Abstract
The purpose of this study was to investigate the hypothesis that cycling efficiency in vivo is related to mitochondrial efficiency measured in vitro and to investigate the effect of training status on these parameters. Nine endurance trained and nine untrained male subjects 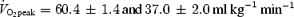 , respectively) completed an incremental submaximal efficiency test for determination of cycling efficiency (gross efficiency, work efficiency (WE) and delta efficiency). Muscle biopsies were taken from m. vastus lateralis and analysed for mitochondrial respiration, mitochondrial efficiency (MEff; i.e. P/O ratio), UCP3 protein content and fibre type composition (% MHC I). MEff was determined in isolated mitochondria during maximal (state 3) and submaximal (constant rate of ADP infusion) rates of respiration with pyruvate. The rates of mitochondrial respiration and oxidative phosphorylation per muscle mass were about 40% higher in trained subjects but were not different when expressed per unit citrate synthase (CS) activity (a marker of mitochondrial density). Training status had no influence on WE (trained 28.0 ± 0.5, untrained 27.7 ± 0.8%, N.S.). Muscle UCP3 was 52% higher in untrained subjects, when expressed per muscle mass (P < 0.05 versus trained). WE was inversely correlated to UCP3 (r = −0.57, P < 0.05) and positively correlated to percentage MHC I (r = 0.58, P < 0.05). MEff was lower (P < 0.05) at submaximal respiration rates (2.39 ± 0.01 at 50%
, respectively) completed an incremental submaximal efficiency test for determination of cycling efficiency (gross efficiency, work efficiency (WE) and delta efficiency). Muscle biopsies were taken from m. vastus lateralis and analysed for mitochondrial respiration, mitochondrial efficiency (MEff; i.e. P/O ratio), UCP3 protein content and fibre type composition (% MHC I). MEff was determined in isolated mitochondria during maximal (state 3) and submaximal (constant rate of ADP infusion) rates of respiration with pyruvate. The rates of mitochondrial respiration and oxidative phosphorylation per muscle mass were about 40% higher in trained subjects but were not different when expressed per unit citrate synthase (CS) activity (a marker of mitochondrial density). Training status had no influence on WE (trained 28.0 ± 0.5, untrained 27.7 ± 0.8%, N.S.). Muscle UCP3 was 52% higher in untrained subjects, when expressed per muscle mass (P < 0.05 versus trained). WE was inversely correlated to UCP3 (r = −0.57, P < 0.05) and positively correlated to percentage MHC I (r = 0.58, P < 0.05). MEff was lower (P < 0.05) at submaximal respiration rates (2.39 ± 0.01 at 50% 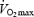 ) than at state 3 (2.48 ± 0.01) but was neither influenced by training status nor correlated to cycling efficiency. In conclusion cycling efficiency was not influenced by training status and not correlated to MEff, but was related to type I fibres and inversely related to UCP3. The inverse correlation between WE and UCP3 indicates that extrinsic factors may influence UCP3 activity and thus MEff in vivo.
) than at state 3 (2.48 ± 0.01) but was neither influenced by training status nor correlated to cycling efficiency. In conclusion cycling efficiency was not influenced by training status and not correlated to MEff, but was related to type I fibres and inversely related to UCP3. The inverse correlation between WE and UCP3 indicates that extrinsic factors may influence UCP3 activity and thus MEff in vivo.
The efficiency of physical work is a measure of the body's effectiveness in converting energy from nutrients into external work. Performed work rate can be determined exactly during cycling and calculated efficiency between subjects varies between 25 and 30% (Gaesser & Brooks, 1975). Mathematical modelling predicts that even small increments in efficiency may lead to major improvements in endurance (Moseley & Jeukendrup, 2001). There is accordingly a great interest in identifying potential determinants that may increase efficiency of physical work. Whole body efficiency is mainly determined by the efficiency of the working muscles, which is influenced by anatomical, biomechanical and biochemical factors (Williams, 1985; Gardner et al. 1989) – all of which could contribute to the intersubject differences in efficiency (Gaesser & Brooks, 1975; Coyle et al. 1992).
An intriguing question, which has been addressed in many studies, is whether cycling efficiency can be increased by training. The topic has been addressed in a number of cross-sectional studies. However, the results are inconclusive in that studies have shown higher (Kunstlinger et al. 1985; Gardner et al. 1989; Gissane et al. 1991), lower (Mallory et al. 2002) or similar (Boning et al. 1984; Nickleberry & Brooks, 1996; Marsh et al. 2000; Moseley & Jeukendrup, 2001) cycling efficiency in endurance trained subjects. Cycling efficiency has in some (Coyle et al. 1992; Horowitz et al. 1994) but not all studies (Medbøx, 1990; Pedersen et al. 2002) been shown to correlate to the relative muscle composition of type I fibres. There are some data, which indicate that endurance trained subjects have a higher proportion of type I fibres than untrained subjects (Fitzsimons et al. 1990; Putman et al. 2004). The higher cycling efficiency in endurance trained subjects, observed in some studies, may therefore be a consequence of fibre type differences between the groups. This possibility has not been adequately addressed in previous studies and further studies are therefore required.
Oxidative phosphorylation (OxPhos) is the main process of ATP production and changes in the efficiency of OxPhos (mitochondrial efficiency: MEff) will therefore have a direct influence on cycling efficiency. MEff is conventionally measured during maximal ADP stimulated respiration (state 3) as the ATP formation per consumed oxygen (P/O ratio) and defines the coupling between ATP formation and substrate oxidation. It is well established that MEff is higher during carbohydrate oxidation than during fatty acid oxidation but previous studies have been unable to detect differences between trained and untrained subjects (Tonkonogi & Sahlin, 1997; Fernstrom et al. 2004). However, during state 3, back-leakage of protons is minimized due to reduced membrane potential and measured MEff therefore corresponds to a theoretical peak value. Proton leak is higher during submaximal respiration and intrinsic differences between trained and untrained muscles in membrane proton conductance could thus become manifest as a difference in MEff during submaximal but not during state 3 respiration. Measurement of MEff during submaximal respiration is technically more difficult than during state 3 respiration and requires a constant rate of ADP infusion (Gnaiger et al. 2000; Mogensen & Sahlin, 2005). Differences in MEff have been proposed to be a major factor in explaining intersubject differences in work efficiency (Schrauwen & Hesselink, 2003). However, until now there have been no studies of MEff at submaximal respiration rates or of the relation between cycling efficiency and MEff.
Dissipation of the proton gradient by leakage or slippage of protons over the mitochondrial membrane will uncouple mitochondrial ATP formation from substrate oxidation and thus reduce MEff (Kadenbach, 2003). The role of uncoupling protein isotype 1 (UCP1) as a mediator of proton leak in brown adipose tissue is well established (Nedergaard & Cannon, 2003), whereas the uncoupling potency of the homologous protein (UCP3), present in skeletal muscle, has been disputed (Nedergaard & Cannon, 2003). However, since cycling efficiency is inversely related to both mRNA of UCP3 (Schrauwen et al. 1999) and UCP3 protein in skeletal muscle (Schrauwen & Hesselink, 2003) the issue deserves further attention. Muscle content of UCP3 is reduced by endurance training (Russell et al. 2003; Fernstrom et al. 2004; Schrauwen et al. 2005) and is lower in type 1 fibres (Russell et al. 2003). The relation between UCP3 and cycling efficiency may therefore be secondary to other factors (i.e. training status or fibre type composition). Further studies are required to investigate this issue.
The purpose of this study was to investigate if (i) the differences between subjects in cycling efficiency can be explained by individual differences in MEff, (ii) if cycling efficiency and MEff are higher in endurance trained subjects than in untrained subjects, and (iii) if cycling efficiency is related to fibre type composition and UCP3 protein. We hypothesized that a high cycling efficiency is caused by a high MEff and that both cycling efficiency and MEff are higher in endurance trained subjects.
Methods
Subjects
Nine trained (mean ± s.e.m., 25.0 ± 0.9 year; 175 ± 1 cm; 72.6 ± 2.0 kg) and nine untrained (mean ± s.e.m. 24.2 ± 0.7 year; 182 ± 3 cm; 88.7 ± 7.6 kg) healthy male subjects volunteered for the study. Inclusion criteria for the trained group were a 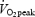 above 55 ml O2 min−1 kg−1 and a high level of habitual physical activity. Inclusion criteria for the untrained group were a
above 55 ml O2 min−1 kg−1 and a high level of habitual physical activity. Inclusion criteria for the untrained group were a 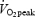 below 45 ml O2 min−1 kg−1 and a low level of habitual physical activity. None of the subjects were competitive cyclists or used cycling as part of their training. Subjects agreed to participate in the experiment after having been informed of the purpose and potential risks involved. The project was approved by the local ethics committee at the Odense University Hospital (VF20040035) and conforms to the Declaration of Helsinki II.
below 45 ml O2 min−1 kg−1 and a low level of habitual physical activity. None of the subjects were competitive cyclists or used cycling as part of their training. Subjects agreed to participate in the experiment after having been informed of the purpose and potential risks involved. The project was approved by the local ethics committee at the Odense University Hospital (VF20040035) and conforms to the Declaration of Helsinki II.
Test protocol
The subjects performed two type of tests, a graded maximal test to determine the 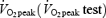 and a submaximal test to determine the cycling efficiency (submaximal efficiency test). Subjects were instructed to avoid exhaustive exercise and to abstain from alcohol 24 h prior to tests. Intake of food, coffee and tea and use of tobacco were not allowed 2 h prior to tests. The instructions were not followed by one subject and his values of in vivo efficiency were therefore excluded. Before each test, the cycle ergometer (Monark model 824E, Sweden) was adjusted to ensure standardized joint angles for all subjects. When the pedal was in its lowest position the subjects were instructed to let the arch of the shoe be in contact with the pedal. This was done to minimize possible technical advantages for those subjects who used cycling as their daily choice of transportation. This standardization of the sitting positions was used throughout all tests, except for the
and a submaximal test to determine the cycling efficiency (submaximal efficiency test). Subjects were instructed to avoid exhaustive exercise and to abstain from alcohol 24 h prior to tests. Intake of food, coffee and tea and use of tobacco were not allowed 2 h prior to tests. The instructions were not followed by one subject and his values of in vivo efficiency were therefore excluded. Before each test, the cycle ergometer (Monark model 824E, Sweden) was adjusted to ensure standardized joint angles for all subjects. When the pedal was in its lowest position the subjects were instructed to let the arch of the shoe be in contact with the pedal. This was done to minimize possible technical advantages for those subjects who used cycling as their daily choice of transportation. This standardization of the sitting positions was used throughout all tests, except for the 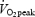 test where subjects were allowed to use toe straps. In both tests, the subjects cycled at a cadence of 80 r.p.m. by the use of a metronome. For calculation of the true cadence, a crank dynamometer (SRM Schoberer Rad Messtechnich, Jülich, Germany) was applied to the ergometer, which recorded the mean cadence over a period of 15 s.
test where subjects were allowed to use toe straps. In both tests, the subjects cycled at a cadence of 80 r.p.m. by the use of a metronome. For calculation of the true cadence, a crank dynamometer (SRM Schoberer Rad Messtechnich, Jülich, Germany) was applied to the ergometer, which recorded the mean cadence over a period of 15 s.
 test
test
The subjects performed a 10-min warm-up at approximately 50% of 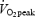 (estimated from expected maximal heart rate and actual heart rate). Hereafter, the work rate was set to elicit 90% of the expected work rate at
(estimated from expected maximal heart rate and actual heart rate). Hereafter, the work rate was set to elicit 90% of the expected work rate at 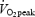 . Work rate increased by 40 watt (W) every minute until exhaustion. The test was terminated when the subjects were unable to maintain the pedalling frequency despite verbal encouragement. During the test, oxygen uptake was measured in 15-s intervals with a mixing chamber online system (Oxycon Pro, Jaeger Germany). Immediately after the test the subjects were asked to indicate the rate of perceived exertion using the 6–20 graded Borg Scale (Borg, 1970). Heart rate was measured continuously (Polar, Kempele, Finland). A capillary blood sample was taken from the finger tip immediately post-exercise and after 2 min recovery and analysed for blood lactate concentration (1500 Sport Lactate Analyser, Yellow Springs Instruments, Yellow Springs, Ohio, USA). The main criteria for the attainment of
. Work rate increased by 40 watt (W) every minute until exhaustion. The test was terminated when the subjects were unable to maintain the pedalling frequency despite verbal encouragement. During the test, oxygen uptake was measured in 15-s intervals with a mixing chamber online system (Oxycon Pro, Jaeger Germany). Immediately after the test the subjects were asked to indicate the rate of perceived exertion using the 6–20 graded Borg Scale (Borg, 1970). Heart rate was measured continuously (Polar, Kempele, Finland). A capillary blood sample was taken from the finger tip immediately post-exercise and after 2 min recovery and analysed for blood lactate concentration (1500 Sport Lactate Analyser, Yellow Springs Instruments, Yellow Springs, Ohio, USA). The main criteria for the attainment of 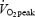 were that exhaustion occurred within 4–8 min and that levelling off was observed in
were that exhaustion occurred within 4–8 min and that levelling off was observed in 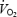 . Additional criteria included respiratory exchange ratio (RER) > 1.10, lactate concentration > 8 mmol l−1 and a ventilatory equivalent for O2 > 30 l l−1.
. Additional criteria included respiratory exchange ratio (RER) > 1.10, lactate concentration > 8 mmol l−1 and a ventilatory equivalent for O2 > 30 l l−1.
Submaximal efficiency test
At least 48 h after the 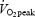 test, subjects performed a submaximal test at three different absolute work rates and at 80%
test, subjects performed a submaximal test at three different absolute work rates and at 80% 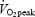 . After adjusting the ergometer, the subjects rested on the ergometer (3 min), performed load-less pedalling (5 min) and cycled at 40, 80 and 120 W (5 min at each work rate). Work rate was hereafter decreased to 40 W for 5 min before adjusting it to 80% of
. After adjusting the ergometer, the subjects rested on the ergometer (3 min), performed load-less pedalling (5 min) and cycled at 40, 80 and 120 W (5 min at each work rate). Work rate was hereafter decreased to 40 W for 5 min before adjusting it to 80% of 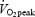 (10 min).
(10 min).
Heart rate and oxygen uptake were determined between 3 and 4.5 min of exercise at fixed work rates and between 4 and 10 min when exercising at 80% 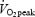 . Capillary blood lactate concentration was determined during the final half minute of exercise at fixed work rates and at 4, 7 and 10 min of exercise at 80% of
. Capillary blood lactate concentration was determined during the final half minute of exercise at fixed work rates and at 4, 7 and 10 min of exercise at 80% of 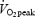 . The submaximal efficiency test was repeated two to four times separated by at least 48 h. All trained subjects completed the submaximal efficiency tests but one untrained subject did not complete the 10 min cycling at 80%
. The submaximal efficiency test was repeated two to four times separated by at least 48 h. All trained subjects completed the submaximal efficiency tests but one untrained subject did not complete the 10 min cycling at 80% 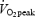 .
.
Determination of efficiency
In its essence, efficiency is a ratio between (external) power output and the ensuing energy expenditure (EE). Efficiency may, however, be expressed in a variety of ways. In this study three constructs of efficiency were employed, gross efficiency (GE), work efficiency (WE) and delta efficiency (DE).
GE is defined as work rate divided by energy expenditure:
Although commonly employed, GE has been criticised for its inclusion in the denominator of energy-delivery processes that do not contribute to production of external work. This objection is allowed for in the construct of WE, which is defined as power output divided by the increase in EE from load-less pedalling to that of the current exercise. It has, however, been suggested (Gaesser & Brooks, 1975) that since the 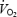 for load-less pedalling often diverges from the general power output–EE relationship, it may be advisable to replace that value with the y-intercept of the work rate–efficiency relationship.
for load-less pedalling often diverges from the general power output–EE relationship, it may be advisable to replace that value with the y-intercept of the work rate–efficiency relationship.
To minimize a potential influence of a 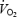 slow component – which might vary between subject groups – the mean EE during the 4th to 5th minute was used in the calculations of GE and WE.
slow component – which might vary between subject groups – the mean EE during the 4th to 5th minute was used in the calculations of GE and WE.
The concept of DE bears resemblance to WE in the sense that delta values for increase in work rate are compared with delta values for increases in EE. However, in contrast to the WE calculation, which is founded upon a single workload, DE calculations are based upon a series of work rates which are then subject to linear regression analysis.
DE is considered by many to be the most valid estimate of muscular efficiency (Gaesser & Brooks, 1975; Coyle et al. 1992). However, despite the seeming solid foundation of the construct, repeated DE measurements have revealed a rather high test–retest variation coefficient and Moseley & Jeukendrup (2001) recommend that estimations of cycling efficiency should not be based upon DE alone. The CV calculated from duplicate measurements in the same subject (when more than two measurements were available two of these were randomly chosen) was 5.79, 5.84 and 6.63% for WE (80 W), GE (80 W) and DE, respectively.
In order to obtain precise values for work rate in the efficiency calculations, power output was calculated from the set work rate and the true cadence as monitored by the SRM crank system. EE was obtained from rate of oxygen uptake using the equations developed by Brouwer (1957). The equation takes the substrate utilization into account by calculating the energetic value of oxygen based on the RER value.
It is interesting to note that the ratio between EE during CHO oxidation and EE during fatty acid oxidation (21.4/19.4 = 1.103) is similar to the ratio between MEff (P/O ratio) with glucose and MEff with palmitate (2.58/2.33 = 1.107 (Brand et al. 1994)).
In the present study, there was some concern that the EE at 40 watts may be influenced by an increased activation of antagonistic muscles and that EE at 120 W in the untrained group may be influenced by anaerobic contribution and fatigue. Based on these arguments we consider WE at 80 W and possibly DE as the most reliable measures of cycling efficiency.
Muscle biopsies and preparation of mitochondria
On a separate day, muscle biopsies were taken from m. vastus lateralis with a modified Bergström needle. After local anaesthesia (2–3 ml, 20 mg ml−1 Carbocain, AstraZeneca AB, Sweden) insertions one-third (±2 cm) of the distance between patella and anterior superior iliac spine were made for subsequent muscle biopsies while the subject rested in the supine position. Two biopsies, one from each leg, were taken. A small part of each biopsy was used for determination of fibre type distribution. The pooled muscle samples were divided into two parts. One part (10–15 mg) was rapidly frozen in liquid nitrogen and stored at −80°C for later determination of enzyme activities and UCP3 protein content. The remaining part (105–250 mg) was weighed and used for mitochondrial isolation as previously described (Tonkonogi & Sahlin, 1997). Briefly, the muscle samples were finely minced and rinsed thoroughly with isolation medium containing (mmol l−1): sucrose, 100; KCl, 100; Tris-HCl, 50; KH2PO4, 1; EGTA, 0.1; and 0.2% BSA; pH 7.40. Hereafter the muscle pieces were incubated in 1 ml of the above-described medium containing 0.2 mg ml−1 bacterial protease (subtilisin Carlsberg; EC 232-752-2, type VIII, Sigma Chemical Co.) dissolved immediately before use. After 2 min of incubation, the minced tissue was homogenized in a water-cooled glass homogenizer with a motor-driven (180 r.p.m.) Teflon pestle. The radial clearance between the pestle and glass was 0.15 mm. The tissue was homogenized for 2 min in intervals of 15 s turning and 5 s rest. The turning direction of the pestle was changed for every interval. Hereafter three volumes of protease free isolation medium were added and the resulting solution was centrifuged at 750 g for 10 min. The pellet was removed and the preserved supernatant was centrifuged at 10 000 g for 10 min. The resulting pellet had a brown core covered by a lighter fluffy layer, which was carefully washed off with the isolation medium. The remaining part of the pellet was resuspended in 650 μl of isolation medium and centrifuged at 7000 g for 3 min. The supernatant was removed and the pellet was washed and resuspended in a suspension medium (about 0.5 μl mg−1 initial muscle) containing (mmol l−1): mannitol, 225; sucrose, 75; Tris, 10; EDTA, 0.1; and 0.2% BSA; pH 7.40. All of the above procedures were carried out at 0–4°C. Part of the mitochondrial suspension was used for assessment of respiratory function and the remaining part was frozen in liquid nitrogen and stored at −80°C for later assay of CS activity.
Mitochondrial respiratory activity
Mitochondrial oxygen consumption was measured polarographically using a Clark-type electrode (DW1 oxygraph, Hansatech Instruments, Norfolk, England). The electrode was surrounded by a temperature controlled water-jacketed glass chamber maintaining the temperature at 25°C. The oxygraph was equipped with magnetic stirring and a gas-tight plunger ensuring a minimum of oxygen diffusion between the chamber solution and the surroundings. For data collection, the electrode was connected to a computer. The measurement was carried out in 485 μl oxygraph medium containing (mmol l−1): mannitol, 225; sucrose, 75; Tris, 10; KCl, 10; K2HPO4, 10; EDTA, 0.1; and MgCl2(6H2O), 0.8; pH 7.0. Pyruvate (5 mmol l−1) + l-malate (2 mmol l−1) was used as substrate. Mitochondrial respiration was initiated by the addition of 12.5 μl of mitochondrial suspension medium. After reaching a stable rate, state 3 was initiated by adding K-ADP dissolved in oxygraph medium (final concentration 0.3 mmol l−1). The concentration of ADP was determined spectrophotometrically (Passonneau & Lowry, 1993). After determination of the maximal respiration (state 3) and respiration without ADP (state 4), the oxygen tension was regained and the submaximal respiration was initiated by low rate ADP infusion. This was accomplished by the use of a microdialysis pump (CMA/Microdialysis CMA/102, CMA/Microdialysis, Solna, Sweden) with a pumping rate ranging from 0.1 to 20 μl min−1. Preliminary tests confirmed the accuracy of the pump at the low pumping rates. For all subjects it was attempted to do three submaximal respiratory measurements at, respectively, low, medium and high pumping rate. However, in some cases the stepwise increase in the pumping rate limited the number of respiratory rates to two measurements. This corresponded to a pumping rate ranging from 0.3 to 2.0 μl min−1. The P/O ratio was calculated as the rate of infused ADP (assuming all added ADP is converted to ATP) divided by the absolute oxygen consumption rate. The steady state respirations were corrected for the added oxygen by the ADP medium and for the effect of oxygen diffusion at different oxygen tensions (determined in separate blank experiments). All measurements were performed at oxygen concentrations ranging from 100 to 250 μmol l−1.
 |
The P/O ratios were plotted against the absolute respiration expressed as respiration per citrate synthase (CS) activity. The resulting data were fitted to a logarithmic function (Intercooled Stata 8.2, StataCorp LP, College Station, TX, USA):
where Y is the P/O ratio and X is the respiration per unit CS, and a and b are constants representing the shape of the non-linear fit and the horizontal magnitude, respectively. It was assumed that the shape of the curve (a-value) was the same for every individual in each group. The mean value of a for the group was used, together with P/O ratio and respiration, to determine the b-value for each subject. A logarithmic curve was thereby calculated for every subject, only differing by the horizontal magnitude of the curve. In this way subjects could be ranged based on three or two measurements in contrast to choosing one measurement as representative of the individual mitochondrial efficiency. The individual logarithmic equation was used to calculate the P/O ratio at 50% of state 3.
CS activity
The CS activity was determined in freeze-dried muscle samples and in the corresponding mitochondrial-rich suspension buffer. CS was used as reference base for the respiratory measurements (i.e. a marker of mitochondrial density). Freeze-dried samples were trimmed free of blood and connective tissue, powdered and homogenized in a Triton solution (containing (mmol l−1): Na2HPO4, 50; EDTA-Na2, 1; and 0.05% Triton X-100; pH 7.0) using a glass–glass homogenizer. Homogenized muscles or dissolved mitochondria were assayed for CS activity spectrophotometrically at 25°C according to the method used by Alp et al. (1976).
Fibre type distribution
Myosin heavy chain (MHC) composition was analysed as previously described (Danieli et al. 1986) and modified for humans (Andersen & Aagaard, 2000). Briefly, muscle homogenate (80 μl) was mixed with 200 μl of sample buffer (10% glycerol, 5%β-mercaptoethanol and 2.3% SDS, 62.5 mmol l−1 Tris and 0.2% bromophenol blue at pH 6.8), boiled in a water bath at 100°C for 3 min and loaded (10–40 μl) on a SDS-PAGE gel (8% polyacrylamide (100: 1 acrylamid:bisacrylamid), 30% glycerol, 67.5 mmol l−1 Tris-base, 0.4% SDS, and 0.1 mol l−1 glycine). Gels were run at 80 V for at least 42 h at 4°C and MHC bands made visible by staining with Coomassie. The gels were scanned (Linoscan 1400 scanner, Heidelberg, Germany) and MHC bands quantified densitometrically (Phoretix 1D, non-linear, Newcastle, UK). MHC II was identified with Western blot using monoclonal antibody (Sigma M 4276) with the protocol Xcell IITM (Invitrogen, Carlsbad, CA, USA).
UCP3 protein content
Determination of UCP3 protein content has been described in detail previously (Tonkonogi et al. 2003). Briefly, portions of freeze-dried muscle were homogenized in ice-cold lysis buffer containing (mmol l−1): Hepes, 2; EDTA, 1; EGTA, 5; MgCl2, 10; β-glycerophosphate, 50; Na3VO4, 1; DTT, 2; 1% Triton X-100; 20 μg ml−1 leuptin; 50 μg ml−1 aprotinin; and 40 μg ml−1 PMSF; pH 7.40. The protein concentration was determined (BCA protein assay 23223 Pierc Cat. 1610737, Bio-Rad Laboratories, Hercules, CA, USA). Homogenates were solubilized in Laemmli sample buffer (containing: 62.5 mmol l−1 Tris-HCl; 2% SDS; 25% glycerol; 0.01% bromphenol blue and 5%β-mercaptoethanol) and denaturized by boiling. A constant amount of protein was added per lane (100 μg) on 12% polyacrylamide gels and separated by SDS-PAGE for 60 min at 135 V. The separated polypeptides were transferred to a PVDF membrane at 10 V for 60 min, and blocked in Tris-buffered saline (TBS) with 5% non-fat milk. Membranes were incubated overnight with polyclonal antibody against UCP3 (Chemicon AB3046), diluted 1: 1000, washed and incubated with secondary antibody goat anti-rabbit (IgG-HRP, NO.sc-2030, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The membrane was again washed and incubated with the chemiluminescence detection reagent (ECL No. RPN 2106; Amersham). Finally, an X-ray film was exposed to the membrane for 50 min. The optical density of the bands was quantified by using Molecular Analyst 1.5 (Bio-Rad) and the density of each sample was related to that of a standard, which was run on the same gel. Samples were analysed for UCP3 protein in duplicate.
Statistics
Data given in the text and tables are presented as means ± s.e.m. Differences in GE and WE between training groups and absolute work rates were tested for statistical significance with a two-way ANOVA with repeated measures. If a main difference was detected the location of significance was determined with the Student-Newman-Keuls post hoc test. Differences between trained and untrained subjects were tested for statistical significance with Student's unpaired t test. Correlation between two variables was tested with Spearman's correlation analysis. The relation between P/O ratio and submaximal respiration rate was analysed with non-linear regression analysis based on a logarithmic function. Statistical significance was accepted at P < 0.05.
Results
Subject characteristics (Table 1)
Table 1.
Group characteristics
| Characteristics | Trained (n = 9) | Untrained (n = 9) | Statistics (trained versus untrained) |
|---|---|---|---|
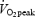 (ml O2 min−1 kg−1) (ml O2 min−1 kg−1) |
60.4 ± 1.4 | 37.0 ± 2.0 | P < 0.001 |
| CS (Units (g d.wt.)−1 min−1) | 119.6 ± 3.0 | 84.9 ± 9.3 | P < 0.01 |
| MHC I (%) | 45.7 (22–60.4) | 42.9 (32.6–53.6) | N.S. |
| (n = 8) |
Values are mean ± s.e.m. except for MHC I, which is shown as mean (range). N.S. not statistically significant.
The trained subjects had a markedly higher oxidative capacity compared to the untrained subjects. This was exemplified by a 63% higher 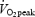 (P < 0.001), and 41% higher CS activity (P < 0.01) (Table 1). Fibre type composition ranged from 22 to 60% MHC I but was not different between trained and untrained subjects (Table 1).
(P < 0.001), and 41% higher CS activity (P < 0.01) (Table 1). Fibre type composition ranged from 22 to 60% MHC I but was not different between trained and untrained subjects (Table 1).
Cycling efficiency (Table 2)
Table 2.
Cycling efficiency and lactate concentration during absolute and relative work rates
| Absolute work rates | Relative work rate | ||||
|---|---|---|---|---|---|
| Training status | 40 W | 80 W | 120 W | 80% 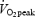
|
|
| Lactate | Trained | 1.4 ± 0.1 | 1.2 ± 0.2 | 1.5 ± 0.2 | 8.5 ± 0.5 |
| (mmol l−1) | Untrained | 1.5 ± 0.2 | 1.8 ± 0.3 | 2.4 ± 0.2* | 6.8 ± 0.6* |
| GE | Trained | 11.5 ± 0.4 | 17.0 ± 0.5a | 19.2 ± 0.3a,b | 21.0 ± 0.3 |
| (%) | Untrained | 11.2 ± 0.6 | 16.0 ± 0.3a | 18.2 ± 0.3a,b | 18.8 ± 0.4* |
| WE | Trained | 25.1 ± 0.5 | 28.1 ± 0.5a | 27.6 ± 0.6a | 25.0 ± 0.4 |
| (%) | Untrained | 25.9 ± 0.4 | 27.7 ± 0.8a | 26.9 ± 0.6a | 24.7 ± 0.4 |
| DE | Trained | 26.7 ± 0.4 | 26.7 ± 0.4 | 26.7 ± 0.4 | — |
| (%) | Untrained | 26.5 ± 0.5 | 26.5 ± 0.5 | 26.5 ± 0.5 | — |
Mean (± s.e.m.) efficiency and blood lactate values from 8 trained and 9 untrained subjects. Blood samples for lactate analysis were taken during the final minute of exercise at the indicated work rate. The relative work rates (80% 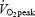 ) corresponds to 254 ± 8 W (trained) and 155 ± 11 W (untrained). Values are mean ± s.e.m. GE, gross efficiency; WE, work efficiency and DE, delta efficiency. Statistical significance was tested with ANOVA (absolute work rates) or unpaired t test (80%
) corresponds to 254 ± 8 W (trained) and 155 ± 11 W (untrained). Values are mean ± s.e.m. GE, gross efficiency; WE, work efficiency and DE, delta efficiency. Statistical significance was tested with ANOVA (absolute work rates) or unpaired t test (80% 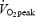 and DE). For details regarding definition and calculation see Methods.
and DE). For details regarding definition and calculation see Methods.
Significant difference from trained (P < 0.05).
Significant difference from 40 W (P < 0.05).
Significant difference from 80 W (P < 0.05).
Oxygen uptake measured while resting on the cycle ergometer (trained 324 ± 25 and untrained 326 ± 37 ml O2 min−1) and during load-less pedalling (trained 867 ± 40 and untrained 904 ± 55 ml O2 min−1) showed no difference between groups. Work rate at 80% 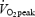 was significantly different between groups (trained 258 ± 8 W and untrained 155 ± 11 W, P < 0.001). EE increased significantly as a function of increased work rate (P < 0.001 for both groups) but there was no difference between groups. The relation between EE and work rate was linear with a high correlation for all subjects (r > 0.99). The y-intercept was calculated for each subject by linear regression and was used in the calculation of the WE (see methods). There was no difference between groups in the y-intercept (trained 185 ± 15 versus untrained 203 ± 17 ml O2 min−1, P > 0.05).
was significantly different between groups (trained 258 ± 8 W and untrained 155 ± 11 W, P < 0.001). EE increased significantly as a function of increased work rate (P < 0.001 for both groups) but there was no difference between groups. The relation between EE and work rate was linear with a high correlation for all subjects (r > 0.99). The y-intercept was calculated for each subject by linear regression and was used in the calculation of the WE (see methods). There was no difference between groups in the y-intercept (trained 185 ± 15 versus untrained 203 ± 17 ml O2 min−1, P > 0.05).
GE increased significantly when work rate increased from 40 to 80 W (P < 0.05) and from 80 to 120 W (P < 0.05) but was not influenced by training status when subjects were exercising at the same absolute work rate. However, during exercise at 80% of 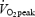 , GE was significantly higher in trained subjects (P < 0.01 versus untrained, Table 2). There was a main effect of work rate on WE (P < 0.05) but not of training status. WE increased significantly between 40 and 80 W (P < 0.05) but not between 80 and 120 W. WE at 80% of
, GE was significantly higher in trained subjects (P < 0.01 versus untrained, Table 2). There was a main effect of work rate on WE (P < 0.05) but not of training status. WE increased significantly between 40 and 80 W (P < 0.05) but not between 80 and 120 W. WE at 80% of 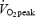 was significantly lower than that at 80 W (P < 0.001) but there was no difference between trained and untrained subjects. Blood lactate increased above 6 mmol l−1 in both trained and untrained subjects during exercise at 80% of
was significantly lower than that at 80 W (P < 0.001) but there was no difference between trained and untrained subjects. Blood lactate increased above 6 mmol l−1 in both trained and untrained subjects during exercise at 80% of 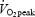 (Table 2) and the lower efficiency than that at 80 W may relate to the slow component of
(Table 2) and the lower efficiency than that at 80 W may relate to the slow component of 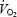 kinetics. DE was calculated as the slope of the linear relationship between EE and work rate (40, 80 and 120 W). Two untrained subjects had high values of blood lactate at 120 W (> 5.0 mmol l−1) and DE was in these subjects calculated from EE at 40 and 80 W. DE for all subjects ranged from 25 to 30% but there was no significant difference between the trained and untrained subjects (P > 0.05, Table 2).
kinetics. DE was calculated as the slope of the linear relationship between EE and work rate (40, 80 and 120 W). Two untrained subjects had high values of blood lactate at 120 W (> 5.0 mmol l−1) and DE was in these subjects calculated from EE at 40 and 80 W. DE for all subjects ranged from 25 to 30% but there was no significant difference between the trained and untrained subjects (P > 0.05, Table 2).
Mitochondrial characteristics (Table 3)
Table 3.
Mitochondrial respiration
| Respiratory parameter | Trained (n = 9) | Untrained (n = 8) | Statistics (trained vs. untrained) |
|---|---|---|---|
| State 3 | 88.7 ± 4.1 | 88.4 ± 8.9 | N.S. |
| State 4 | 6.5 ± 0.4 | 7.2 ± 1.1 | N.S. |
| RCI ratio | 14.0 ± 1.1 | 13.0 ± 1.0 | N.S. |
| P/O ratio (state 3) | 2.47 ± 0.02 | 2.50 ± 0.06 | N.S. |
Values are mean ± s.e.m. State 3 is the maximal ADP stimulated respiration with pyruvate ( + malate) and state 4 is the mitochondrial respiration without ADP. RCI ratio is the state 3-value divided by the state 4-value. P/O ratio (state 3) is the amount of ADP used per oxygen atom during state 3. Respiration rates (state 3 and state 4) are presented as nmol O2 min−1 U CS−1. N.S: not statistically significant.
Mitochondrial yield, calculated from the fraction of muscle CS activity recovered in isolated mitochondria, averaged 15% (range 7–26%) in the trained and 16% (range 9–23%) in the untrained group. Respiratory control index (RCI) and P/O ratio, measured during state 3–4 transition were high and demonstrate a close coupling between reduction of oxygen and phosphorylation of ADP (Table 3). There was no difference between groups in the RCI ratio or the P/O ratio. When the respiration was expressed per kilogram muscle mass there was a significant difference in the mitochondrial respiration between the trained and untrained groups (trained 2.4 ± 0.1 versus untrained 1.7 ± 0.2 mmol O2 min−1 (kg wet wt)−1, P < 0.05). In contrast, when the respiration was expressed relative to CS activity, there was no significant difference between the trained and untrained group in state 3 or state 4 respirations (Table 3).
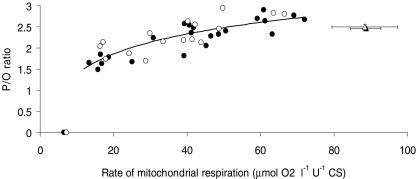
MEff (P/O ratio) was low at low rates of respiration but increased asymptotically towards that at state 3 when respiration increased (Fig. 1). There was no difference between the trained and untrained group in the shape or horizontal magnitude of the curve (a and b constants) and the curve fit is therefore shown for the group as a whole (Fig. 1). Average MEff at 50% of state 3 respiration was 2.39 ± 0.01 for the whole material (n = 17) which was significantly lower (P < 0.05) than that at state 3 (2.48 ± 0.01).
Figure 1. Relationship between MEff (P/O ratio) and rate of mitochondrial respiration.
Submaximal respiration is denoted by • (trained, n = 9) and ○ (untrained, n = 8) and was induced by constant rate of ADP infusion (2–3 rates per subject). Values during state 3 respiration (mean ± s.e.m.) are denoted by ▴ (trained) and ▵ (untrained). Values during state 4 respiration (mean ± s.e.m.) are shown on the x-axis. Data were statistically analysed with non-linear regression analysis based on a logarithmic function. There was no statistical significant difference between the trained and untrained subjects and the line represents the logarithmic fit for the entire group of subjects.
UCP3 protein
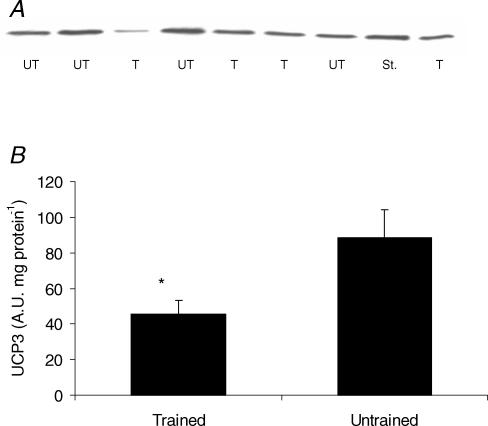
Untrained subjects had 52% more UCP3 protein per muscle weight compared to the trained group (P < 0.05) (Fig. 2B) and the difference was even more pronounced (300% higher) when CS activity was used as reference base (i.e. UCP3 per mitochondrial volume) (P < 0.01) (data not shown). Neither MEff (determined at submaximal or state 3 respiration) nor state 4 respiration was correlated to muscle UCP3. Furthermore, in contrast to previous findings using immunofluoroscence (Russell et al. 2003) there was no correlation between fibre type composition (%MHC I) and UCP3 (r = −0.36, P = 0.16).
Figure 2. Muscle content of UCP3 protein.
A, a representative gel with samples from 4 untrained (UT), 4 trained (T) subjects and one standard (St.). B, UCP3 protein content (A.U., arbitrary units) (mean ± s.e.m.) from trained (n = 9) and untrained (n = 9) subjects. *Significant difference (P < 0.05).
Correlation between cycling efficiency, UCP3, fibre type composition, and MEff
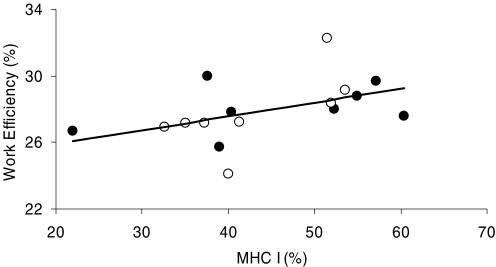
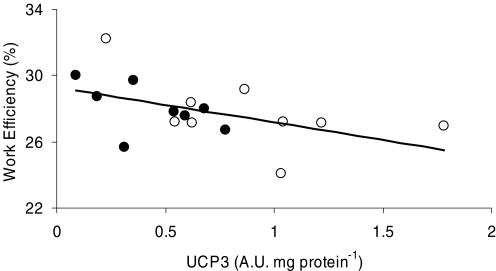
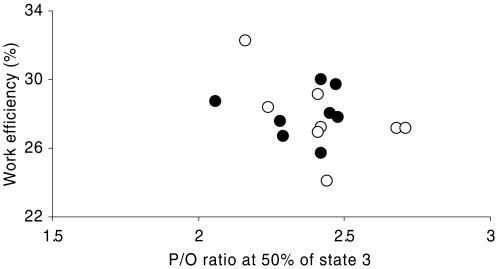
Cycling efficiency, measured as WE, demonstrated a low but statistically significant correlation with fibre type composition (% MHC I) both at 80 (r = 0.58, P < 0.05, Fig. 3) and 120 W (r = 0.58, P < 0.05), and tended to correlate to percentage MHC I, when measured as DE (r = 0.41, P = 0.12). Furthermore, cycling efficiency was significantly correlated to the amount of UCP3 when measured as WE at 80 (r = −0.57, P < 0.05, Fig. 4) and 120 W (r = −0.71, P < 0.01) or as DE (r = −0.50, P < 0.05). However, WE at 80% 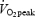 was correlated neither to percentage MHC I nor to UCP3 (P > 0.05). Furthermore, there was no correlation between cycling efficiency and MEff (r = −0.26, P > 0.05, Fig. 5).
was correlated neither to percentage MHC I nor to UCP3 (P > 0.05). Furthermore, there was no correlation between cycling efficiency and MEff (r = −0.26, P > 0.05, Fig. 5).
Figure 3. Correlation between work efficiency and percentage MHC I.
Values are from 8 trained (•) and 8 untrained (○) subjects at 80 W. WE was significantly correlated to the percentage of MCH I (r = 0.58, P < 0.05).
Figure 4. Correlation between work efficiency and UCP3 protein content.
Values are mean ± s.e.m. from 8 trained (•) and 9 untrained (○) subjects at 80 W. WE was significantly correlated to the amount of UCP3 protein content (r = −0.57, P < 0.05).
Figure 5. Comparison between work efficiency and MEff (P/O ratio) at 50% of state 3.
Values are from 8 trained (•) and 8 untrained (○) subjects at 80 W. Work efficiency was not significantly correlated to MEff measured at submaximal rates.
Discussion
The main new finding in the present study was that cycling efficiency was inversely correlated to UCP3 protein content but not to MEff. In agreement with some previous findings, we found a correlation between cycling efficiency and the proportion of type I fibres. Furthermore, we were unable to find a difference between trained and untrained subjects in cycling efficiency or MEff.
Mitochondrial efficiency
The present results are consistent with previous findings that increased aerobic training status is associated with marked increases in muscle oxidative power but that endurance training has no influence on muscle respiratory parameters (state 3 and OxPhos) when these are related to mitochondrial volume (CS activity) (Table 2) (Wibom et al. 1992; Tonkonogi & Sahlin, 1997; Tonkonogi et al. 2000; Fernstrom et al. 2004). MEff is conventionally measured as the P/O ratio during state 3. The results from this study confirms previous findings (Wibom et al. 1992; Tonkonogi & Sahlin, 1997; Tonkonogi et al. 2000; Fernstrom et al. 2004) that MEff during state 3 is not affected by training status. However, membrane potential will decrease during state 3 respiration and the back-leakage of protons will be reduced or abolished (Jarmuszkiewicz et al. 2004). Therefore, during state 3, the influence of intrinsic uncoupling factors will be diminished and potential differences in P/O ratio between two populations of mitochondria will be difficult to observe. During two-legged exercise 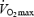 is limited by oxygen supply and not by maximal oxidative power of the working muscle (Andersen & Saltin, 1985). Mitochondria are therefore respiring at submaximal rates even during maximal exercise and it is therefore more physiological to determine MEff during submaximal respiration rates. The present study is the first to report MEff during submaximal respiration in human skeletal muscle. The result clearly shows that MEff is reduced at submaximal rates of respiration but approaches that at state 3 when respiration rate increases (Fig. 2). Similar results have been presented in isolated mitochondria from rodent liver and muscle (Willis & Jackman, 1994; Gnaiger et al. 2000; Mogensen & Sahlin, 2005). In accordance with the present results, these studies have shown a curvilinear increase in the P/O ratio as a function of increased respiration. Furthermore, our results demonstrate that MEff is unaffected by training status also at submaximal respiration rates. Thus, from the present study it can be concluded that under standardized in vitro conditions proton leak is not different in trained and untrained subjects (Fig. 1).
is limited by oxygen supply and not by maximal oxidative power of the working muscle (Andersen & Saltin, 1985). Mitochondria are therefore respiring at submaximal rates even during maximal exercise and it is therefore more physiological to determine MEff during submaximal respiration rates. The present study is the first to report MEff during submaximal respiration in human skeletal muscle. The result clearly shows that MEff is reduced at submaximal rates of respiration but approaches that at state 3 when respiration rate increases (Fig. 2). Similar results have been presented in isolated mitochondria from rodent liver and muscle (Willis & Jackman, 1994; Gnaiger et al. 2000; Mogensen & Sahlin, 2005). In accordance with the present results, these studies have shown a curvilinear increase in the P/O ratio as a function of increased respiration. Furthermore, our results demonstrate that MEff is unaffected by training status also at submaximal respiration rates. Thus, from the present study it can be concluded that under standardized in vitro conditions proton leak is not different in trained and untrained subjects (Fig. 1).
From the present study it is clear that there is no correlation between MEff and cycling efficiency, either at maximal or at submaximal respiration rates (Fig. 5). These results suggest that intrinsic mitochondrial uncoupling factors do not influence individual cycling efficiency. However, it cannot be excluded that the mitochondrion is a determining factor for the efficiency of muscular work during in vivo conditions, where various extrinsic factors may influence the leak and slippage of protons through the inner membrane. It is well-known proton leak is influenced by factors like fatty acids, free radicals, adenine nucleotides and membrane potential (Kadenbach, 2003; Brand & Esteves, 2005). These factors could vary between subjects and influence mitochondrial efficiency in vivo thereby affecting the individual efficiency of the muscular work.
If the non-linear relationship between P/O ratio and intensity of the mitochondrial respiration is present in vivo one would expect a lower mitochondrial efficiency and cycling efficiency during low intensity exercise than during higher intensities. The present results demonstrate a lower WE at the lowest work rate (40 W) than at 80 and 120 W. Although a lower MEff may contribute to the low WE at 40 W we consider an increased coactivation of antagonistic muscles to be more important. This is also supported by the high 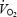 during load-less pedalling. Previous studies have shown that DE decreases with increments in work rate (Gaesser & Brooks, 1975). Furthermore, Marcinek, 2004) measured P/O ratio in resting muscle in vivo with a method based on infrared spectroscopy and 31P MRS. The P/O ratio was estimated to about 2.1 in resting mouse muscle presumably with FA as the main substrate. Theoretically, maximal P/O ratio during FA oxidation is 2.33 (Brand et al. 1994) and the results imply that the degree of uncoupling in vivo is low despite low rates of respiration. The present results from isolated mitochondria would predict a much lower P/O ratio at rest. OxPhos is stimulated both by a push mechanism (increases in redox drive, i.e. NADH/NAD+) and a pull mechanism (increases in ADP or ADP/(ATP × Pi)) (Kushmerick & Crow, 1983). An increased redox drive will increase mitochondrial membrane potential and stimulate proton leak and electron slip (Kadenbach, 2003). Our experiments with isolated mitochondria were performed with maximal redox drive (saturated substrate concentrations), whereas the conditions in vivo may involve a lower redox drive. Measurements of NADH in human muscle support this line of reasoning in that NADH and thus the redox drive was much lower during exercise at 40%
during load-less pedalling. Previous studies have shown that DE decreases with increments in work rate (Gaesser & Brooks, 1975). Furthermore, Marcinek, 2004) measured P/O ratio in resting muscle in vivo with a method based on infrared spectroscopy and 31P MRS. The P/O ratio was estimated to about 2.1 in resting mouse muscle presumably with FA as the main substrate. Theoretically, maximal P/O ratio during FA oxidation is 2.33 (Brand et al. 1994) and the results imply that the degree of uncoupling in vivo is low despite low rates of respiration. The present results from isolated mitochondria would predict a much lower P/O ratio at rest. OxPhos is stimulated both by a push mechanism (increases in redox drive, i.e. NADH/NAD+) and a pull mechanism (increases in ADP or ADP/(ATP × Pi)) (Kushmerick & Crow, 1983). An increased redox drive will increase mitochondrial membrane potential and stimulate proton leak and electron slip (Kadenbach, 2003). Our experiments with isolated mitochondria were performed with maximal redox drive (saturated substrate concentrations), whereas the conditions in vivo may involve a lower redox drive. Measurements of NADH in human muscle support this line of reasoning in that NADH and thus the redox drive was much lower during exercise at 40% 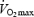 than that at 75 and 100%
than that at 75 and 100% 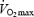 (Sahlin et al. 1987). The difference between our data in vitro predicting a low P/O ratio in resting muscle and that determined in vivo demonstrating a high P/O ratio may therefore relate to differences in redox drive.
(Sahlin et al. 1987). The difference between our data in vitro predicting a low P/O ratio in resting muscle and that determined in vivo demonstrating a high P/O ratio may therefore relate to differences in redox drive.
Effect of training status on cycling efficiency
Cycling efficiency has in the present study been calculated in different ways (GE, WE and DE). GE describes the external power output as the percentage of the total EE, thereby not subtracting for basal EE. The observed increase in GE at increasing work rate and the higher GE in trained subjects versus untrained subjects (Table 2) at the same relative work rate (80% 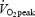 ) can therefore, to a large extent, be explained by a decreased relative significance of basal EE. However, in contrast to the present data, GE has in some studies been found to increase after training (Gardner et al. 1989; Coyle, 2005) or to be higher in endurance trained subjects (Kunstlinger et al. 1985; Gissane et al. 1991) even when measured at the same absolute work rate. However, it cannot be excluded that the higher GE in trained subjects is caused by decreased influence of the non-exercise component of EE rather than by an increased muscular efficiency.
) can therefore, to a large extent, be explained by a decreased relative significance of basal EE. However, in contrast to the present data, GE has in some studies been found to increase after training (Gardner et al. 1989; Coyle, 2005) or to be higher in endurance trained subjects (Kunstlinger et al. 1985; Gissane et al. 1991) even when measured at the same absolute work rate. However, it cannot be excluded that the higher GE in trained subjects is caused by decreased influence of the non-exercise component of EE rather than by an increased muscular efficiency.
WE and DE are alternative ways to calculate cycling efficiency which, in contrast to GE, are independent of basal EE. The findings of the present study where WE and DE were unaffected by training status (Table 2) agree with the majority of previous studies (Gaesser & Brooks, 1975; Boning et al. 1984; Nickleberry & Brooks, 1996; Marsh et al. 2000) but are in contrast to studies where increased training status resulted in lower (Mallory et al. 2002) or higher DE (Coyle, 2005). Mallory et al. (2002) showed an inverse relationship between DE and 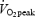 in a group of 22 subjects including both males and females with aerobic capacities ranging from 34.3 to 59.2 ml O2 min−1 kg−1. It is well known that females have a lower
in a group of 22 subjects including both males and females with aerobic capacities ranging from 34.3 to 59.2 ml O2 min−1 kg−1. It is well known that females have a lower 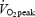 (ml O2 min−1 kg−1) compared with males, and there are some data which indicate that net efficiency is higher in females than in males (Bosco et al. 1980). It is therefore possible that the negative correlation between DE and
(ml O2 min−1 kg−1) compared with males, and there are some data which indicate that net efficiency is higher in females than in males (Bosco et al. 1980). It is therefore possible that the negative correlation between DE and 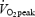 is explained by sex differences. A recent longitudinal study (Coyle, 2005) showed that intense endurance training over a period of many years can increase DE. The physiological adaptation behind this increment was suggested to be a change in the fibre type distribution towards a higher amount of more efficient type 1 fibres. However, since fibre type composition was not measured and only one subject was included, the results of this study need to be verified with more subjects and measurement of fibre type composition.
is explained by sex differences. A recent longitudinal study (Coyle, 2005) showed that intense endurance training over a period of many years can increase DE. The physiological adaptation behind this increment was suggested to be a change in the fibre type distribution towards a higher amount of more efficient type 1 fibres. However, since fibre type composition was not measured and only one subject was included, the results of this study need to be verified with more subjects and measurement of fibre type composition.
Fibre type distribution and cycling efficiency
Work efficiency was correlated to the relative volume of type 1 fibres (% MHC I, Fig. 3) and is consistent with some (Coyle et al. 1992; Horowitz et al. 1994; Hansen et al. 2002) but not all (Medbø, 1990; Pedersen et al. 2002) studies. The higher cycling efficiency in subjects with a high percentage of type 1 fibres may be related to a higher contractile efficiency (work per ATP usage) and/or a higher MEff (ATP formation per oxygen used) of type 1 fibres. In previous studies it has often been assumed that type 1 fibres have a higher MEff than type 2 fibres (Krustrup et al. 2004). Three studies have compared MEff in rat skeletal muscle mainly composed of type 1 or type 2 fibres. Jackman & Willis (1996) showed a low P/O ratio in isolated mitochondria from type 2 fibres when pyruvate + malate was used in combination with glycerol-3-phosphate. However, they also showed a higher P/O ratio in type 2 fibres when pyruvate + malate was used alone. These results are in contrast to a study by Pande & Blanchaer (1971) showing no difference between mitochondria isolated from red and white muscle irrespectively of the substrate used. Results from our laboratory are consistent with the latter study and demonstrate that in rat muscle there is no significant difference in MEff between type 1 and type 2 fibres, either at submaximal or at maximal state 3 respiration (Mogensen & Sahlin, 2005). Furthermore, the present study could not show a significant correlation between percentage MHC I and MEff in mitochondria isolated from human skeletal muscle. The idea that MEff is higher in mitochondria from type 1 fibres is based on limited evidence and can therefore be questioned.
Most animal studies investigating the thermodynamic efficiency of muscle contraction have shown that type 1 fibres are more efficient than type 2 fibres (Smith et al. 2005 and references herein). However, as previously discussed (Medbø, 1990) there could be differences between human and animal type 2 fibres. In animals, such as rats and mice, type 2 fibres are only active during short bursts of intense work, in contrast to humans where type 2 fibres are active also during endurance exercise (Krustrup et al. 2004). The efficiency of type 1 and 2 fibres has been measured in human single fibres and it was shown that the thermodynamic efficiency of the cross-bridge cycle does not differ between type 1 and type 2 fibres (He et al. 2000). The highest efficiency for type 1 fibres was attained at low to moderate contractile velocities whereas the highest efficiency for type 2 fibres was attained at high velocities of contraction. Based on these results it seems logical to assume that as work rate increases and more type 2 fibres are recruited one would need to increase the cadence in order to find the optimal efficiency. Several studies in humans have also demonstrated that the most efficient cadence increases with increasing work rates (Foss & Hallen, 2004 and references herein). Furthermore, the correlation between cycling efficiency and percentage MHC I is abolished if the subjects freely can chose their own cadence (Hansen et al. 2002). This indicates that an increased recruitment of type 2 fibres at high work rates would be associated with reduced efficiency if the cadence is maintained constant and below the optimal cadence. Thus, it may be argued that the observed correlation between cycling efficiency and percentage MHC I is caused by a more optimal cadence for type 1 fibres than for type 2 fibres.
UCP3 protein and cycling efficiency
Muscle UCP3 was lower in the group of trained subjects (Fig. 2), which is consistent with previous studies (Russell et al. 2003; Fernstrom et al. 2004; Schrauwen et al. 2005). Cycling efficiency, measured both as WE and DE, was inversely correlated to UCP3. The finding appears to be in agreement with the results from Schrauwen & Hesselink, 2003), showing a very strong inverse correlation between GE and UCP3 protein content (r = −0.97). GE was in this study measured in seven moderately active male subjects cycling for 2 h at a constant relative work rate (50% of 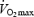 ) and thus at different absolute work rates. It is evident from the present results (Table 2) as well as from other studies (Gaesser & Brooks, 1975; Nickleberry & Brooks, 1996) that GE increases when the absolute work rate increases. In a group of subjects, this will result in a positive correlation between the aerobic capacity and GE. Furthermore, it is also well known that UCP3 protein decreases as a function of increased aerobic capacity (Fig. 2) (Russell et al. 2003; Fernstrom et al. 2004; Schrauwen et al. 2005). These two correlations will unavoidably create a dependency between GE (if measured at the same relative work rates) and UCP3 content, probably explaining the observed strong negative correlation between GE and UCP3 (Schrauwen & Hesselink, 2003). Although the results of Schrauwen & Hesselink (2003) can be questioned on methodological grounds, the present results are in conformity and demonstrate that cycling efficiency measured both as WE and DE is inversely correlated with UCP3 protein content in muscle.
) and thus at different absolute work rates. It is evident from the present results (Table 2) as well as from other studies (Gaesser & Brooks, 1975; Nickleberry & Brooks, 1996) that GE increases when the absolute work rate increases. In a group of subjects, this will result in a positive correlation between the aerobic capacity and GE. Furthermore, it is also well known that UCP3 protein decreases as a function of increased aerobic capacity (Fig. 2) (Russell et al. 2003; Fernstrom et al. 2004; Schrauwen et al. 2005). These two correlations will unavoidably create a dependency between GE (if measured at the same relative work rates) and UCP3 content, probably explaining the observed strong negative correlation between GE and UCP3 (Schrauwen & Hesselink, 2003). Although the results of Schrauwen & Hesselink (2003) can be questioned on methodological grounds, the present results are in conformity and demonstrate that cycling efficiency measured both as WE and DE is inversely correlated with UCP3 protein content in muscle.
Since the discovery of UCP3 in 1997 (Boss et al. 1997), numerous studies have investigated the potential function of this mitochondrial protein. Currently two theories have gained considerable interest, stating (a) that UCP3 could reduce mitochondrial generation of reactive oxygen species (Krauss et al. 2005) and (b) that UCP3 could reduce accumulation of non-esterfied fatty acids in the mitochondrial matrix (Schrauwen et al. 2001). While these two theories are supported by experimental evidence, the importance of UCP3 for whole body EE and mitochondrial efficiency remains unclear. Studies using UCP3 knockout mice have shown an unaltered body weight, exercise tolerance and FA oxidation (Vidal-Puig et al. 2000) and normal resting oxygen consumption and respiratory exchange ratio (Gong et al. 2000). On the other hand overexpression of the UCP3 gene has resulted in hyperphagic and lean mice (Clapham et al. 2000). Recently a study by MacLellan et al. (2005) showed that increased UCP3 content in rat myoblasts facilitated FA oxidation and decreased ROS production, whereas uncoupled respiration was unaffected. The absence of firm evidence of UCP3 mediating mitochondrial uncoupling has led to the discussion of whether UCP3 really is capable of uncoupling OxPhos (Nedergaard & Cannon, 2003). In the present study neither uncoupled respiration, measured as state 4, nor MEff at submaximal respiration was correlated to UCP3 protein. These results speak against a role of UCP3 in mitochondrial uncoupling and it is possible that the observed correlation between cycling efficiency and UCP3 protein, instead of being a cause and effect, is the result of a correlation between cycling efficiency and percentage MHC I (Fig. 3). However, the absence of a correlation between UCP3 and percentage MHC I (r = −0.35, P > 0.05) speaks against this idea. In analogy with the previous discussion, it cannot be excluded that the absence of UCP3 activating factors in vitro (e.g. fatty acids and reactive oxygen species) explains why there is no correlation between UCP3 and MEff or between UCP3 and state 4 respiration.
Summary
The main purpose of the present study was to investigate if differences between subjects in cycling efficiency could be explained by individual differences in MEff. The results showed that there was no correlation between cycling efficiency and MEff as determined in vitro. However, cycling efficiency was correlated to percentage MHC I and inversely correlated UCP3 protein content. Both these correlations are interesting but further research is required to establish the mechanism. MEff assessed at submaximal respiratory intensities was lower than that at state 3. Despite the lack of correlation between cycling efficiency and MEff determined in vitro it cannot be excluded that extrinsic factors influence MEff in vivo and that this explains the correlation between WE and UCP3.
Acknowledgments
The authors wish to thank Arne Gaarn, Benthe Jørgensen, Chris Christensen and Gitte Schell Klemmesen for excellent technical assistance. The work was supported financially by Statens Sundhedsvidenskabelige Forskningsråd and Kulturministeriets Udvalg for Idrætsforskning and by the Swedish Research Council (project 13020).
References
- Alp PR, Newsholme EA, Zammit VA. Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J. 1976;154:689–700. doi: 10.1042/bj1540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boning D, Gonen Y, Maassen N. Relationship between work load, pedal frequency, and physical fitness. Int J Sports Med. 1984;5:92–97. doi: 10.1055/s-2008-1025887. [DOI] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Bosco C, Komi PV, Sinkkonen K. Mechanical power, net efficiency and muscle structure in male and female middle distance runners. Scand J Sports Sci. 1980;12:288–294. [Google Scholar]
- Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- Brand MD, Chien LF, Ainscow EK, Rolfe DF, Porter RK. The causes and functions of mitochondrial proton leak. Biochim Biophys Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brouwer E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl. 1957;6:795–802. [PubMed] [Google Scholar]
- Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- Coyle EF. Improved muscular efficiency displayed as Tour de France champion matures. J Appl Physiol. 2005;98:2191–2196. doi: 10.1152/japplphysiol.00216.2005. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- Danieli BD, Zerbato E, Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986;138:981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Fernstrom M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol. 2004;554:755–763. doi: 10.1113/jphysiol.2003.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons DP, Diffee GM, Herrick RE, Baldwin KM. Effects of endurance exercise on isomyosin patterns in fast- and slow-twitch skeletal muscles. J Appl Physiol. 1990;68:1950–1955. doi: 10.1152/jappl.1990.68.5.1950. [DOI] [PubMed] [Google Scholar]
- Foss O, Hallen J. The most economical cadence increases with increasing workload. Eur J Appl Physiol. 2004;92:443–451. doi: 10.1007/s00421-004-1175-5. [DOI] [PubMed] [Google Scholar]
- Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol. 1975;38:1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- Gardner AW, Poehlman ET, Corrigan DL. Effect of endurance training on gross energy expenditure during exercise. Hum Biol. 1989;61:559–569. [PubMed] [Google Scholar]
- Gissane C, Corrigan DL, White JA. Gross efficiency responses to exercise conditioning in adult males of various ages. J Sports Sci. 1991;9:383–391. doi: 10.1080/02640419108729898. [DOI] [PubMed] [Google Scholar]
- Gnaiger E, Mendez G, Hand SC. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci U S A. 2000;97:11080–11085. doi: 10.1073/pnas.97.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong DW, Monemdjou S, Gavrilova O, Leon LR, Marcus-Samuels B, Chou CJ, et al. Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J Biol Chem. 2000;275:16251–16257. doi: 10.1074/jbc.M910177199. [DOI] [PubMed] [Google Scholar]
- Hansen EA, Andersen JL, Nielsen JS, Sjogaard G. Muscle fibre type, efficiency, and mechanical optima affect freely chosen pedal rate during cycling. Acta Physiol Scand. 2002;176:185–194. doi: 10.1046/j.1365-201X.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JF, Sidossis LS, Coyle EF. High efficiency of type I muscle fibers improves performance. Int J Sports Med. 1994;15:152–157. doi: 10.1055/s-2007-1021038. [DOI] [PubMed] [Google Scholar]
- Jackman MR, Willis WT. Characteristics of mitochondria isolated from type I and type IIb skeletal muscle. Am J Physiol. 1996;270:C673–C678. doi: 10.1152/ajpcell.1996.270.2.C673. [DOI] [PubMed] [Google Scholar]
- Jarmuszkiewicz W, Navet R, Alberici LC, Douette P, Sluse-Goffart CM, Sluse FE, et al. Redox state of endogenous coenzyme q modulates the inhibition of linoleic acid-induced uncoupling by guanosine triphosphate in isolated skeletal muscle mitochondria. J Bioenerg Biomembr. 2004;36:493–502. doi: 10.1023/B:JOBB.0000047331.25248.7a. [DOI] [PubMed] [Google Scholar]
- Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004;447:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- Kunstlinger U, Ludwig HG, Stegemann J. Force kinetics and oxygen consumption during bicycle ergometer work in racing cyclists and reference-group. Eur J Appl Physiol Occup Physiol. 1985;54:58–61. doi: 10.1007/BF00426299. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Crow MT. Regulation of energetics and mechanics by myosin light chain phosphorylation in fast-twitch skeletal muscle. Fed Proc. 1983;42:14–20. [PubMed] [Google Scholar]
- MacLellan JD, Gerrits MF, Gowing A, Smith PJ, Wheeler MB, Harper ME. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005;54:2343–2350. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- Mallory LA, Scheuermann BW, Hoelting BD, Weiss ML, McAllister RM, Barstow TJ. Influence of peak VO2 and muscle fiber type on the efficiency of moderate exercise. Med Sci Sports Exerc. 2002;34:1279–1287. doi: 10.1097/00005768-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Marcinek DJ. Mitochondrial dysfunction measured in vivo. Acta Physiol Scand. 2004;182:343–352. doi: 10.1111/j.1365-201X.2004.01372.x. [DOI] [PubMed] [Google Scholar]
- Marsh AP, Martin PE, Foley KO. Effect of cadence, cycling experience, and aerobic power on delta efficiency during cycling. Med Sci Sports Exerc. 2000;32:1630–1634. doi: 10.1097/00005768-200009000-00017. [DOI] [PubMed] [Google Scholar]
- Medbø JI. Type I and Type II fibres work with the same mechanical efficiency during bicycling. In: Marechal G, Buonocore C, editors. Muscle and Motility. Hampshire, Andover, UK: Intercept Limited; 1990. pp. 303–308. [Google Scholar]
- Mogensen M, Sahlin K. Mitochondrial efficiency in rat skeletal muscle: influence of respiration rate, substrate and muscle type. Acta Physiol Scand. 2005;185:229–236. doi: 10.1111/j.1365-201X.2005.01488.x. [DOI] [PubMed] [Google Scholar]
- Moseley L, Jeukendrup AE. The reliability of cycling efficiency. Med Sci Sports Exerc. 2001;33:621–627. doi: 10.1097/00005768-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The ‘novel’‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- Nickleberry BL, Jr, Brooks GA. No effect of cycling experience on leg cycle ergometer efficiency. Med Sci Sports Exerc. 1996;28:1396–1401. doi: 10.1097/00005768-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Pande SV, Blanchaer MC. Carbohydrate and fat in energy metabolism of red and white muscle. Am J Physiol. 1971;220:549–553. doi: 10.1152/ajplegacy.1971.220.2.549. [DOI] [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. Enzymatic Analysis. A Practical Guide. Totowa, NJ, USA: Humana Press; 1993. [Google Scholar]
- Pedersen PK, Sorensen JB, Jensen K, Johansen L, Levin K. Muscle fiber type distribution and nonlinear VO2-power output relationship in cycling. Med Sci Sports Exercise. 2002;34:655–661. doi: 10.1097/00005768-200204000-00015. [DOI] [PubMed] [Google Scholar]
- Putman CT, Xu X, Gillies E, MacLean IM, Bell GJ. Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur J Appl Physiol. 2004;92:376–384. doi: 10.1007/s00421-004-1104-7. [DOI] [PubMed] [Google Scholar]
- Russell AP, Wadley G, Hesselink MK, Schaart G, Lo S, Leger B, et al. UCP3 protein expression is lower in type I, IIa and IIx muscle fiber types of endurance-trained compared to untrained subjects. Pflugers Arch. 2003;445:563–569. doi: 10.1007/s00424-002-0943-5. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Katz A, Henriksson J. Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem J. 1987;245:551–556. doi: 10.1042/bj2450551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, Hesselink M. Uncoupling protein 3 and physical activity: the role of uncoupling protein 3 in energy metabolism revisited. Proc Nutr Soc. 2003;62:635–643. doi: 10.1079/PNS2003277. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Russell AP, Moonen-Kornips E, Boon N, Hesselink MK. Effect of 2 weeks of endurance training on uncoupling protein 3 content in untrained human subjects. Acta Physiol Scand. 2005;183:273–280. doi: 10.1111/j.1365-201X.2004.01393.x. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Saris WH, Hesselink MK. An alternative function for human uncoupling protein 3: protection of mitochondria against accumulation of nonesterified fatty acids inside the mitochondrial matrix. FASEB J. 2001;15:2497–2502. doi: 10.1096/fj.01-0400hyp. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Troost FJ, Xia J, Ravussin E, Saris WH. Skeletal muscle UCP2 and UCP3 expression in trained and untrained male subjects. Int J Obes Relat Metab Disord. 1999;23:966–972. doi: 10.1038/sj.ijo.0801026. [DOI] [PubMed] [Google Scholar]
- Smith NP, Barclay CJ, Loiselle DS. The efficiency of muscle contraction. Prog Biophys Mol Biol. 2005;88:1–58. doi: 10.1016/j.pbiomolbio.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, et al. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiol Scand. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Walsh B, Svensson M, Sahlin K. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. J Physiol. 2000;528:379–388. doi: 10.1111/j.1469-7793.2000.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol. 1992;73:2004–2010. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]
- Williams KR. The relationship between mechanical and physiological energy estimates. Med Sci Sports Exerc. 1985;17:317–325. [PubMed] [Google Scholar]
- Willis WT, Jackman MR. Mitochondrial function during heavy exercise. Med Sci Sports Exerc. 1994;26:1347–1353. [PubMed] [Google Scholar]