Abstract
Recently, it has been shown that the neonatal immune environment can have significant programming effects on the adult neuroimmune response. A single neonatal immune challenge with the bacterial mimetic lipopolysaccharide (LPS) can alter the neuroendocrine, neurochemical and febrile responses to a subsequent, homotypic (LPS) immune challenge as adults. As the programming effects of viral stimuli during this neonatal period are unknown, we tested whether the viral mimetic polyinosinic–polycytidylic acid (PolyIC), administered on postnatal day 14 (P14) would alter the adult neuroimmune responses to a subsequent PolyIC challenge. Our results show that animals treated neonatally with PolyIC had significantly attenuated febrile responses to an adult PolyIC challenge, which coincided with a heightened corticosteroid response. When the corticosteroid receptor blocker RU486 was administered prior to the adult PolyIC challenge, animals treated neonatally with PolyIC no longer displayed attenuated febrile responses. Similar responses to an adult LPS challenge have been seen in animals that were exposed neonatally to LPS, indicating that both neonatal immune stimuli elicit highly similar programming effects on the adult neuroimmune responses. However, we find that neither neonatal PolyIC nor neonatal LPS challenges led to an alteration in the adult febrile or corticosteroid responses to a heterotypic adult immune challenge, indicating that the programming effects of the neonatal immune environment are stimulus specific, and do not alter the adult responses to other immune stimuli.
The neuroimmune response is characterized by a bi-directional communication between peripheral immune cells and the central nervous system. Of particular importance during times of infection is the activation of the centrally mediated febrile and corticosterone responses, which aid the innate immune system in the effective clearance of the pathogen while limiting the extent of inflammatory damage (Schobitz et al. 1994; Jiang et al. 1999). Inhibition of these innate immune responses has been shown to significantly affect the morbidity and mortality with infection (Kluger et al. 1998; Jiang et al. 2000; Nadeau & Rivest, 2003).
There is growing evidence that the neonatal immune environment can program the subsequent neuroimmune responsiveness of the adult. A single challenge with the bacterial endotoxin lipopolysaccharide (LPS) during the neonatal period can significantly alter the febrile, neuroendocrine, neurochemical and behavioural responses of the adult (Shanks et al. 2000; Boisse et al. 2004; Ellis et al. 2005; Spencer et al. 2005). A heightened neuroendocrine response, resulting in increased levels of corticosterone, has been shown to underlie the attenuated inflammatory responses to an adult LPS challenge (Ellis et al. 2005), highlighting that programming of these centrally mediated responses by the neonatal immune environment can alter other facets of the innate immune response as well.
However LPS, derived from gram-negative bacteria, represents only one type of immune stimulus, which is mediated by the toll-like receptor (TLR)-4 pathways (Tapping et al. 2000). Viral immune stimuli, which may be even more prevalent in the developed world, represent another important type of immune stimulus, yet the effects of these stimuli occurring neonatally remain largely unknown. Polyinosinic–polycytidylic acid (PolyIC), a synthetic double stranded RNA molecule used as a viral mimetic, acts via the TLR-3 pathway (Alexopoulou et al. 2001) to elicit febrile, cytokine and hypothalamic–pituitary–adrenal (HPA) responses (Rotondo et al. 1988; Milton et al. 1992; Fortier et al. 2004), thus representing a distinct immunological stimulus from LPS. It is important to note that although PolyIC and LPS are recognized by different immune receptors, they utilize similar mechanisms to activate their neuroimmune responses; hence there exists a potential for either of these immune stimuli given neonatally to also program the adult neuroimmune responses to heterotypic immune stimuli.
In light of this, we set out to test whether (1) neonatal PolyIC challenge has programming effects on the adult innate immune responses to PolyIC, and (2) whether either a neonatal PolyIC or LPS challenge could influence the innate immune responses to a dissimilar (heterotypic) adult immune challenge.
Methods
Animals
Pregnant Sprague-Dawley rats (Charles River, Canada) were maintained at 22°C on a 12-h light–dark cycle (07.00 h–19.00 h), where food and water were available ad libitum. Ten days after birth (P10) all litters were culled to 12 pups. On P14, six pups per litter were randomly selected, and LPS (100 μg kg−1, i.p.; Sigma, E. coli, serotype 026:B6) or PolyIC (1 mg kg−1, i.p.; Sigma) was administered in sterile saline (1 μl (g body wt)−1). These doses have been previously shown to elicit moderate febrile responses (Fortier et al. 2004; Ellis et al. 2005). Six control pups per litter were administered an equivalent volume of sterile, pyrogen free saline. Litters were weaned at P21 (keeping only male rats) and animals were housed two to three per cage until they reached 10–12 weeks of age. Animals were then housed two per cage during all temperature recordings, and were individually housed for blood sampling. In a separate group of experiments, naive adult rats (P42) were given either a PolyIC injection (1 mg kg−1, i.p.) or saline as controls and were left for 8 weeks (until P96). All procedures were in accordance with the Canadian Council on Animal Care regulations and were approved by the local University of Calgary animal care committee.
Body temperature recording
Adult male rats that had been challenged neonatally with LPS, PolyIC or saline were anaesthetized with halothane (induced at 4%, maintained at 2%) and temperature data loggers (SubCue Inc., Calgary, Canada) were surgically implanted, using aseptic techniques, into the abdomen. After a 5-day recovery, adult rats received either LPS (50 μg kg−1, i.p.) or PolyIC (500 μg kg−1, i.p.) dissolved in sterile saline. Both of these doses have been previously shown to elicit a reliable febrile response in adult rats (Boisse et al. 2004; Fortier et al. 2004). Body temperature measurements were taken every 15 min for a period of 8 h.
Corticosterone ELISA
In a separate group of adult animals from the same neonatal treatment groups, indwelling jugular catheters were surgically implanted (Thrivikraman et al. 2002) into the right jugular vein under halothane anaesthesia (induced at 4%, maintained at 2%). Following 4 days of recovery jugular catheters were attached to a cannula to allow blood to be taken without disturbing the animals. A blood sample (300 μl) was taken immediately before an LPS (50 μg kg−1, i.p.) or PolyIC (500 μg kg−1, i.p.) injection, then every 30 min for a period of 4 h Blood was collected in heparinized tubes and immediately centrifuged at 4°C, and plasma was snap frozen in liquid nitrogen and stored at −80°C. A corticosterone ELISA kit (DE3600 R & D systems Inc., Minneapolis, MN, USA) was used to assay the plasma samples for free corticosterone. The precision and sensitivity of this assay was: interassay variability, 7.8–13.1%CV; intra-assay variability, 6.7–8.0%CV; and sensitivity, 27 pg ml−1.
RU486 treatment
Adult animals that were treated neonatally with either saline or PolyIC had abdominal temperature data loggers implanted to measure core body temperature. After 1 week recovery, animals were injected with 50 mg kg−1 RU486 (Mifepristone, Sigma, St Louis, MO, USA) or vehicle (DMSO). This dose of RU486 has been shown to block both the peripheral and central effects of circulating corticosterone (Nadeau & Rivest, 2003; Ellis et al. 2005) and to be without effect on normal body temperature (Ellis et al. 2005). Thirty minutes after injection of the RU486 or vehicle, all animals received PolyIC (500 μg kg−1, i.p.) and temperature was recorded every 15 min for a period of 8 h. The vehicle has previously been shown to have no effect on the febrile responses to adult immune stimuli (Ellis et al. 2005).
Data analysis
Fever data were calculated as a cumulative fever index (area under the curve) between 60 and 480 min following either LPS or PolyIC injection (a time after the stress induced hyperthermia has subsided) and compared using Student's unpaired t test. Average baseline temperatures (time −60 to time 0 min) were statistically compared by Student's unpaired t test. All corticosterone data were compared with a two-way repeated measures analysis of variance, with a Student-Neuman-Keuls post hoc comparison. Significance was accepted at P < 0.05.
Results
Neonatal PolyIC challenge attenuates the febrile responses to PolyIC in adults
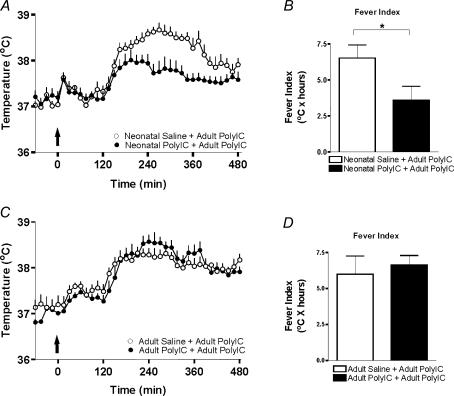
We measured the febrile responses to PolyIC in adult animals that had been treated neonatally with either PolyIC or saline. The neonatal PolyIC challenge did not affect adult baseline body temperatures or the stress induced hyperthermia elicited by handling and injection, when compared to neonatal saline treated controls (Fig. 1A, baseline; 37.2 ± 0.2°C versus 37.0 ± 0.1°C, hyperthermia; 37.6 ± 0.1°C versus 37.6 ± 0.1°C, n = 7, P > 0.05). However, when animals that had received PolyIC as neonates were given a homotypic adult immune challenge (PolyIC), they displayed significantly attenuated febrile responses compared to those that had received saline neonatally (fever index; Fig. 1B).
Figure 1. Neonatal PolyIC attenuates febrile response to adult PolyIC.
A, body temperature responses to an adult PolyIC challenge in animals that had been treated neonatally with either saline or PolyIC. Arrow indicates time of injection. B, animals that had been treated neonatally with PolyIC display significantly attenuated febrile responses to an adult PolyIC challenge compared to animals that had received saline neonatally (fever index; n = 7/group, P < 0.05). C, body temperature responses to an adult PolyIC challenge in animals that had received either PolyIC or saline 2 months earlier. D, there is no alteration in the adult febrile response to PolyIC when the first challenge occurs outside the neonatal period (fever index; n = 7/group, P > 0.05).
To determine whether these attenuated fevers require immune exposure during a susceptible period of development, young adult animals that had received no neonatal treatment were challenged with PolyIC (1 mg kg−1, i.p.) or saline on P42. On P96, these adult animals received a PolyIC challenge (500 μg kg−1, i.p.) and body temperature was recorded over a period of 8 h. As can be seen in Fig. 1C and D, the body temperature responses were similar in the P96 adult animals regardless of whether they had previously been challenged with PolyIC.
A neonatal PolyIC challenge sensitizes the corticosterone response to an adult PolyIC challenge
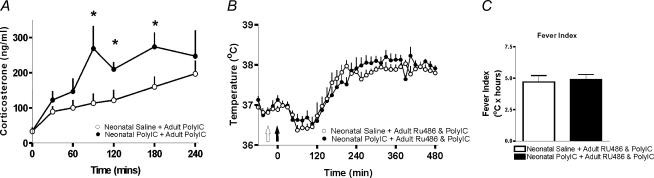
Given the potent antipyretic properties of corticosteroids, we asked whether animals treated neonatally with PolyIC would display increased corticosterone responses to a homotypic adult immune challenge (PolyIC) when compared to animals that had received saline neonatally. While basal levels of free corticosterone were similar, animals that received PolyIC neonatally displayed a significantly greater corticosterone response from 90 to 180 min after the adult Poly IC challenge compared to the animals that received saline neonatally (Fig. 2A).
Figure 2. Heightened corticosterone response underlies attenuated fever.
A, adult corticosterone responses to an adult PolyIC challenge in animals that had been challenged neonatally with either saline or PolyIC. Animals that had been treated neonatally with PolyIC displayed significantly elevated corticosterone levels from 90 to 240 min following an adult PolyIC challenge, when compared to animals that had received saline neonatally (n = 8/group, P < 0.05). B, body temperature responses to an adult PolyIC challenge in animals that had been treated neonatally with either saline or PolyIC. RU486 (open arrow) was given 30 min before the adult PolyIC injection (filled arrow). C, the attenuated adult febrile responses to PolyIC seen previously in animals that had been treated neonatally with PolyIC are completely abolished by RU486 (fever index; n = 7/group, P > 0.05).
Blocking the corticosterone effects with RU486 abolishes the attenuated febrile response to PolyIC as adults
To assess whether the attenuated febrile responses to the adult PolyIC challenge were due to the increased corticosterone response in animals that had been treated neonatally with PolyIC, a glucocorticoid receptor blocker (RU486) was administered 30 min before the adult PolyIC challenge. When RU486 was given prior to the adult PolyIC challenge, animals that had been treated neonatally with either saline or PolyIC displayed similar febrile responses (Fig. 2B and C).
The adult febrile and corticosterone responses are sensitized only to a homotypic immune challenge
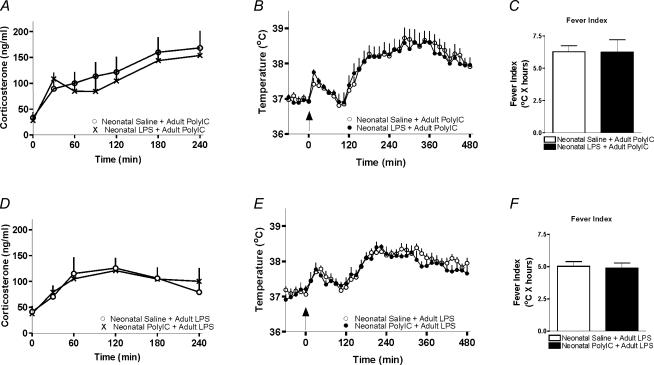
In light of the overlapping intracellular and neurochemical mediators used by both PolyIC and LPS, it was important to assess if either LPS or PolyIC immune challenges occurring neonatally could alter responses to a heterotypic adult immune challenge. Animals which received LPS neonatally displayed unaltered febrile and corticosterone responses to a heterotypic immune challenge (i.e. PolyIC) as adults, compared to animals that had been treated neonatally with saline (Fig. 3A–C). Similarly, animals that had received PolyIC neonatally also displayed unaltered febrile and corticosterone responses to a heterotypic immune challenge (i.e. LPS) as adults, compared to animals that had been treated neonatally with saline (Fig. 3D–F).
Figure 3. Lack of programming by heterotypic immune stimuli.
A and D, animals that had been treated neonatally with LPS display similar corticosterone responses to an adult PolyIC challenge when compared to animals that had received saline neonatally (panel A, n = 6/group, P > 0.05). Similarly, animals that had received PolyIC neonatally displayed similar corticosterone responses to an adult LPS challenge when compared to animals that had received saline neonatally (panel D, n = 6/group, P > 0.05). B and E, body temperature responses to an adult immune challenge in animals that had been treated neonatally with PolyIC, LPS or saline. Animals that had been treated neonatally with LPS display similar febrile responses to an adult PolyIC challenge when compared to animals that had received saline neonatally (fever index; panel C, n = 7/group, P > 0.05). Similarly, animals that had received PolyIC neonatally displayed similar febrile responses to an adult LPS challenge when compared to animals that had received saline neonatally (fever index; panel F, n = 7/group, P > 0.05).
Discussion
This study highlights several important findings regarding the effects of early life immune stimuli on the innate, neuroimmune response of adults. First, we have shown that a single neonatal immune challenge with the viral mimetic PolyIC has long-lasting effects on the febrile and corticosterone responses to a homotypic adult immune challenge. Thus, neonatal programming of the innate immune response is not only limited to the gram-negative bacterial responses previously reported (Boisse et al. 2004; Shanks et al. 2000), but can also be achieved with viral immune stimuli. We further showed that the attenuated febrile response was due to an increased corticosterone response to the adult PolyIC challenge, as blocking the effects of this enhanced response with the glucocorticoid receptor blocker RU486 abolished the attenuated febrile responses. Perhaps most remarkable is the fact that animals which were challenged neonatally with PolyIC displayed unaltered febrile and corticosterone responses to a heterotypic immune challenge (LPS) as adults. Similarly, animals challenged neonatally with LPS also displayed unaltered febrile and corticosterone responses to a heterotypic immune challenge (PolyIC) as adults. These results indicate that there is a stimulus specific alteration in the neuroimmune response, in that the neonatal immune challenge only alters subsequent responses to that same immune stimulus, and not to a heterotypic immune challenge.
This stimulus specificity was quite unexpected. From the present results with a neonatal PolyIC challenge, and those previously published on neonatal LPS (Ellis et al. 2005), it appears that both types of immune stimuli elicit highly similar programming effects on the adult neuroimmune responses to homotypic stimuli (i.e. suppression of the febrile response by an increased corticosterone response). Interestingly, the innate immune responses to both PolyIC and LPS require pro-inflammatory cytokine production (Luheshi, 1998; Fortier et al. 2004), require the action of PGE2 within the brain (Rotondo et al. 1988; Abul et al. 1997; Ivanov & Romanovsky, 2004) and are negatively regulated by corticosteroids (Abul et al. 1987), indicating that the mechanisms of fever induction and suppression are similar between the two types of immune stimuli. However, one major difference between these two immune stimuli is their recognition by the innate immune system. It is known that the responses to PolyIC are mediated by the TLR-3, while those to LPS are mediated by the TLR-4 (O'Neill et al. 2003), and thus any alteration in the expression, binding or intracellular signalling of either TLR pathway may underlie the stimulus specificity elicited by either a neonatal PolyIC or LPS challenge (Boisse et al. 2004; Ellis et al. 2005). Future studies will be needed to confirm the role of these TLRs in the programming of the adult immune response.
The stimulus specificity highlighted in the present study has also been seen in the phenomenon known as endotoxin tolerance, where an initial immunological challenge with LPS can desensitize subsequent innate immune responses to LPS, without affecting the responses to other TLR stimuli, such as PolyIC (Brint et al. 2004) and muramyl dipeptide (Roth et al. 1997). However, our results show that the attenuated neuroimmune responses are only seen when the first immune stimulus occurs during the neonatal period, suggesting that a developmental phenomenon underlies these programming effects, rather than a form of immunological tolerance.
Long-term alterations initiated by an early life LPS challenge are not limited to neuroimmune responses, but can also affect recovery after cerebral ischaemia (Spencer et al. 2006), impair tumour immunity (Hodgson et al. 2001), attenuate allergic sensitization (Blumer et al. 2005) and inhibit the development of experimental arthritis (Shanks et al. 2000). Additionally, it has been shown that neonatal immune challenges alter memory, cognitive ability and pain sensitivity as adults (Gilmore et al. 2003,2005; Zuckerman & Weiner, 2005; Bilbo et al. 2005; Boisse et al. 2005). Taken together, these studies indicate that early life bacterial stimuli can lead to altered susceptibility to inflammatory and psychological disorders later in life. In light of the present findings regarding the programming effects of neonatal PolyIC, it will be important for future studies to examine the effects, if any, that early life viral immune challenges have on these other centrally mediated responses. Due to the stimulus specificity highlighted in the present study, contrasting the changes elicited by either a neonatal PolyIC or LPS challenge can help to identify key elements that underlie these neonatal programming events.
Acknowledgments
This work was supported by CIHR. S.E. is supported by NSERC & AHFMR. Q.J.P. is supported by AHFMR.
References
- Abul H, Davidson J, Milton AS, Rotondo D. Dexamethasone pre-treatment is antipyretic toward polyinosinic: polycytidylic acid, lipopolysaccharide and interleukin 1/endogenous pyrogen. Naunyn Schmiedebergs Arch Pharmacol. 1987;335:305–309. doi: 10.1007/BF00172802. [DOI] [PubMed] [Google Scholar]
- Abul HT, Davidson J, Milton AS, Rotondo D. Prostaglandin E2 enters the brain following stimulation of the acute phase immune response. Ann N Y Acad Sci. 1997;813:287–295. doi: 10.1111/j.1749-6632.1997.tb51707.x. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin Exp Allergy. 2005;35:397–402. doi: 10.1111/j.1365-2222.2005.02184.x. [DOI] [PubMed] [Google Scholar]
- Boisse L, Mouihate A, Ellis S, Pittman QJ. Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J Neurosci. 2004;24:4928–4934. doi: 10.1523/JNEUROSCI.1077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisse L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119:133–141. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O'Neill LA, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–1521. doi: 10.1096/fj.04-3569fje. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic: polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I: C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr Res. 2001;50:750–755. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–1270. doi: 10.1128/iai.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Detolla L, Van Rooijen N, Singh IS, Fitzgerald B, Lipsky MM, Kane AS, Cross AS, Hasday JD. Febrile-range temperature modifies early systemic tumor necrosis factor alpha expression in mice challenged with bacterial endotoxin. Infect Immun. 1999;67:1539–1546. doi: 10.1128/iai.67.4.1539-1546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann N Y Acad Sci. 1998;856:224–233. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- Luheshi GN. Cytokines and fever. Mechanisms and sites of action. Ann N Y Acad Sci. 1998;856:83–89. doi: 10.1111/j.1749-6632.1998.tb08316.x. [DOI] [PubMed] [Google Scholar]
- Milton NG, Hillhouse EW, Milton AS. Activation of the hypothalamo-pituitary-adrenocortical axis in the conscious rabbit by the pyrogen polyinosinic: polycytidylic acid is dependent on corticotrophin-releasing factor-41. J Endocrinol. 1992;135:69–75. doi: 10.1677/joe.0.1350069. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Brown Z, Ward SG. Toll-like receptors in the spotlight. Nat Immunol. 2003;4:299. doi: 10.1038/ni0403-299. [DOI] [PubMed] [Google Scholar]
- Roth J, Aslan T, Storr B, Zeisberger E. Lack of cross tolerance between LPS and muramyl dipeptide in induction of circulating TNF-alpha and IL-6 in guinea pigs. Am J Physiol. 1997;273:R1529–R1533. doi: 10.1152/ajpregu.1997.273.4.R1529. [DOI] [PubMed] [Google Scholar]
- Rotondo D, Abul HT, Milton AS, Davidson J. Pyrogenic immunomodulators increase the level of prostaglandin E2 in the blood simultaneously with the onset of fever. Eur J Pharmacol. 1988;154:145–152. doi: 10.1016/0014-2999(88)90091-x. [DOI] [PubMed] [Google Scholar]
- Schobitz B, Reul JM, Holsboer F. The role of the hypothalamic-pituitary-adrenocortical system during inflammatory conditions. Crit Rev Neurobiol. 1994;8:263–291. [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600206. in press. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge – effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2000;165:5780–5787. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Huot RL, Plotsky PM. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc. 2002;10:84–94. doi: 10.1016/s1385-299x(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]