Le Prell et al., 2003). A similar constellation of effects is reported here after selective LOC lesion (e.g. Figure 8).
The complex cytochemistry and pharmacology of the LOC system make it difficult to predict the effects of its removal. Activating the LOC pathway via electrical stimulation of the inferior colliculus suggests there are two functional subsystems capable of eliciting slow suppressive or excitatory effects on cochlear nerve activity (Groff and Liberman 2003). Correspondingly, the LOC system is cytochemically heterogeneous: co-localization studies suggest that a majority population of cholinergic cells also contain GABA, while a minority population is dopaminergic (Darrow et al. 2006a). There is also immunohistochemical evidence for peptide transmitters (urocortin, CGRP and opioids) in LOC terminals: although evidence is incomplete, these transmitters may also co-localize in the cholinergic terminals (see Eybalin 1993 for review). Pharmacological studies with agonists and antagonists of putative LOC neurotransmitters have suggested both excitatory and inhibitory effects on cochlear nerve activity. Cochlear perfusion of certain opioid agonists enhance cochlear nerve gross potentials (Sahley et al., 1991). ACh perfusion increased both spontaneous and glutamate-induced spiking (Felix and Ehrenberger 1992). In contrast, GABA did not affect spontaneous activity, but decreased glutamate-induced, and ACh-induced activity (Felix and Ehrenberger 1992; Arnold et al. 1998), while dopamine decreased both spontaneous and sound-driven activity (d'Aldin et al. 1995; Oestreicher 1997; Ruel et al. 2001). CGRP application increased spontaneous rates, and decreased mechanically driven rates, of primary afferents in the lateral line (Bailey and Sewell 2000). Conversely, targeted gene deletion of CGRP in mice decreased suprathreshold neural responses, without corresponding changes in DPOAE amplitudes (Maison et al. 2003).
Previous lesion studies have taken four approaches to studying LOC function: 1) cut the entire OC bundle, thereby interrupting both MOC and LOC fibers (e.g. Liberman 1990; Zheng et al. 1999); 2) cut only the crossed OC bundle, thereby interrupting 2/3 of the MOC while sparing the LOC (e.g. Kujawa and Liberman 1997); 3) stereotaxically lesion the LSO/LOC system (Le Prell et al. 2003); or 4) perfuse the cochlea with agents designed to selectively destroy one class of LOC neurons (Le Prell et al 2005). Data from the first two approaches suggest that the LOC can modulate spontaneous and sound-evoked discharge rates in cochlear nerve fibers without large effects on threshold or tuning (Liberman 1990; Walsh et al. 1998). In chinchillas, complete de-efferentation (approach 1) increased sound-driven discharge rates (Zheng et al. 1999), suggesting that resting tone in the LOC pathway tends to suppress sound-evoked responses, consistent with the post-lesion enhancement of ABR amplitudes seen here (Figure 8). In contrast, in guinea pig, LOC lesion (approach 3) decreased the amplitudes of cochlear neural response (Le Prell et al. 2003), suggesting that resting LOC tone tends to enhance auditory nerve responses. Destruction of dopaminergic neurons (approach 4) also led to decreases in neural responses (Le Prell et al. 2005), which is contrary to expectations, given that dopamine is inhibitory (Ruel et al 2001). The authors suggested that other co-localized transmitters were destroyed, However, it is possible that the neurotoxin also killed many non-dopaminergic neurons given that the authors report a large scale loss of efferents in the IHC area, yet other reports confirm that in guinea pig, as in mouse, the dopaminergic neurons comprise a small subset of the LOC efferent population (Mulders and Robertson, 2004; Darrow et al 2006).
The fact that LOC destruction in some experiments can increase, and in others decrease, neural excitability, may simply reflect the existence of multiple LOC subgroups with both excitatory and inhibitory effects on cochlear nerve response. If the balance between the resting activation levels, or relative sizes or degrees of co-localization, of different cytochemical subgroups is different in different species, or is differentially affected by different anesthetics, one might expect such qualitatively different effects of LOC destruction, especially when the destruction is subtotal, as in all the relevant experiments. Correspondingly, the possibility must be considered that there are species differences in the protective effects we have now documented in mouse.
B. Acoustic injury and olivocochlear feedback
OHC dysfunction plays a major role in the genesis of both temporary threshold shifts (TTSs) and permanent threshold shifts (PTSs): e.g. slow OHC depolarizations are well correlated with TTS magnitude (Cody and Russell 1985), and the loss of OHCs and/or damage to their stereocilia are well correlated with PTS magnitude (Liberman and Dodds 1984). A longstanding theory of MOC function is that it protects the cochlea from both TTS and PTS via its actions on OHCs (Handrock and Zeisberg 1982; Rajan 1988; Reiter and Liberman 1995; Kujawa and Liberman 1997; Maison and Liberman 2000; Maison et al. 2002). The idea has been supported by four types of experimental findings: 1) the degree of TTS is decreased when the OC bundle is electrically stimulated simultaneously with the acoustic overexposure (Rajan 1988), 2) in animals with chronic OC bundle section, including both MOC and LOC components, deefferented ears are more vulnerable to both TTS and PTS (Handrock and Zeisberg 1982; Kujawa and Liberman 1997); 3) the strength of the MOC reflex (measured as a acoustically driven suppression of DPOAE amplitude) is strongly correlated to vulnerability (Maison and Liberman 2000); and, most definitively, 4) transgenic mice with over-expression of the ACh receptor in OHCs show enhanced MOC effects coupled with enhanced resistance to both TTS and PTS (Maison et al. 2002).
Although MOC-mediated cholinergic effects on OHCs can clearly reduce acoustic vulnerability, there are hints in previous lesion studies that some of the increased vulnerability seen after complete de-efferentation is attributable to loss of the LOC system. For example, when acoustic vulnerability was assessed in totally de-efferented guinea pigs, neurally derived threshold shifts were 10-15 dB higher than OHC-derived shifts (a disparity not present in normal noise-exposed animals), and vulnerability was not significantly affected by the midline lesion, which interrupts 2/3 of the MOC while sparing the LOC (Kujawa and Liberman 1997). However, this and other prior evidence linking the LOC system to protection from acoustic injury is indirect.
Neuronal damage appears to play an important role in the genesis of noise-induced TTSs (Liberman and Mulroy 1982; Robertson 1983; Puel et al. 1998), and the targeting of cochlear neurons by the LOC system makes LOC-mediated protection from acoustic injury an attractive hypothesis. A common cochlear pathology seen in the first 24 hrs after acoustic overexposure is swelling of cochlear nerve dendrites in the IHC area (Liberman and Mulroy 1982; Robertson 1983; Puel et al. 1998). This type of dendritic swelling can also be observed in cochleas perfused with glutamate agonists, but without noise exposure (Puel et al. 1994). Furthermore, when noise is presented with simultaneous intra-cochlear perfusion of a glutamate antagonist, there is less threshold shift (after washout of the glutamate antagonist) and fewer vacuoles (Puel et al. 1998). These observations suggest that dendritic swelling is a type of excitotoxicity brought on by excessive release of glutamate from the IHC. The LOC's dopaminergic component is hypothesized to mediate this protective effect: a) dendritic vacuolizations in the IHC area have been observed in cochleas perfused with a dopamine antagonist (without exposure to intense noise; Ruel et al. 2001) and b) cochlear perfusion of dopamine agonists prior to noise exposure reduced the amount of noise induced threshold shift and ischemia-induced neuronal swelling (d'Aldin et al. 1995). Given that the LOC system targets IHCs as well as auditory nerve dendrites (Liberman et al. 1990), this LOC-mediated protection could be mediated presynaptically, e.g. by reducing glutamate release from the IHC, or it could result from post-synaptic modulation of the Ca++ entry known to mediate excitotoxicity in other systems.
Previous work in our laboratory has documented the presence of IHC area vacuoles in mice with 40 dB of TTS, i.e. similar in magnitude to that produced in this study (Wang et al. 2002). The dramatic nature of dendritic swelling suggests that it should be accompanied by a loss of synaptic transmission between the IHC and cochlear nerve: electron microscopic images show loss of intracellular components from, and membrane rupture of, the post-synaptic afferent terminal (Robertson 1983; Puel et al. 1998; Ruel et al. 2001; Le Prell et al 2004). It follows that loss of synaptic transmission should contribute to the acute threshold shift, and that TTS should be larger in neural measures (e.g. ABRs) than in OHC-based measures (e.g. DPOAEs). In the present study, LOC lesions resulted in enhanced ABR threshold shifts re DPOAE threshold shifts, consistent with a significant component of “additional” TTS attributable to a dysfunction at the level of the inner hair cell or cochlear nerve. In contrast, mice with normal LOC innervation showed almost identical degrees of ABR and DPOAE shift (Fig. 9), suggesting that, with an intact LOC system, all the functionally important changes underlying the TTS were present at the level of OHC-dominated active cochlear mechanics.
If dendritic vacuolization is a functionally relevant contributor to TTS, why do ears with an intact LOC system show comparable ABR and DPOAE shifts (Mills 2003; Wang et al. 2002; Maison et al. 2003; Kujawa and Liberman 2006)?. In comparing ABR- and DPOAE-based threshold shifts it is important to consider the relative insensitivity of neural-based sound-evoked gross potentials such as ABRs to a distributed loss of neural elements. In adult chinchillas with selective IHC loss, which is functionally similar to primary neuronal degeneration as far as ABR generation is concerned, a distributed loss of 50% of the responding neurons in a particular cochlear region resulted in a neural-based gross potential shift of <6 dB (Liberman et al. 1997). This can be understood by considering that a sound level increase of 6 dB can double the discharge rate of individual cochlear neurons near threshold (e.g. Winter et al. 1990) and thereby compensate for the loss of half the responsive neural elements. This line of argument resolves the apparent paradox that significant numbers of dysfunctional-looking cochlear nerve dendrites can be observed in normal animals with TTS, without a significant degree of “additional” threshold shift in ABRs re DPOAEs.
The insensitivity of neural-based metrics to distributed IHC loss, and subsequently to neural degeneration, also implies that the 10-15 dB of “additional” ABR shift observed in LOC-lesioned ears (Fig. 9) must correspond to a very large number of dysfunctional auditory nerve fibers. Furthermore, considering that the LOC lesions in this report were incomplete, an average of ∼50% destruction from base to apex (Fig. 5), the data also suggest a very strong anti-excitotoxic effect of an intact LOC system. Given the existing pharmacological data implicating dopaminergic transmission in blocking dendritic swelling (Ruel et al. 2001), and given the recent report that the mouse LOC system consists of two functional subsystems (one cholinergic and one dopaminergic, (Darrow et al. 2006a)), it is tempting to speculate that the loss of the dopaminergic component is responsible for the anti-excitotoxic effects described here. On the other hand, there is also a growing literature, in other systems, on the neuroprotective and anti-excitotoxic effects of cholinergic transmission through nicotinic ACh receptors (Dajas-Bailador and Wonnacott, 2004), both by post-synaptic effects (eliciting changes in expression of Ca++-buffering proteins) as well as by pre-synaptic receptors (reducing glutamate release). RT-PCR studies suggest expression of a variety of nicotinic ACh receptors in spiral ganglion cells (Bao et al. 2005), the major LOC target.
It has been suggested that OC-mediated protection is an epiphenomenon rather then a functional role that evolved because it confers selective advantage, given that the traumatic acoustic exposures used in “protection” experiments are well above levels that ever existed in the pre-industrial age (Kirk and Smith 2003). The present study uses sound pressures that are significantly lower than those used in previous studies of MOC protection (94 dB vs. ≥ 105 dB SPL) and thus makes it more plausible that anti-excitotoxicity is one of the functional roles of the LOC system, rather than an epiphenomenon. Furthermore, the incompleteness of the present lesions and the magnitude of the protective effect they reveal make it likely that significant protective effects may be present for exposures at even lower sound pressures.
Acknowledgements
Research supported by grants from the NIDCD: RO1 DC00188, P30 DC05209 and T32 DC0038. We gratefully acknowledge the generous assistance provided by Drs. S. Bledsoe and C. Le Prell in applying their stereotaxic injection techniques to the mouse.
Footnotes
In a larger sample of cases, summarized in a study of the LOC role in balancing bilateral neural excitability (Darrow et al. 2006b), a complementary reduction was seen in the ABR amplitudes in the contralateral ears of LOC Hit cases. We hypothesize that the lack of contralateral effects in the smaller subset of animals used in these acoustic injury experiments reflects (chance) differences in the degree to which the crossing ascending projections to the contralateral LSO were interrupted by the lesions.
References Cited
- Adams JC. Sound stimulation induces Fos-related antigens in cells with common morphological properties throughout the auditory brainstem. J Comp Neurol. 1995;361:645–668. doi: 10.1002/cne.903610408. [DOI] [PubMed] [Google Scholar]
- Arnold T, Oestreicher E, Ehrenberger K, Felix D. GABAA receptor modulates the activity of inner hair cell afferents in guinea pig cochlea. Hear Res. 1998;125:147–153. doi: 10.1016/s0378-5955(98)00144-0. [DOI] [PubMed] [Google Scholar]
- Bailey GP, Sewell WF. Calcitonin Gene-Related Peptide suppresses Hair Cell Responses to Mechanical Stimulation in the Xenopus Lateral Line Organ. J Neuroscience. 2000;20:5163–5169. doi: 10.1523/JNEUROSCI.20-13-05163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit beta2 in the maintenance of spiral ganglion neurons during aging. J Neurosci. 2005;25:3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol. 1987;260:605–618. doi: 10.1002/cne.902600412. [DOI] [PubMed] [Google Scholar]
- Brown MC. Fiber pathways and branching patterns of biocytin-labeled olivocochlear neurons in the mouse brainstem. J Comp Neurol. 1993;337:600–613. doi: 10.1002/cne.903370406. [DOI] [PubMed] [Google Scholar]
- Campbell JP, Henson MM. Olivocochlear neurons in the brainstem of the mouse. Hear Res. 1988;35:271–274. doi: 10.1016/0378-5955(88)90124-4. [DOI] [PubMed] [Google Scholar]
- Cody AR, Russell IJ. Outer hair cells in the mammalian cochlea and noise-induced hearing loss. Nature (Lond) 1985;315:662–665. doi: 10.1038/315662a0. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ., Jr. Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J Physiol. 2003;548:307–312. doi: 10.1113/jphysiol.2003.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- d'Aldin C, Puel JL, Leducq R, Crambes O, Eybalin M, Pujol R. Effects of a dopaminergic agonist in the guinea pig cochlea. Hear Research. 1995;90:202–211. doi: 10.1016/0378-5955(95)00167-5. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol. 2006;498:403–414. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback: evidence for a role in balancing interaural sensitivity. Nature Neuroscience (In Press) 2006b doi: 10.1038/nn1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- Felix D, Ehrenberger K. The efferent modulation of mammalian inner hair cell afferents. Hear Research. 1992;64:1–5. doi: 10.1016/0378-5955(92)90163-h. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; New York: 1997. [Google Scholar]
- Guinan J. Physiology of Olivocochlear Efferents. In: Dallos AP P, Fay RR, editors. The Cochlea. Springer; NY: 1996. pp. 435–502. [Google Scholar]
- Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J Neurophysiol. 2003;90:3178–3200. doi: 10.1152/jn.00537.2003. [DOI] [PubMed] [Google Scholar]
- Handrock M, Zeisberg J. The influence of the efferent system on adaptation, temporary and permanent threshold shift. Arch Otorhinolaryngol. 1982;234:191–195. doi: 10.1007/BF00453630. [DOI] [PubMed] [Google Scholar]
- Henderson D, Subramaniam M, Papazian M, Spongr VP. The role of middle ear muscles in the development of resistance to noise induced hearing loss. Hear Res. 1994;74:22–28. doi: 10.1016/0378-5955(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Liscum A, van Adel B, Ito M. Projections from the superior olive and lateral lemniscus to tonotopic regions of the rat's inferior colliculus. Hear Res. 1998;116:43–54. doi: 10.1016/s0378-5955(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Kirk EC, Smith DW. Protection from acoustic trauma is not a primary function of the medial olivocochlear efferent system. J Assoc Res Otolaryngol. 2003;4:445–65. doi: 10.1007/s10162-002-3013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: effects of chronic de-efferentation and sham surgery. J Neurophysiol. 1997;78:3095–3106. doi: 10.1152/jn.1997.78.6.3095. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Shore SE, Hughes LF, Bledsoe SC., Jr. Disruption of lateral efferent pathways: functional changes in auditory evoked responses. J Assoc Res Otolaryngol. 2003;4:276–290. doi: 10.1007/s10162-002-3018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, Dolan DF, Bledsoe SC, Jr, Moody DB. Chronic excitotoxicity in the guinea pig cochlea induces temporary functional deficits without disrupting otoacoustic emissions. J Acoust Soc Am. 2004;116:1044–56. doi: 10.1121/1.1772395. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Halsey K, Hughes LF, Dolan DF, Bledsoe SC., Jr. Disruption of lateral olivocochlear neurons via a dopaminergic neurotoxin depresses sound-evoked auditory nerve activity. J Assoc Res Otolaryngol. 2005;6:48–62. doi: 10.1007/s10162-004-5009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Chesney CP, Kujawa SG. Effects of selective inner hair cell loss on DPOAEs in carboplatin-treated chinchillas. Auditory Neurosci. 1997;3:255–268. [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: Quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Mulroy MJ. Acute and chronic effects of acoustic trauma: cochlear pathology and auditory-nerve pathophysiology. In: Hamernik RP, Henderson D, Salvi R, editors. New Perspectives on Noise-Induced Hearing Loss. Raven Press; New York: 1982. pp. 105–135. [Google Scholar]
- Liberman MC. Effects of chronic cochlear de-efferentation on auditory-nerve response. Hear Res. 1990;49:209–224. doi: 10.1016/0378-5955(90)90105-x. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a non-invasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Luebke AE, Liberman MC, Zuo J. Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J Neurosci. 2002;22:10838–10846. doi: 10.1523/JNEUROSCI.22-24-10838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in mouse: immunocytochemical maps, crossed vs. uncrossed contributions and colocalization of ACh, GABA, and CGRP. J Comp Neurol. 2003;455:406–416. doi: 10.1002/cne.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Emeson RB, Adams JC, Luebke AE, Liberman MC. Loss of alpha CGRP reduces sound-evoked activity in the cochlear nerve. J Neurophysiol. 2003;90:2941–2949. doi: 10.1152/jn.00596.2003. [DOI] [PubMed] [Google Scholar]
- Mills DM. Differential responses to acoustic damage and furosemide in auditory brainstem and otoacoustic emission measures. J Acoust Soc Am. 2003;113:914–924. doi: 10.1121/1.1535942. [DOI] [PubMed] [Google Scholar]
- Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Dopaminergic olivocochlear neurons originate in the high frequency region of the lateral superior olive of guinea pigs. Hear Res. 2004;187:122–130. doi: 10.1016/s0378-5955(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neuroscience. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen KK, Roth K. Histochemical localization of cholinesterases in the cochlear nuclei of the cat, with notes on the origin of acetylcholinesterase-positive afferents and the superior olive. Brain Res. 1969;16:165–185. doi: 10.1016/0006-8993(69)90092-4. [DOI] [PubMed] [Google Scholar]
- Oestreicher E, Arnold W, Ehrenberger K, Felix D. Dopamine regulates the glutamatergic inner hair cell activity in guinea pigs. Hear Res. 1997;107:46–52. doi: 10.1016/s0378-5955(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d'aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- Puel JL, Pujol R, Tribillac F, Ladrech S, Eybalin M. Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol. 1994;341:241–256. doi: 10.1002/cne.903410209. [DOI] [PubMed] [Google Scholar]
- Rajan R. Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity. I. Dependence on electrical stimulation parameters. J Neurophysiol. 1988;60:549–568. doi: 10.1152/jn.1988.60.2.549. [DOI] [PubMed] [Google Scholar]
- Reiter ER, Liberman MC. Efferent-mediated protection from acoustic overexposure: Relation to slow effects of olivocochlear stimulation. J Neurophysiol. 1995;73:506–514. doi: 10.1152/jn.1995.73.2.506. [DOI] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear Res. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Ruel J, Nouvian R, Gervais d'Aldin C, Pujol R, Eybalin M, Puel JL. Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea. European Journal of Neuroscience. 2001;14:977–986. doi: 10.1046/j.0953-816x.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Bennett TM, Woolf NK, Axelsson A. Protection from noise induced hearing loss by prior exposure to a nontraumatic stimulus: Role of the middle ear muscles. Hear Res. 1994;72:23–28. doi: 10.1016/0378-5955(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Sahley TL, Kalish RB, Musiek FE, Hoffman DW. Effects of opioid drugs in auditory evoked potentials suggests a role of lateral olivocochlear dynorphins in auditory function. Hear Res. 1991;55:133–142. doi: 10.1016/0378-5955(91)90099-u. [DOI] [PubMed] [Google Scholar]
- Shibayama H, Sakai T, Yohro T. Central distribution of the stapedius motoneurons in the rat--a study of topographical anatomy and HRP transport experiments. Kaibogaku Zasshi. 1990;65:120–133. [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, McFadden SL, Liberman MC. Long-term effects of sectioning the olivocochlear bundle in neonatal cats. J Neurosci. 1998;18:3859–3869. doi: 10.1523/JNEUROSCI.18-10-03859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter IM, Robertson D, Yates GK. Diversity of characteristic frequency rate-intensity functions in guinea pig auditory nerve fibers. Hear Res. 1990;45:191–202. doi: 10.1016/0378-5955(90)90120-e. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Henderson D, McFadden SL, Ding DL, Salvi RJ. Auditory nerve fiber responses following chronic cochlear de-efferentation. J Comp Neurol. 1999;406:72–86. doi: 10.1002/(sici)1096-9861(19990329)406:1<72::aid-cne5>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]


