Abstract
In locomotion, the flexor muscles of the leg are mainly concerned with the relatively constant task of raising the foot, whereas the extensors have the more variable task of support and propulsion at different speeds. This suggests that the way in which the fusimotor system works may differ between the two muscle groups. Observations previously made of the static and dynamic γ-motor firing patterns in the ankle extensor medial gastrocnemius (MG) have therefore been repeated in the flexor tibialis anterior (TA). One or more single γ-motor axons, dissected from a small filament of TA nerve, were recorded simultaneously with a number of single spindle afferents in dorsal rootlets. Cats were decerebrated and locomoted spontaneously on a treadmill. Identification of each γ-motor axon depended on relating the changes in firing caused by midbrain stimulation to the changes in static and dynamic behaviour of the spindle afferents in response to repetitive ramp and hold stretches. Static γ axons all showed a smooth modulation in frequency, increasing in phase with muscle shortening, superimposed on a minimum frequency of about 20–30 impulses s−1. Dynamic γ axons showed interrupted firing with the frequency rising abruptly from zero at the onset of shortening, and falling again to zero shortly after the onset of lengthening. The frequency during the active periods was relatively constant, even when movement amplitudes varied. The basic similarity in the static and dynamic gamma discharge patterns for the two muscles suggests that the strategy of γ-motor control is common to both flexors and extensors. The static γ pattern is thought to be a ‘temporal template’ of the expected movement, effectively expanding the dynamic response range of the spindles in active movements. The dynamic γ pattern sensitizes the primary afferents to detect the onset of muscle lengthening and to detect departures from the intended movement trajectory.
The basic motor pattern in mammalian locomotion consists of sequences of activation of the various limb muscles determined by the activity of a central pattern generator (for reviews see Grillner, 1975; Orlovsky et al. 1999). The timing of contraction of the muscles has been carefully recorded by electromyography in many studies (e.g. Engberg & Lundberg, 1969; Goslow et al. 1973) and it has become clear that it is strongly influenced by sensory input, especially proprioceptive (Pearson et al. 2003). One important source of this input is muscle spindle activity, and substantial advances have been made in understanding the controlling function of spindles by means of chronic unitary afferent recordings in normally locomoting cats (see Prochazka et al. 1976; Hulliger et al. 1989; Loeb & Duysens, 1979). However, since spindle activity not only depends not only on muscle length changes but is also strongly influenced by static and dynamic γ-motor output, we need to know in turn how this output is controlled, and certain interpretation of the chronic spindle recordings in these terms has proved difficult. What is required is direct recording of the activity of γ motoneurones in naturally moving animals, but no method for doing this has yet been devised.
A partial solution is to employ cats decerebrated at a level which permits spontaneous locomotion (Severin et al. 1967), but eliminates any conscious sensibility. In this preparation one hindlimb can be partially denervated and immobilized to permit dissection of single muscle spindle afferents from dorsal root filaments and to record the discharge patterns while the animal walks with the other three legs on a treadmill. The earlier studies by this method (e.g. Perret & Buser, 1972; Perret & Berthoz, 1973) relied on simple examination of spindle records, later supplemented by testing spindle sensitivity by sinusoidal stretching (Cabelguen, 1981; Taylor et al. 1985). Recently, the interpretation of spindle recordings has been enhanced by recording the active movements at the ankle and subsequently, after suppressing fusimotor activity, reproducing the same movements by means of a servo apparatus (Taylor et al. 2000a). The difference in spindle discharge between the active and passive movements was taken to indicate the underlying fusimotor activity pattern. The conclusion from this study was that the static γ-motor discharge to ankle flexors and extensors was tonically increased and modulated in parallel with the step cycle, with increasing discharge during muscle shortening. Dynamic sensitivity of spindle Ia afferents to muscle lengthening was increased, but it was not possible to say whether this was due to tonic increase in dynamic γ-motor discharge or to some fluctuating pattern. Direct recordings from triceps γ-motor axons in the decerebrate cat were first reported by Murphy et al. (1984), and these showed that the firing of both static and dynamic types was modulated. This approach has been used again recently by combining isolation of single γ-motor fibres from small filaments of the medial gastrocnemius (MG) muscle nerve with multiple single unit spindle records (Taylor et al. 2000b). The γ-motor axons were identified as static or dynamic by observing the effect of midbrain stimulation while recording the fusimotor effects on muscle spindle responses to passive stretch. Most of the static γ axons (type 1) showed strong modulation in parallel with muscle shortening as predicted by the spindle observations, but there was also a minority population (type 2) less strongly modulated and with a different timing. It emerged that dynamic γ-motor axons were silent during part of the cycle, but fired at high frequency during the transition from muscle shortening to lengthening. This appeared to be favourable for sensitizing the Ia afferents to the onset of muscle stretch (Taylor et al. 2000b; for review see Taylor et al. 2004a).
These direct γ-motor recordings (Taylor et al. 2000b) were made exclusively from the MG nerve, because of its relatively good accessibility. However, they left some doubt regarding fusimotor patterns to flexor muscles since independent observations had suggested that flexors and extensors might have different patterns of control (Murphy & Hammond, 1993). The present experiments were therefore designed to obtain equivalent data from γ-motor axons recorded in small filaments of the nerve to the ankle flexor, tibialis anterior (TA). A preliminary report has appeared in abstract form (Taylor et al. 2004b).
Methods
The preparation of the animals and the procedures for recording signals, applying stimuli and inducing locomotion have been published previously (Taylor et al. 2000a,b) and have been approved by the UK Home Office and by the institutional Ethical Committee. Anaesthesia was induced by means of 5% halothane vapour in equal parts of nitrous oxide and oxygen passed into a 30 l box at a rate of 10 l min−1. Anaesthesia was then continued with 1.5–2% halothane in the N2O–O2 mixture via a face mask during cannulation of the trachea. Thereafter the same mixture was administered via the cannula. All the surgical procedures were carried out under this anaesthesia with monitoring of arterial blood pressure, end-tidal PCO2 and rectal temperature. Adequate depth of anaesthesia was also confirmed by loss of corneal reflex and flexion withdrawal reflex of the forelimb. The preparation included cannulation of the left carotid artery for blood pressure (BP) recording and placing a loop around the right carotid so that this could be occluded just before the decerebration. The left hindlimb was extensively denervated except for the MG and TA muscles. Their nerves were enclosed in recording cuffs about 30 mm proximal to the muscles. The cuffs contained three electrodes with 2 mm spacing. The outer two were joined as the reference. Pairs of wire were also inserted in the MG and TA for recording EMG. The wires were of fine-stranded and Teflon- insulated stainless steel (Cooner Wire, Chatsworth, USA type AS631). They were bared for 5 mm and inserted separately with injection needles and spaced about 5 mm apart in the belly of the muscles, and then stitched in place to the skin. They were designed to detect activity in the bulk of the muscle. The signals were AC amplified, full-wave rectified and low-pass filtered with a corner frequency of 30 Hz.
After surgical preparation, animals were mounted in a stereotaxic frame and supported above a treadmill belt. A urethral catheter was inserted to allow free drainage of the bladder, and a rectal probe used to monitor body temperature, maintained by radiant heat lamps. Fluid was given by intravenous infusion of 10 ml doses of 5% dextran in saline as required. The left hindlimb was kept clear of the treadmill belt by fixation at the mid-femur and at the lower end of the tibia, leaving the foot free to flex and extend about the ankle through the action of the MG and TA. The left foot was secured with two plastic cable ties to a light lever which was pivoted coaxially with the ankle joint on a lightweight instrument potentiometer, so that active movements could be transduced without significant loading, and recorded on magnetic tape. The recording of active movements could be subsequently played back through an electromagnetic servo, which was locked to the potentiometer shaft, to reproduce the active movements passively.
Several (up to six) single muscle spindle afferents were recorded from a cut dorsal root filament exposed by a left-sided unilateral laminectomy, leaving most of the roots intact. Afferents were principally from the TA, but also included some from the MG. To facilitate recording without movement artefacts, silver hook electrodes were mounted in a plastic holder sutured to the interspinous ligament, so that the electrodes and the vertebral processes moved as one during locomotion. The afferents were characterized by their response to muscle stretch (ankle rotation), muscle twitch and conduction velocity.
Once a suitable set of single afferent units had been isolated, premammillary decerebration was carried out, with complete removal of the brain rostral to the plane of section, in order to render the animal totally insentient. The administration of the halothane–N2O–O2 mixture was discontinued and the activity of one or several single motor axons recorded from a small fascicle separated from the TA nerve and cut, leaving the rest of the nerve intact. Not more than 10–20% of the muscle nerve was interrupted. Efferent axons which fired spontaneously or in response to cutaneous or brainstem stimulation were accepted as γ-motor if their conduction velocity (measured by backward spike triggered averaging from a nerve cuff electrode placed about 30 mm centrally) lay between 12 and 40 m s−1. When the γ recording showed one unit very clearly distinguished from the background, a simple window discriminator was used. When several units were present, another discriminator with a second, movable window was employed to give more selective discrimination. The reliability of the process was checked by constant monitoring on an oscilloscope, and by watching the instantaneous frequency traces displayed by the computer. The unitary nature of the γ-motor axon recordings was confirmed by verifying that no very short interspike intervals occurred. As well as the direct recording on computer files, through a CED 1401-plus interface and a computer running Spike2 software (CED Ltd, Cambridge, UK), data were also tape recorded so that they could be reviewed, and in some cases additional motor axons were discriminated off-line.
Well co-ordinated locomotor movements of three legs usually occurred spontaneously on running the treadmill about 30 min after discontinuing the anaesthesia. In one of the 14 cases, stimulation in the midbrain locomotor region (MLR) was required to start locomotion, and in four cases stimulation increased the strength of the movements and caused the animal to raise and place its paws more naturally. Following Shik et al. (1966), the location of the MLR was taken to be that of the cuneiform nucleus and stimulus trains (20 Hz, 0·2 ms, 0·2–2·0 V negative, electrode resistance 104Ω) were applied in that region on the right side. In the left hindlimb, rhythmic movements at the ankle alternated with those on the other side.
Identification of γ axons as static or dynamic was carried out as previously described (Taylor et al. 2000b). In brief, stimulation (as above) in the region 1–3 mm dorsal to the MLR, in periods when locomotor movements were absent, produced fusimotor activation, with different involvement of static and dynamic systems according to electrode position and stimulus strength. The effects of this stimulation on the γ units were observed while applying continous ramp and hold muscle stretches and recording the changes in spindle afferent firing patterns. Signs of γs activation were taken to be increases in afferent firing frequency and irregularity, especially during the muscle shortening, affecting both spindle primary and secondary endings. Signs of γd activation were increases in dynamic response to stretch and in dynamic index (Brown et al. 1965) affecting only b1b2c primary afferents. Those γ units that showed increases in firing accompanying signs of static activity were taken to belong to a population of γs axons supplying the muscle. Similarly, those γ units whose firing frequency correlated with signs of dynamic activity were taken to be representative of the γd supply.
Afferent and efferent recordings were made simultaneously, first during periods of walking on the treadmill. Subsequently, fusimotor activity was suppressed with two or three intravenous doses of 12 mg kg−1 of sodium pentobarbitone, and the afferent recording continued while the previously recorded ankle movements were reproduced passively. During these periods it was confirmed that the dose of pentobarbitone was adequate to suppress EMG and the firing of the γ-motor axons. By subtracting for each spindle the afferent firing recorded during the passive movements from the firing recorded during active movements, a difference signal was obtained, which under certain conditions is linearly related to the static fusimotor output (see Taylor et al. 2000b). This proposal is considered further below in the Discussion.
Finally, the effects of intravenous succinylcholine (200 µg kg−1) were examined in order to determine the functional contacts of each afferent on bag1 and bag2 intrafusal muscle fibres (Taylor et al. 1992a,b). It was important to stimulate the muscle (10 Hz for 30 s) before injecting the succinylcholine, to ensure adequate muscle blood flow. The ramp and hold muscle stretches were applied by the servo mechanism rotating the ankle in order to stretch the muscle by 5 mm in 1 s, hold for 1.5 s and return in 1 s. The stretches were repeated continuously every 6 s during the succinylcholine test. Ventilation was maintained artificially during the short period following succinylcholine administration, during which respiratory muscles were paralysed.
At the end of the experiments animals were killed by means of an i.v. overdose of pentobarbitone.
Results
Identification of γ-motor type
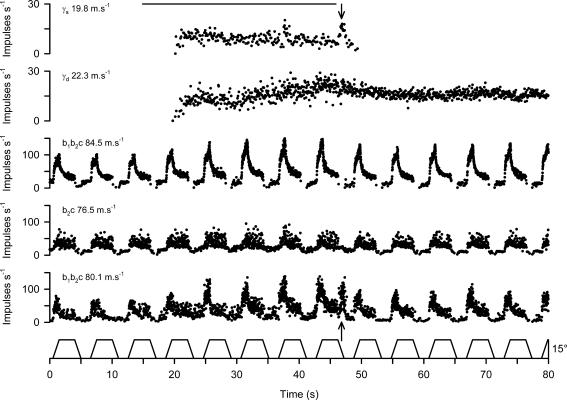
Gamma motor axons were characterized as static or dynamic as illustrated in Fig. 1, which shows the effect of stimulating in the region 1–2 mm dorsal to the cuneiform nucleus on two γ-motor axons, and three muscle spindle primary afferents recorded simultaneously during continuous ramp and hold stretches. During the stimulation period, both efferents commenced firing, with the upper γ axon continuing to do so throughout with a slightly falling frequency, while the lower γ axon showed a progressive increase in its firing rate. The upper one ceased shortly after the end of the stimulation, but the other maintained its tonic firing. In two of the spindle afferents (upper and lower of the three), subsequent testing with succinylcholine showed strong increases in dynamic sensitivity characteristic of contacts of the Ia afferent on a bag1 fibre (b1b2c type afferent), and this indicates that they have the potential to respond to γd excitation. Both showed a progressive increase in dynamic sensitivity to successive ramp stretches throughout the period of stimulation. After the end of stimulation both afferents show an increased dynamic sensitivity to ramp stretches with respect to the prestimulation period. The middle spindle afferent was diagnosed with succinylcholine to be of b2c type, and this, rather than the removal of γd input through cutting some of the motor supply, explains why it could not show a dynamic effect (Taylor et al. 1992a,b). The enhanced dynamic sensitivity of the upper and lower afferents continuing throughout the period of firing of the lower γ axon strongly suggests that this γ axon is one of a population of the dynamic type. During the period of stimulation there is clear evidence of increased static activity acting on the lowermost afferent, as seen by the increase in background firing rate, the maintained firing during the muscle shortening period and the increased variability. A similar, but smaller effect is seen on the middle afferent. When stimulation ceased, both afferents showed a removal of static activity. The paralleling of the static behaviour of these afferents with that of the upper γ axon makes it seem very likely therefore that the upper γ axon is one of a population of static type. The above observations are supported by the analysis presented in Table 1. The measures presented there are as defined previously (Taylor et al. 1992a,b) as follows. Initial frequency (IF) is the frequency in the 0.5 s before the onset of each stretch and is increased mainly by static effects. Peak frequency (PF) is very dependent on dynamic activity, but is also affected by static firing. Static index (SI), the firing frequency 0.5 s after the maximum stretch is reached is affected by both static and dynamic activity. Dynamic difference (DD) is PF – IF and has proved to be a very good index of bag1 fibre contraction. Dynamic index (DI) is PF – SI and is the classical index of dynamic activity (Crowe & Matthews, 1964). These measures were derived from averages of three ramp stretch responses before, during and after the stimulation period. The most notable features of the tabulated data are the increased and maintained values of DD and DI in the upper and lower afferents during the stimulation and poststimulation periods, and the increase in IF in the lower and middle units lasting only during the stimulation period.
Figure 1. Identification of static and dynamic γ axons in TA.
The records from above downward are the stimulus signal, instantaneous frequency of two γ axons, and three muscle spindle afferents and the TA stretch waveform (ankle angle). The stimulus was a train of pulses (0.2 ms, 10 Hz, 2.0 V) delivered dorsal to the cuneiform nucleus on the right hand side. The conduction velocities of all the axons are shown inset on the left. The spindle primary afferents are designated as b1b2c or b2c as found by succinylcholine testing. The arrows indicate the burst of firing in the lowermost spindle coinciding with a burst in the upper γ axon.
Table 1.
Measures of ramp and hold stretch parameters during control (C), brainstem stimulation (S) and poststimulation (P) periods for the three afferents shown in Fig. 1
| IF | PF | SI | DD | DI | ||
|---|---|---|---|---|---|---|
| b1b2c (84.5) | C | 18.6 | 90 | 43.0 | 71.4 | 47.0 |
| S | 16.7 | 132.2 | 61.8 | 115.5 | 70.4 | |
| P | 12.9 | 115.4 | 48.2 | 102.9 | 67.2 | |
| b2c (76.5) | C | 13.0 | 42.8 | 34.7 | 29.8 | 8.1 |
| S | 20.6 | 51.3 | 43.0 | 30.7 | 8.3 | |
| P | 11.0 | 43.4 | 34.7 | 32.4 | 8.7 | |
| b1b2c (80.1) | C | 7.8 | 53.7 | 25.4 | 45.9 | 28.3 |
| S | 24.4 | 95.3 | 50.5 | 70.9 | 44.8 | |
| P | 7.2 | 71.4 | 26.6 | 64.2 | 44.8 |
Left hand column shows unit type (conduction velocity in m s−1). See text for further details.
An additional feature of note was the pronounced burst of firing in the lowermost spindle record during release of stretch at the time marked 47 s. This is characteristic of a burst of static activity and occurred at the same time as a distinct burst in the upper γ record. It is also noteworthy that although the middle and lower afferents can both show static effects, the effect of the γs burst at 47 s is not seen in the middle one. It is known that γs effects vary according to the relative influence on bag2 and chain intrafusal fibres. Whereas chain fibres are very effective in passing on rapid modulations of γs frequency to afferents, bag2 fibres are not (Durbaba et al. 2001,2003). The other, smaller, spontaneous burst in the upper γ axon does not produce a detectable effect on the afferents because it occurs during the muscle stretch, when dynamic effects dominate. It may be questioned why the upper afferent shows no sign of the static effect. Possibly this is a consequence of the cutting of a part of the motor supply to the muscle, which has by chance removed the static γ axons supplying that spindle. In the analysis of such records it is the positive responses that are important. Failure of a particular spindle afferent to show an effect is of no particular significance.
Using the above method 23 γ-motor fibres were identified as static and six as dynamic. Mean conduction velocities were γs 20.1 m s−1 (s.d. 5.39) and γd 20.6 ms−1 (s.d. 7.15). The difference was not significant (t = 0.21, P = 0.83). The remaining 18 units could not be identified with certainty, usually because a clear static or dynamic effect could not be produced by any form of stimulation.
Patterns of γ-static firing
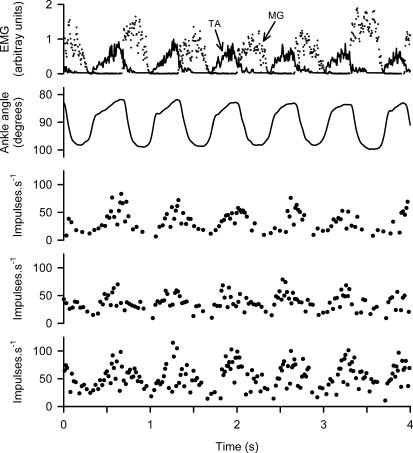
Figure 2 shows data from an experiment in which three single γs axons were discriminated from one muscle nerve filament and regular spontaneous locomotor movements occurred. The upper trace shows alternating EMG activity in the TA (continuous) and MG (broken). The ankle angle record below shows that TA muscle shortening (upward dorsiflexion, reduction of the internal ankle angle) occurs in three phases. The first, slow part occurs passively as the MG activity declines. This is followed by a rapid phase in the early part of TA contraction. Finally, there is a continuing slower shortening caused by the greater part of TA contraction. The single phase of TA lengthening corresponds to the MG contraction. The three lower traces are the instantaneous frequency plots from the three γs axons. They all show continuously modulated discharge with frequency rising to 75–100 impulses s−1 during TA shortening, and falling to 10–20 impulses s−1 during lengthening.
Figure 2. Firing patterns of γs axons during spontaneous locomotion.
Traces from above downward are: EMG of TA (continuous) and MG (broken), ankle angle with TA shortening upwards, instantaneous frequency records of three single γs axons in a TA nerve filament. Note that all three γs axons are modulated in the same way with increasing discharge during TA muscle shortening.
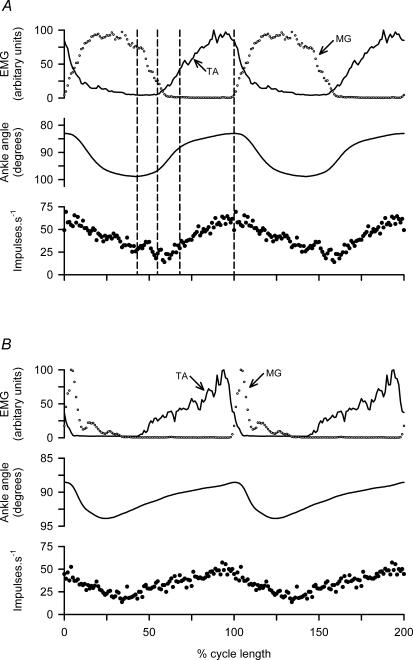
The relationships are more clearly displayed in Fig. 3A in which cycle averages have been constructed from 20 cycles of the data from Fig. 2. The cycles have been aligned on the instant of TA minimum length, and the cycle lengths normalized (mean duration 640 ms). For clarity, two cycles of the resulting averages have been plotted (cf. Shefchyk & Jordan, 1985), and vertical dashed lines drawn to show (a) the onset of the slow phase of TA shortening coinciding with the beginning of reduction in MG EMG; (b) the onset of the rapid phase of TA shortening at the onset of TA EMG; (c) the end of the rapid shortening; and (d) the peak of shortening. The lowermost record is now the ensemble averaged frequency of the three single γs axons. There is a close parallel between the γs frequency and the TA shortening record. The rising phase of the γs frequency also closely resembles the TA EMG trace, but the falling phase is slower and more prolonged. The generality of these findings is confirmed by similar records from another experiment illustrated in Fig. 3B, in which another three single γs axons were isolated. In this experiment the walking pattern was somewhat different, the cycles being longer (mean 800 ms) with the flexion phase relatively prolonged and the active extension shorter than in Fig 3A. It is notable that, despite these differences, the relationships of the mean γs firing frequency to the TA shortening record and to the EMG are essentially the same as described for Fig. 3A.
Figure 3. Ensemble cycle averages of γs firing.
A, data analysed from the three simultaneously recorded γs units of Fig. 2 and B, from three γs units in another experiment, also with spontaneous locomotion. In each panel the uppermost traces show averaged EMG from TA (continuous line) and MG (○), the middle trace shows ankle angle corresponding to TA shortening upward, and the lowest trace averaged instantaneous frequency of the three units. The cycles (20) were aligned for averaging on the TA minimum length point and normalized in time; 1.5 cycles of the average are plotted for clarity and the points are 100 cycle−1. Mean cycle times were A: 640 ms and B: 800 ms. The broken lines in panel A indicate the three phases of TA muscle shortening.
Relationship of γs patterns to spindle difference records
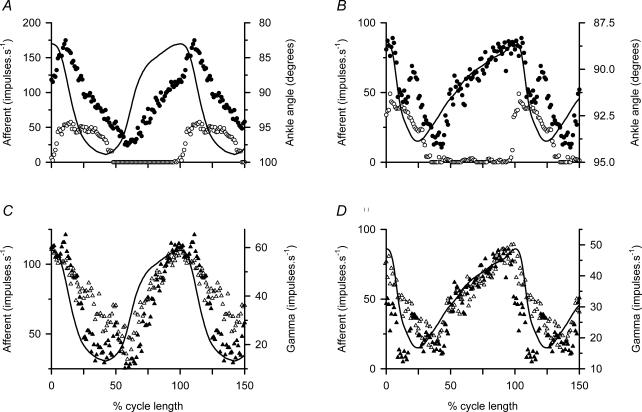
Previous experiments have indicated that, in muscle spindle secondary afferents, firing frequency is largely determined by the linear sum of γs discharge and muscle length. By this we mean that if a suitably scaled pattern of γs discharge is added to the scaled muscle length record then the sum predicts the firing pattern in response to the combination of the length change and the γs discharge. In primary afferents there is an additional component due to dynamic sensitivity to muscle lengthening (Taylor et al. 2000a,b). The present experiments provided the opportunity to check this finding further. Figure 4A and C shows data taken from the experiment of Fig. 3A, and Fig. 4B and D shows data from that of Fig. 3B. In Fig. 4A, the ensemble average of the firing of two b1b2c primary afferents is shown for active movements (•), and for the same movements imposed passively (○). The continuous line shows ankle rotation with TA shortening upwards. In Fig. 4C the difference between the active and passive responses is shown (▴) superimposed on the ensemble average of the three γs units recorded simultaneously during the active movements (▵). These records and the TA muscle shortening record have been scaled to reveal the close similarities between them. The data in Fig. 4B and D are displayed in the same way, the afferent records being the ensemble averages from three b1b2c primary afferents and the γs record being the ensemble average from three γs units. Both experiments confirm the close resemblance of the spindle difference records to the γs discharge patterns, which in turn essentially match the TA muscle shortening. It is noteworthy that these data from TA do not show the phase advance of the γs pattern relative to muscle shortening which was characteristic of MG records (Taylor et al. 2000b).
Figure 4. Comparison of spindle difference signal with γs firing.
A and C, cycle-averaged data from the experiment illustrated in Fig. 3A; B and D similarly from the experiment of Fig. 3B. All panels show ankle angle as a continous line with TA shortening upward. In A and B• indicates afferent frequency during active locomotor movements. ○, the equivalent during the passively repeated movements. C and D, the corresponding afferent difference signal (active response – passive response) as ▴ and the averaged γs firing as ▵. The afferents in A and C were ensemble averages of two b1b2c primaries, and in B and D they were ensemble averages of three b1b2c primaries. In each case the γs records were ensembles of three units.
The afferent records of Fig. 4 are all from b1b2c primary spindle endings, because it happened that no suitable secondary afferent recordings were available. As a consequence, a complication arises in the interpretation because of the dynamic sensitvity to lengthening present in primary endings, but not in secondaries. This is revealed by the distinct peak in the active spindle discharge seen in Fig. 4A and B at the onset of muscle lengthening. This is only partially cancelled by subtraction of the passive records so that the difference records of Fig. 4C and D shows deviations from the γs ensemble records at this point in the cycle. Evidently the spindle dynamic sensitivity is a little higher in the active than in the passive conditions, as would be expected if there were some γd activity during the active movements.
Fluctuation in the γs output
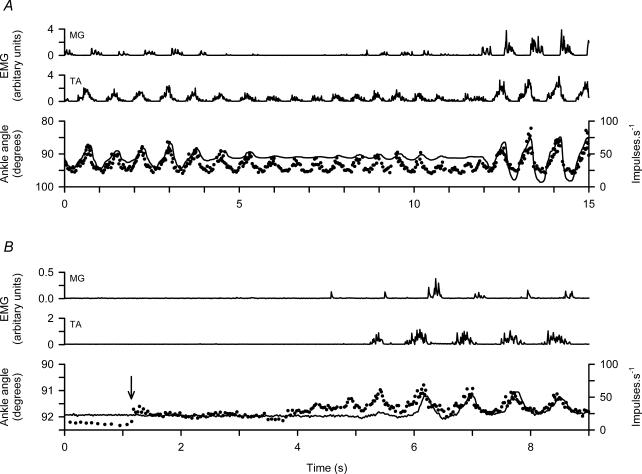
It sometimes happens that the amplitude of the walking movements varies spontaneously, as shown in Fig. 5A. In the period from 4 to 12 s the movements are much reduced. EMG activity in the MG is more reduced than in the TA, so that the ankle position becomes biased towards dorsiflexion. At the same time the amplitude of the modulation of the γs frequency is reduced, roughly in parallel with the movement and TA EMG amplitude. Despite the fact that the modulation depth of the γs frequency varies from 20 to 60 impulses s−1, the lowest frequency in each cycle is constant at about 25 impulses s−1.
Figure 5. Variation in γs firing during spontaneously varying locomotion.
A and B, data from two different experiments. Each shows, from above downwards, MG and TA EMG, ankle rotation with TA shortening upward (continuous line) and instantaneous firing frequency of a TA γs unit. In the bottom panel the arrow indicates the starting of the treadmill.
The γs firing pattern commonly differs from that of the α-motor activity as indicated by the EMG. Thus, in Fig. 5B the onset of a period of walking (initiated by starting the treadmill as indicated by arrow) starts with the γs unit frequency rising from 11 to 24 impulses s−1, and then after 3 s starts to show some signs of modulation before there is any detectable activity in the TA EMG. This indicates some degree of independence of the α- and γs-motor systems. Once the locomotor rhythm is established, the γs frequency, the shortening and the EMG records fall into line.
Patterns of γ-dynamic firing
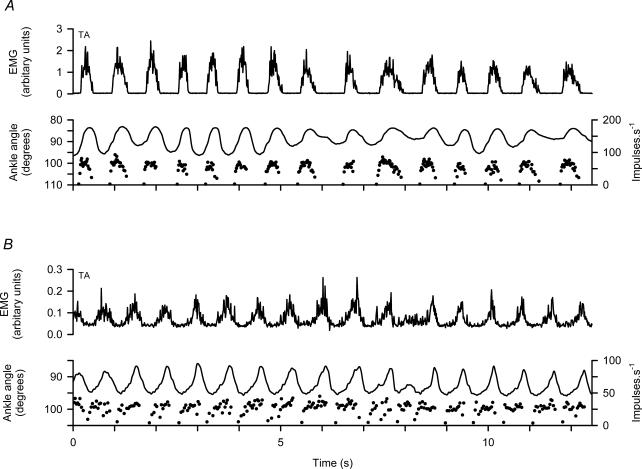
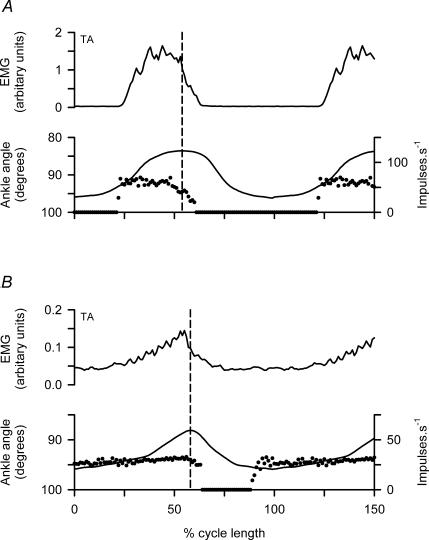
The six γd axons recorded all showed a modulated pattern of discharge with frequency elevated during EMG activity and muscle shortening, but there were some distinct differences from the γs pattern. Figure 6 illustrates this with two unit recordings in panels A and B, respectively. The most obvious difference is that γd axons fire during muscle contraction but fall silent in the intervening periods, whereas the γs axons fire throughout the cycle with the modulation superimposed on a minimum frequency. Another point is that the frequency of γd axons in each cycle rises very rapidly at the onset and then varies relatively little, despite clear changes in EMG and movement amplitude, until the occurrence of the less rapid offset. The timing of the γd discharge is more clearly shown in Fig. 7A and B for cycle averages of the two units of Fig. 6A and B, respectively. The sudden onset of firing occurs distinctly before the start of EMG activity and the muscle shortening, and the end of the firing occurs shortly after onset of muscle lengthening (broken vertical lines). The relatively flat top of the γd discharge frequency is also clearly shown. These features are essentially the same as previously reported for γd axons supplying the MG (Taylor et al. 2000b).
Figure 6. Patterns of firing of γd axons during spontaneous locomotion.
A and B, data from two different experiments. Each shows, from above downwards, TA EMG, ankle rotation and instantaneous frequency of a TA γd axon. Note in each cycle the rapid onset of firing from a silent background and the relative constancy during muscle shortening.
Figure 7. Cycle averages of TA γd related to EMG and active muscle shortening.
A and B, cycle averages from the data of Fig. 7A and B using 9 cycles and 12 cycles, respectively. Muscle length is indicated by internal ankle angle with shortening upwards. Cycles were aligned with the moment of maximal muscle length and were normalized to show 1.5 cycles (mean durations 740 and 735 ms). The broken lines indicate the start of muscle lengthening. Note the sudden onset of firing from a silent background, the relatively constant maintained frequency and the cessation of firing shortly after the start of lengthening.
Discussion
Whereas extensor muscles have to be controlled during locomotion to provide support and thrust, the flexor muscles such as TA have the relatively simple task of raising and swinging the leg forward, with no particular requirement to deal with changing loads. Indeed, it is generally held that adjustment of the speed of gait depends on variation of the extensor rather than the flexor phase (Engberg & Lundberg, 1969). It follows that some differences might be expected to exist in the control of the two muscle groups and, in particular, some differences in the patterns of fusimotor control of their muscle spindles. The present experiments have now provided direct recordings of TA γ-motor axons, coupled with simultaneous spindle afferent recordings, to contrast with previous recordings from the MG (Taylor et al. 2000b).
An important consideration is the validity of the method of identification of each γ-motor axon as static or dynamic. The ideal method of identification would be to stimulate the axon and observe its action on a muscle spindle afferent response to stretch. However, this would require recording from and stimulating the same single axon in continuity. This is achievable with difficulty in ventral rootlets (Noth, 1983), but probably not in the conditions of the present experiments. Another method (Murphy et al. 1984) was based on the observation that in MG γ axons, one of two patterns of locomotor activity was associated with a high impulse rate at rest and the other with a low resting rate. In separate experiments it was then established that γ axons with high resting rates were dynamic and the others static in their effects on spindle primary afferents. In our previous γ recordings in extensor muscles (Taylor et al. 2000b), we could not distinguish two groups according to resting frequency and there is no reason to suppose that it would be possible to do so in flexor muscles, as their γ axons are often silent in the absence of locomotion (see Fig. 1). It is for these reasons that we have developed the method of brainstem stimulation to evoke differential effects on static and dynamic γ axon firing (Taylor et al. 1992c). The particular effects are diagnosed by observing the effects on ramp stretch responses of a set of spindle afferents and correlating them with the firing of the simultaneously recorded γ axons. An important assumption is that the members of a population of γ axons of a particular type behave similarly. Although we have no direct evidence to this effect, there is certainly no evidence against it, and the consistency of the effects observed on a number of afferents recorded simultaneously is as expected on this basis. Moreover, it is difficult to explain the regular observation of particular patterns of γ motoneurone firing associated with the identification as static or dynamic if the method were not reliable.
The main findings for the two muscles are similar. The γs firing is strongly and smoothly modulated in parallel with muscle length changes, increasing with muscle shortening and with the minimum frequency not falling below 20 impulses s−1. The amplitude of the modulation varies in proportion to the amplitude of the movements. In both muscles, the strength of the static modulation is usually enough in these experiments to lead to a net rise in spindle afferent firing frequency during muscle shortening. Only when shortening is particularly fast, do the afferents pause. This is in line with the observations of Prochazka et al. (1979) who showed that when muscles shorten at rates greater than 0.2 resting lengths s−1, intrafusal contraction is not fast enough to prevent afferent silencing. We have previously shown for MG that when spindle recordings are made during natural movements, the recorded movements can be reproduced passively after supressing fusimotor activity, and the response to passive movements recorded. The difference of the active record minus the passive (referred to as the difference signal) represents the effect of fusimotor activity, principally static. The validity of this approach has now been verified for TA as well as for MG (Taylor et al. 2000b), by showing the very close similarity of the difference signal to the γs firing profile. As indicated above, the difference signal may fail to predict the γs firing when the rate of shortening is too great. Also, during rapid active lengthening, the dynamic sensitivity of primary afferents (especially in the presence of γd firing) leads to a peak in the difference signal which departs from the γs profile. However, these effects do not cause any problem in interpretation, and have been discussed in some detail previously (Ellaway et al. 2002; Taylor, 2002).
A possibility that has to be considered is that during active contractions stretching of the series elastic component would cause greater shortening of the spindles than indicated by the total muscle plus tendon length, as set out by Hoffer et al. (1989). Thus the passive playback of the muscle length may not give an accurate reflection of the muscle spindle length changes that occurred in the active state. In turn, the afferent discharge during passive playback might be higher than expected and hence the difference signal may underestimate the fusimotor output. While this is a valid concern in general, we believe that it is not an important consideration in the present experiments because the contractions are essentially isotonic. We are encouraged in this view by the consistency of the relation of the difference signal to the simultaneously recorded static gamma ensembles and to the muscle shortening record.
The γd patterns also appear to be similar in the two muscles. The units are silent during most of the lengthening phase and start to fire abruptly with the onset of shortening. The frequency rapidly rises to a maximum, stays relatively constant then falls to zero shortly after the onset of lengthening. This is a pattern which we have previously referred to as interrupted, as distinct from the smoothly modulated pattern of γs units (Taylor et al. 2000b). It is worth noting that while the γs firing pattern could be predicted quite accurately from the spindle difference signal, the γd pattern could not. The observed dynamic behaviour of primary afferents during locomotion could have been explained by the existence of tonic γd firing, when in fact direct recording shows that the firing is interrupted. This interrupted pattern has been shown to be well-suited to preparing the primary afferents to respond strongly with a burst of firing at the onset of lengthening (Taylor et al. 2000b).
Though the general patterns of γ-motor activity are similar in TA and MG, some clear differences have emerged. Whereas the modulaton pattern for the MG is phase-advanced with respect to the shortening record, the TA pattern is not. This was first noticed in the spindle afferent studies (Taylor et al. 2000a), but is now confirmed by direct recording. The functional implications of this difference are uncertain, but it may be that the requirement for the MG to bear load means that it is an advantage to start the intrafusal shortening ahead of the extrafusal. Another important difference is that in the MG, two types of γs firing pattern were observed (Taylor et al. 2000b). Type 1 was just as described above with strong, smooth modulation in parallel with the muscle shortening. The other, type 2 was less strongly modulated and had a quite different relation to shortening. The present study of γs recordings in the TA has found only type 1 behaviour. The reason for this difference is not known. It was suggested that type 2 axons might be directed preferentially to bag2 intrafusal fibres, but the absence of this pattern in the TA would be difficult to rationalize on this basis. It may be connected rather with the fact that the MG acts across two joints and in normal locomotion, with movement at both the knee and ankle, a greater complexity of control is required. This question might be resolved by making similar recordings from γ-motor axons supplying the soleus, an extensor acting across one joint only.
The present experiments confirm the deductions of Perret & Buser (1972) and of Cabelguen (1981), but additionally show details of static and dynamic motor activity, which could not be determined from indirect observations. The only other data available on direct recordings of TA γ-motor axons were obtained by Murphy & Hammond (1993). Two patterns of firing were observed and termed phasic and tonic, and these were thought to be static and dynamic, respectively, on the basis of comparisons with the spindle afferent recordings of Cabelguen (1981) and of Perret & Berthoz (1973). In fact, the two patterns recorded by Murphy & Hammond (1993) strongly resemble those in the present data, but are identified by a more direct and independent method as ‘tonic’ being γs and ‘phasic’ being γd. It should be recalled that the identification in the present experiments is backed up by the clear resemblance of the γs pattern to the active minus passive spindle difference signal. Clearly, the method used to distinguish static and dynamic γ-motor axons is of prime importance in the interpretation of the γ-motor recordings. The other direct γ-motor recordings of interest are from the flexor hallucis longus and flexor digitorum longus (Murphy, 2002). Again the ‘tonic’ and ‘phasic’ patterns were observed, but no reference was made to their identification as static or dynamic. It has to be emphasized that, despite the anatomically based naming of these muscles as flexors, they actually contribute to the support and thrust of the limb, and hence must be regarded as physiological extensors. Indeed, their activity is essentially coincident with that of the soleus and MG (O'Donovan et al. 1982).
All the direct observations that have been made of γ-motor activity, including the present ones, involve considerable distortion of the normal physiology, for example by decerebration and denervation and fixation of the limb. In the future there may be developments that allow recordings in more normal states while still retaining the ability to analyse the behaviour of several isolated spindle afferents (see Durbaba et al. 2005). However, there is reasonable agreement between a number of different studies on extensor muscles, and we have now established that the patterns of γ-motor activity in locomotion are very similar in ankle flexors and extensors. Static units show a marked modulation superimposed on a tonic level. Their firing frequency fluctuates in parallel with the muscle shortening record and with some phase advance in extensors, but not in flexors. Dynamic units fire with an interrupted pattern, their frequency rising rapidly from zero to a plateau during muscle contraction and falling to zero again during relaxation. The basic similarity of fusimotor patterns in flexors and extensors, despite their tasks being substantially different, implies that certain general principles govern the use of the fusimotor system. The proposal that we have made previously, is that the static fusimotor pattern seems to represent a ‘temporal template’ of the intended pattern of active muscle shortening. (By intended in this context we mean the pattern that has been determined by processes of adaptation and is currently incorporated in some centrally generated motor sequence.) The potential value of such a strategy is that the muscle spindles may normally contribute little to the generation of contraction force, but if shortening is unexpectedly opposed then the rising γs firing will cause substantial increase in spindle afferent activity, which can reflexly compensate for the loading. The extra spindle firing could also be used in central adaptive mechanisms to modify subsequent motor patterns. If the additional spindle activity were sufficient it might alert the animal to the unexpected obstruction and cause a change in the strategy of the movement. Similar conclusions were derived from studies of rhythmic jaw movements in the lightly anaesthetized cat (Taylor et al. 1997). The significance of the interrupted pattern of dynamic fusimotor firing in rhythmic movements has been suggested to be that it primes the primary spindle afferents to provide a very distinct signal to the central pattern generator indicating the onset of muscle lengthening. In this way the rhythm of the pattern generator would be harmonized with the natural frequency of oscillation of the limb and thereby enhance the economy of the movement.
Acknowledgments
This research was supported by a grant from the Wellcome Trust. Our thanks are due to Dr Abdul Sami for skilled assistance.
References
- Brown MC, Crowe A, Matthews PBC. Observations on the fusimotor fibres of the tibialis posterior muscle of the cat. J Physiol. 1965;177:140–159. doi: 10.1113/jphysiol.1965.sp007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelguen J-M. Static and dynamic fusimotor controls in various hindlimb muscles during locomotion activity in the decorticate cat. Brain Res. 1981;213:83–97. doi: 10.1016/0006-8993(81)91249-x. [DOI] [PubMed] [Google Scholar]
- Crowe A, Matthews PBC. The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. J Physiol. 1964;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbaba R, Taylor A, Ellaway PH, Rawlinson S. Modulation of primary afferent discharge by dynamic and static gamma motor axons in cat muscle spindles in relation to the intrafusal fibre types activated. J Physiol. 2001;532:563–574. doi: 10.1111/j.1469-7793.2001.0563f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbaba R, Taylor A, Ellaway PH, Rawlinson S. The influence of bag2 and chain intrafusal muscle fibers on secondary spindle afferents in the cat. J Physiol. 2003;550:263–278. doi: 10.1113/jphysiol.2002.031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbaba R, Taylor A, Ellaway PH, Rawlinson S. Comparison of static fusimotor patterns in the fixed and unfixed hindlimb of the locomoting decerebrate cat. J Physiol. 2005;567P:C108. [Google Scholar]
- Ellaway PH, Taylor A, Durbaba R, Rawlinson S. Role of the fusimotor system in locomotion. Adv Exp Med Biol. 2002;508:335–342. doi: 10.1007/978-1-4615-0713-0_39. [DOI] [PubMed] [Google Scholar]
- Engberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969;75:614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973;141:1–42. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: Central mechanisms and reflex interaction. Physiol Rev. 1975;55:247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Caputi AA, Pose IE, Griffiths RI. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res. 1989;80:75–85. doi: 10.1016/s0079-6123(08)62201-3. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Dürmüller N, Prochazka A, Trend P. Flexible fusimotor control of muscle spindle feedback during a variety of natural movements. Prog Brain Res. 1989;80:87–101. doi: 10.1016/s0079-6123(08)62202-5. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Duysens J. Activity patterns in individual hindlimb primary and secondary afferents during normal movements in unrestrained cats. J Neurophysiol. 1979;42:420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Murphy PR. Tonic and phasic discharge patterns in toe flexor gamma motoneurons during locomotion in the decerebrate cat. J Neurophysiol. 2002;87:286–294. doi: 10.1152/jn.00917.2000. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Hammond GR. The locomotor discharge characteristics of ankle flexor γ-motoneurones in the decerebrate cat. J Physiol. 1993;462:59–70. doi: 10.1113/jphysiol.1993.sp019543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Stein RB, Taylor J. Phasic and tonic modulation of impulse rates in γ-motoneurones during locomotion in premammillary cats. J Neurophysiol. 1984;52:228–243. doi: 10.1152/jn.1984.52.2.228. [DOI] [PubMed] [Google Scholar]
- Noth J. Autogenetic inhibition of extensor γ-motoneurones revealed by electrical stimulation of group I fibres in the cat. J Physiol. 1983;342:51–65. doi: 10.1113/jphysiol.1983.sp014839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan MJ, Pinter MJ, Dum RP, Burke RE. Actions of FDL and FHL muscles in intact cats: functional dissociation between anatomical synergists. J Neurophysiol. 1982;47:1126–1143. doi: 10.1152/jn.1982.47.6.1126. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagna TG, Grillner S. Neuronal Control of Locomotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- Pearson KG, Misiaszek JE, Hulliger M. Chemical ablation of sensory afferents in the walking system of the cat abolishes the capacity for functional recovery after peripheral nerve lesions. Exp Brain Res. 2003;150:50–60. doi: 10.1007/s00221-003-1445-1. [DOI] [PubMed] [Google Scholar]
- Perret C, Berthoz A. Evidence of static and dynamic fusimotor actions on the spindle response to sinusoidal stretch during locomotor activities in the cat. Exp Brain Res. 1973;18:178–188. doi: 10.1007/BF00234722. [DOI] [PubMed] [Google Scholar]
- Perret C, Buser P. Static and dynamic fusimotor activity during locomotor movements in the cat. Brain Res. 1972;40:165–169. doi: 10.1016/0006-8993(72)90123-0. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Stephens JA, Wand P. Muscle spindle discharge in normal and obstructed movements. J Physiol. 1979;287:57–66. doi: 10.1113/jphysiol.1979.sp012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Westerman RA, Ziccone SP. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976;39:1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Severin FV, Orlovskii GN, Shik ML. Work of the muscle spindle receptors during controlled locomotion. Biofizika. 1967;12:502–511. [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in α-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electrical stimulation of the mid-brain. Biofizika. 1966;11:756–765. [PubMed] [Google Scholar]
- Taylor A. Give proprioceptors a chance. Adv Exp Med Biol. 2002;508:327–334. doi: 10.1007/978-1-4615-0713-0_38. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH. Direct and indirect assessment of α-motor firing patterns. Can J Physiol Pharmacol. 2004a;82:793–802. doi: 10.1139/y04-053. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, Rawlinson S. Patterns of fusimotor activity during locomotion in the decerebrate cat deduced from recordings from hindlimb muscle spindles. J Physiol. 2000a;522:515–532. doi: 10.1111/j.1469-7793.2000.t01-3-00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, Rawlinson S. Flexor α-motor patterns during locomotion. J Physiol. 2004b;555P:C168. [Google Scholar]
- Taylor A, Durbaba R, Rodgers JF. The classification of afferents from muscle spindles of the jaw-closing muscles of the cat. J Physiol. 1992a;456:609–628. doi: 10.1113/jphysiol.1992.sp019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Ellaway PH, Durbaba R, Rawlinson S. Distinctive patterns of static and dynamic gamma motor activity during locomotion in the decerebrate cat. J Physiol. 2000b;529:825–836. doi: 10.1111/j.1469-7793.2000.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Hidaka O, Durbaba R, Ellaway PH. Fusimotor influences on jaw muscle spindle activity during swallowing-related movements in the cat. J Physiol. 1997;503:157–168. doi: 10.1111/j.1469-7793.1997.157bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Rodgers JF, Durbaba R, Fowle AJ. The classification of hindlimb muscle spindle afferents in the cat by the influence of the intrafusal fibres. J Physiol. 1992b;456:629–644. doi: 10.1113/jphysiol.1992.sp019357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Rodgers JF, Fowle AJ, Durbaba R. Interpretation of spindle afferent recordings according to intrafusal fibre influence. In: Jami L, Pierrot-Desseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992c. pp. 105–112. [Google Scholar]
- Taylor J, Stein RB, Murphy RB. Impulse rates and sensitivity to stretch of soleus muscle spindle afferent fibers during locomotion in premammillary cats. J Neurophysiol. 1985;53:341–360. doi: 10.1152/jn.1985.53.2.341. [DOI] [PubMed] [Google Scholar]