Abstract
Recent evidence suggests that alterations in ionic conductances in spinal motoneurones, specifically the manifestation of persistent inward currents, may be partly responsible for the appearance of hyperexcitable reflexes following spinal cord injury (SCI). We hypothesized that such alterations would manifest as temporal facilitation of stretch reflexes in human SCI. Controlled, triangular wave, ankle joint rotations applied at variable velocities (30–120 deg s−1) and intervals between stretches (0.25–5.0 s) were performed on 14 SCI subjects with velocity-dependent, hyperexcitable plantarflexors. Repeated stretch elicited significant increases in plantarflexion torques and electromyographic (EMG) activity from the soleus (SOL) and medial gastrocnemius (MG). At higher velocities (≥ 90 deg s−1), reflex torques declined initially, but subsequently increased to levels exceeding the initial response, while mean EMG responses increased throughout the joint perturbations. At lower velocities (≤ 60 deg s−1), both joint torques and EMGs increased gradually. Throughout a range of angular velocities, reflex responses increased significantly only at intervals ≤ 1 s between stretches and following at least four rotations. Ramp-and-hold perturbations used to elicit tonic stretch reflexes revealed significantly prolonged EMG responses following one or two triangular stretches, as compared to single ramp-and-hold excursions. Post hoc analyses revealed reduced reflex facilitation in subjects using baclofen to control spastic behaviours. Evidence of stretch reflex facilitation post-SCI may reflect changes in underlying neuronal properties and provide insight into the mechanisms underlying spastic reflexes.
Following brain or spinal cord injury (SCI), a prominent feature of the ‘upper motor neurone syndrome’ is spasticity (Katz & Rymer, 1989), characterized as velocity-dependent, hyperexcitable stretch reflexes (Lance, 1980). Spasticity is but one component of a spectrum of involuntary motor behaviours broadly characterized as spastic hypertonia (Priebe et al. 1996; Meythaler, 2001), which can include hyperactive multijoint reflexes (spasms), agonist–antagonist co-contraction, and dystonia or abnormal posturing. Although there are changes in passive muscle stiffness following neurological injury (Dietz et al. 1981; Lieber et al. 2004), the pathology and primary mechanisms underlying stretch reflex hyperexcitability (i.e. spasticity) are neural in origin (Sheean, 2002; Kamper et al. 2003). Specific potential mechanisms responsible for spasticity include alterations in motoneuronal excitability (Katz & Rymer, 1989; Heckman et al. 2005) or augmented synaptic inputs with muscle stretch (Dietrichson, 1971a,b; Iles & Roberts, 1986; Koerber et al. 1994; Calancie et al. 2002; Jankowska & Hammar, 2002), although the contributions of each are unknown.
Animal models of SCI have provided substantial insight into mechanisms underlying exaggerated spinal reflexes. For example, following chronic (> 2 weeks), complete spinalization at the S2–S3 spinal cord level in the rat, the animal's tail demonstrates signs of hyperreflexia, including clonus and spasms (Bennett et al. 1999, 2004). Such behaviours occur concomitantly with alterations in intrinsic motoneurone (MN) properties. In particular, persistent inward (Ca2+ and Na+) currents (PICs) are observed in MNs and may be partly responsible for the spastic behaviours (Li & Bennett, 2003). Following blockade of action potentials, PICs are often observed as voltage-gated, sustained periods of depolarization (i.e. plateau potentials) that can amplify and prolong the effects of brief excitatory inputs (Crone et al. 1988; Hounsgaard et al. 1988; Bennett et al. 2001). In addition, PIC activation is time dependent, manifesting as temporal facilitation (i.e. wind-up or warm-up) of neuronal excitability following brief repeated excitation. Such behaviour is characterized by a decrease in the depolarization required to activate PICs (i.e. reduction of PIC threshold) with repeated stimuli, with little increase in PIC magnitude (Svirskis & Hounsgaard, 1997; Bennett et al. 1998). As such, wind-up is observed as a time-dependent reduction in the synaptic input required for previously quiescent MNs to reach firing threshold, thereby resulting in earlier recruitment and a net amplification of the motor output (Bennett et al. 1998).
In mammalian spinal MNs, the presence of PICs is facilitated by descending serotonergic and noradrenergic inputs from brainstem pathways (Conway et al. 1988; Hounsgaard et al. 1988), and may reduce the need for continuous synaptic drive during sustained voluntary contractions (Kiehn & Eken, 1998). In the absence of such inputs, as occurs following acute SCI, PICs are typically observed only following exogenous application of monoamines. Two to four weeks following SCI, however, PICs re-emerge spontaneously (i.e. without descending modulatory inputs) and simultaneously with the onset of hyperreflexia (Bennett et al. 2001).
Consistent with the above results, the presence of PIC-like phenomena has been implicated in the manifestation of involuntary spinal reflexes in humans following SCI. For example, low-threshold, percutaneous muscle stimulation elicits sustained motor output that outlasts the stimulus period (Nickolls et al. 2004), although there is no apparent relationship with spastic motor activity. Motor unit discharge patterns observed during long-lasting spasms reveal low discharge rates with extremely low variability, and are thought to originate from intrinsic MN PICs (Gorassini et al. 2004). In addition, repeated electrical stimuli at the medial arch of subjects with complete SCI elicits temporal facilitation of hyperactive flexion reflexes (or flexor spasms; Hornby et al. 2003; Schmit et al. 2003), the time constant of which is similar to wind-up observed in spinal neurones displaying PICs (Svirskis & Hounsgaard, 1997; Bennett et al. 1998). The combined results suggest that PIC-like activity in spinal circuits can be elicited in subjects with SCI and may contribute to long-lasting spasms. Whether such behaviours contribute to velocity-dependent, hyperexcitable stretch reflexes (i.e. spasticity) following human SCI is unknown.
The following study was undertaken to determine if exaggerated stretch reflexes observed in plantarflexors of individuals with SCI demonstrate properties consistent with PIC-like activity. Specifically, we hypothesized that the reflex motor output to repeated plantarflexor stretch in individuals with spasticity following chronic SCI would demonstrate temporal facilitation. Repeated joint rotation at variable velocities and intervals between perturbations were performed, while joint torques and EMG activity of selected lower extremity muscles were collected. Evidence of stretch reflex facilitation may provide information regarding the mechanisms underlying spasticity following SCI.
Methods
Subjects
Individuals with SCI (>6 months duration) were recruited through the outpatient clinics of the Rehabilitation Institute of Chicago. Subjects with sagittal plane passive ankle range of motion >40 deg were included, with the presence of increased passive and/or reflex responses to manually imposed plantarflexor stretch. Specifically, subjects with an Ashworth score ≥ 1 (Ashworth, 1964), or those individuals with the presence of plantarflexor clonus (i.e. involuntary rhythmic joint oscillation initiated by rapid, maintained stretch; Benz et al. 2005) were eligible. Exclusion criteria included multiple CNS lesions, lower extremity nerve injury, including subjects with a neurological level of injury below the 10th thoracic level, or a history of lower extremity fracture. Informed consent was obtained for each subject, and all procedures were conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Northwestern University.
Fourteen subjects (13 males) classified as motor complete (n = 8) or incomplete SCI were enrolled in this study (see Table 1 for summary). Mean subject age was 36 years and mean duration post injury was 81 months. Six individuals were prescribed the anti-spastic medication baclofen. Thirteen subjects presented with sustained clonus >10 s duration, while the other had an Ashworth score of 2. As a comparison, five subjects (4 males; ages 28–45 years) without neurological injury were tested for reflex responses to repeated plantarflexor stretch.
Table 1.
Subject characteristics for stretch reflex wind-up
| Subj. | Age (years) | Duration of SCI (months) | Neuro- logical level | ASIA classifi- cation | Anti-spasticity medications |
|---|---|---|---|---|---|
| 1 | 40 | 42 | C6 | A | 110 mg baclofen |
| 2 | 35 | 136 | T6 | A | None |
| 3 | 47 | 24 | T10 | A | None |
| 4 | 30 | 48 | C8 | C | 160 mg baclofen |
| 5 | 41 | 324 | C2 | D | 60 mg baclofen |
| 6 | 49 | 48 | C7 | B | 120 mg baclofen |
| 7 | 26 | 46 | T2–4 | C | None |
| 8 | 44 | 36 | C6 | D | None |
| 9 | 25 | 14 | C8 | C | None |
| 10 | 36 | 16 | T4 | A | None |
| 11 | 25 | 70 | C5 | A | 160 mg baclofen |
| 12 | 34 | 65 | T4 | A | None |
| 13 | 32 | 62 | C4–5 | C | None |
| 14 | 39 | 204 | T7 | A | 100 mg baclofen |
Subject characteristics for assessment of stretch reflex wind-up are provided, including age, duration of spinal cord injury (SCI), neurological level and classification, as determined by the American Spinal Injury Association (ASIA; Maynard et al. 1997), and total daily dose of anti-spastic medication (specifically baclofen).
Experimental design
The details of the experimental set-up have been previously described (Hornby et al. 2003; Schmit et al. 2003). Briefly, participants were seated in an adjustable height chair of the testing apparatus (Biodex Rehabilitation Testing System 2; Biodex Medical Systems, Shirley, NY, USA). The foot of the tested extremity was secured to a footplate, which was attached to a 6 degrees of freedom load cell to calculate ankle, knee and hip torques. All force–torque signals were low-pass filtered (200 Hz), and sampled at 1000 Hz.
Surface electromyograms (EMGs) were recorded from the medial gastrocnemius (MG) and soleus (SOL) of the tested extremity. In addition, EMGs from the tibialis anterior, vastus lateralis, rectus femoris and medial hamstrings were recorded to detect multijoint spasms. Active electrodes (model DE2.1, Delsys, Boston, MA, USA) were applied to the skin over the muscle. Signals were amplified (×1000), filtered (20–450 Hz), and sampled at 1000 Hz on the same computer system as that used to acquire the torque data.
With subjects instructed to relax, stretch reflexes were elicited through controlled ankle rotations imposed by the Biodex using constant-velocity (‘ramp’) perturbations. The range of movement was 30 deg during all testing, with the maximum dorsiflexion (DF) angle established at 5 deg from end range (range: 80–105 deg). The knee of each participant was flexed (range 45–85 deg) such that maximal ankle range of motion into DF could be elicited (i.e. with a shortened MG muscle length). Starting position was in the most plantarflexed position and MG or SOL reflex activity was robust in all subjects.
Experimental protocol
Three separate protocols were performed on each subject and tested in the order described below. The first protocol (14 SCI subjects, 5 intact subjects) examined the effects of repeated, ramp perturbations on stretch reflex responses. Six consecutive cycles of DF and plantarflexion (PF) movements at constant angular velocities (30, 60, 90, 120 deg s−1) were performed with no delay between movement directions. Trials at each velocity were randomized and performed 3 times with >2 min between stretches to minimize reflex adaptation. Any trial with detectable EMG activity prior to the first stretch reflex was rejected.
The second protocol (13 subjects) investigated reflex responses to repeated, constant velocity (120 deg s−1) movements into DF followed by movements into PF at variable velocities. Each perturbation sequence consisted of six consecutive DF movements at 120 deg s−1 followed immediately by PF perturbations at 60, 30, 15, 10 or 6 deg s−1 (intervals between stretches were 0.5, 1, 2, 2.86 or 5 s, respectively).
The third protocol (9 subjects) investigated the effects of repeated joint rotation on the magnitude and duration of stretch reflex responses during ‘ramp-and-hold’ perturbations (i.e. tonic stretch reflexes). Ramp-and-hold perturbations consisted of 120 deg s−1 movements into DF followed by a static hold at the end range of perturbation. Such movements were applied singly or following one or two additional triangular wave rotations at 120 deg s−1.
Data collection and analysis
During repeated joint rotation, SOL and MG EMG responses were present primarily during DF perturbations, with less activity evident with PF movements, particularly during the first few rotations. The 60 Hz noise was removed from the EMG signals using a band-stop filter at 55–65 Hz (4th order Butterworth filter applied backward and forward to remove phase delays; filtfilt function in Matlab; Mathworks, Inc., Natick, MA, USA). The signal was rectified and smoothed using a 4th order, 10 Hz low-pass Butterworth filter (also applied forward and backward), and the area of the smoothed signal was calculated for both the DF and PF excursions. For single and repeated ramp-and-hold perturbations, SOL and MG EMG were often present following cessation of movement during the final DF hold position. Integrated EMG area was calculated for 3 s, starting from the beginning of the final joint rotation into DF (0.25 s) extending into the hold phase (2.75 s).
Sagittal plane angles of the hip, knee and ankle, and segment lengths of the thigh, shank, and foot-to-load cell were determined to calculate joint torques using equations previously described (Schmit et al. 2000). All torques were low-pass filtered at 20 Hz using an 8th order Butterworth filter with zero phase delay (filtfilt function). Passive and gravitational torques were obtained from the slow stretch perturbations and subtracted from the rapid stretches to calculate the reflex response. A 4th order polynomial was fitted to the data and the coefficients were used to calculate the passive torques for the fast movement trials, which accounted for gravitational torques and at least partially removed passive elastic resistance (see Discussion). Stretches were segmented for each DF and PF excursion, minimum and maximum torques were identified, and peak torque was defined as the difference in the values. Small inertial artefacts were assumed constant between repeated perturbations at similar velocities. For ramp-and-hold perturbations (protocol 3), the peak torque and integrated area under the torque signal for 2.75 s following the last joint rotation was calculated.
Statistical analysis
For repeated joint rotations, the first six reflex responses (torque and EMG) were analysed for the DF direction. The velocity dependence of the reflex responses was assessed using the peak torque and SOL and MG EMG during the first DF perturbation of each sequence. EMG responses from other muscles and hip and knee torques were typically minimal in most subjects and are not included in data presentation. Peak rectified root mean square (RMS) EMG was used for calculation of velocity-dependent measurements, and is presented in absolute values. Correlation coefficients were determined to identify relationships between angular velocity and reflex responses. Subsequently, a multiple regression analysis was used to determine the relationship of SOL and MG activity to joint torque. For repeated stretch reflex responses (protocols 1 and 2), EMG responses were normalized to the initial (1st) response, and are expressed as a percentage in all repeated perturbation conditions. For ramp-and-hold perturbations (protocol 3), peak torque, integrated torque area, and EMG integrated area during the initiation of the final DF excursion to 3 s into the hold phase were analysed. Joint torque responses are presented in absolute values. Joint torques and EMG responses were compared statistically using repeated measures ANOVAs with significance noted at P < 0.05, with post hoc Tukey-Kramer analyses to determine individual differences.
Results
Responses to repeated stretch reflex elicitation at different velocities
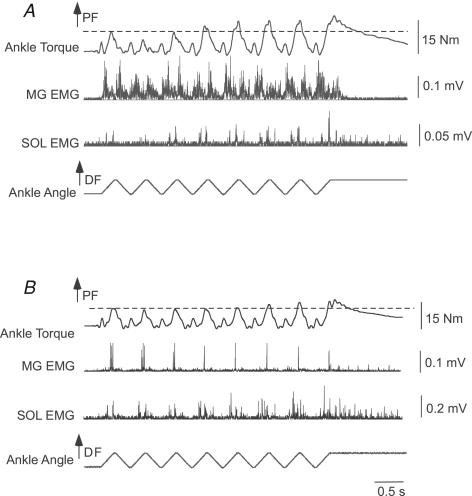
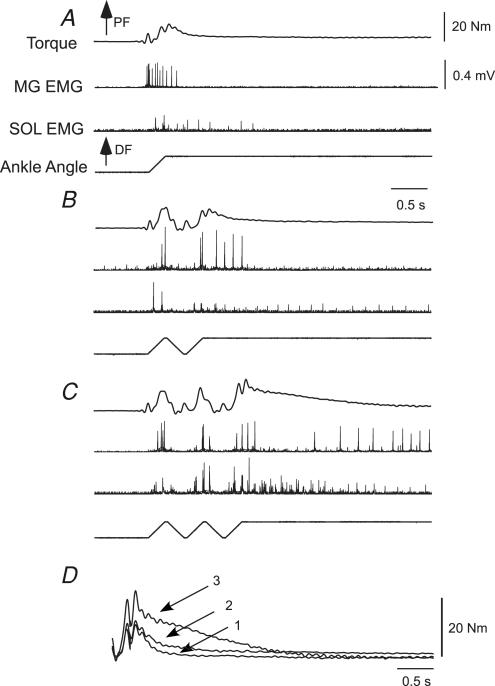
In the first protocol, stretch reflex responses were elicited for at least six consecutive cycles at four different angular velocities (30–120 deg s−1). Figure 1 demonstrates responses to 120 deg s−1 repeated stretch reflex perturbations in two different subjects with motor incomplete (Fig. 1A) and complete (Fig. 1B) SCI. For the first subject, PF torque and SOL and MG activity increased during the first joint rotation (i.e. indicative of a stretch reflex response), although both torque and SOL activity were smaller in response to the second stretch. With repeated perturbations, SOL activity and torque increased to levels exceeding the initial response, while MG remained constant. In the second subject, gradual increases in joint torque and SOL activity were observed, while MG decreased during the trials and did not appear related to joint torque. In both subjects, however, joint torque and SOL (but not MG) activity remained elevated above baseline levels during the final joint perturbation, in which the ankle was held into dorsiflexion (eliciting a prolonged tonic stretch reflex).
Figure 1. Example of reflex facilitation with 120 deg s−1 repeated triangular wave perturbations performed in two subjects.
A, in the first subject (subject 8), an initial depression and gradual increase in joint torque was observed, with soleus (SOL) activity modulated with the torque responses. In contrast, MG was active during both dorsiflexion (DF) and plantarflexion (PF) perturbations, and did not appear to be related to joint torque. B, in the second subject (subject 2), PF torque demonstrated slight increases with repeated stretch, while SOL activity gradually increased. In contrast, MG responses were reduced during the repeated stretch. Changes in MG activity out of proportion with alterations in joint torque may be indicative of the flexed knee posture of subjects during testing.
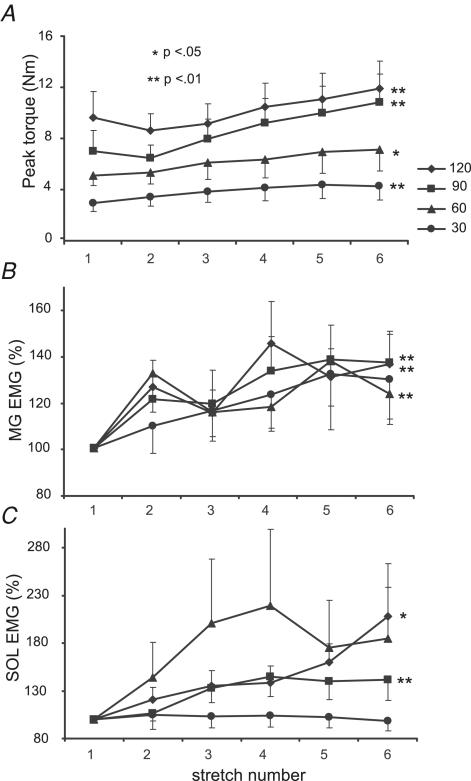
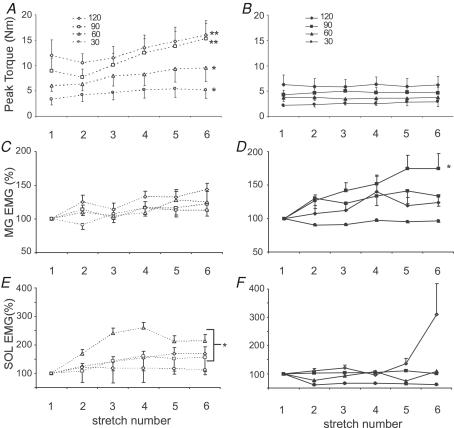
Mean torque responses to repeated PF stretches were similar to the individual data shown in Fig. 1. Following initial responses, averaged joint torques elicited at higher velocities (≥ 90 deg s−1) decreased upon the 2nd stretch, followed by a gradual increase with subsequent rotations (Fig. 2A). A repeated measures ANOVA revealed significant increases in torque responses at both 120 deg s−1 (2nd and 3rd responses versus 6th response) and at 90 deg s−1 (1st and 2nd responses versus 5th and 6th stretches; both P < 0.01). At slower velocities (≤ 60 deg s−1), mean joint torques increased gradually with no initial decline (P < 0.01). Differences were observed between the 1st and 2nd versus 5th and 6th responses.
Figure 2. Mean torque and EMG responses to repeated stretch at 30–120 deg s−1.
A, means and s.e.m. of ankle torques in 14 subjects during 6 repeated stretches at 120, 90, 60 and 30 deg s−1. Significant increases in torque are shown at all velocities (P ≤ 0.01 for 120, 90 and 30 deg s−1 and P < 0.05 at 60 deg s−1). B, normalized medial gastrocnemius (MG) EMG for 13 subjects with significant differences at 120, 90 and 60 deg s−1, P < 0.01. C, normalized SOL EMG in 11 subjects with reliable recordings, with significance shown for 120 deg s−1, P < 0.05, and 90 deg s−1, P < 0.01. Peak torques declined immediately following the initial stretch but demonstrated gradual increases with additional stretches. In contrast, EMG activity gradually increased with repeated stretch.
In contrast to joint torques, SOL and MG activity increased gradually at all velocities (Fig. 2B and C). Significant increases were apparent at ≥ 90 deg s−1 for both muscles, although the differences were greater for MG (P < 0.01 for both velocities) versus SOL (P < 0.05 at 120 deg s−1, P < 0.01 at 90 deg s−1). The depression of joint torque responses for the 2nd stretch reflex at higher velocities with increasing EMG probably indicates adaptive processes at the muscle and joint connective tissue (Nuyens et al. 2002). At slower speeds (≤ 60 deg s−1), only MG EMG activity was significant at 60 deg s−1 (P < 0.01; SOL EMG P = 0.06 at 60 deg s−1). Despite the slight differences in reflex responses demonstrated between the subjects in Fig. 1AversusFig. 1B, there were only small, non-significant differences in the extent of facilitation between motor complete versus incomplete subjects.
As observed in Fig. 1A for MG activity, one possible cause for the observed reflex facilitation may be the progressive depolarization of PF motor pools, such that synaptic summation or supra-threshold increases in MN PICs may contribute to the gradual increase in reflex excitability. While the use of EMG cannot detect gradual, subthreshold depolarization, SOL and MG EMG activity was analysed 100 ms prior to each joint rotation to detect if supra-threshold activation increased between joint rotation. With repeated stretch at all velocities, there was no significant increase in SOL and MG EMG prior to each stretch as compared to the level of EMG activity before the first stretch, when the muscle was quiescent (repeated measures ANOVA). Further, at 120 deg s−1 (i.e. the shortest interval between stretches), four subjects demonstrated elevated MG or SOL activity prior to the second versus first stretch (determined by EMG activity >2 standard deviations above baseline). Following removal of these subjects from the data set, a significant reflex facilitation in EMG activity during the stretch was still observed (P < 0.05). The data indicate that increased reflex responses are unlikely to be due to supra-threshold summation of MN activity, although subthreshold facilitation of PICs or synaptic summation cannot be ruled out.
Velocity dependence of stretch reflex behaviours
The velocity dependence of the stretch reflexes is suggested in Fig. 2A by the increasing joint torques at higher angular velocities. To quantify this association, peak joint torques and peak rectified, smoothed EMGs from the MG and SOL during the initial DF perturbation at all velocities were quantified and normalized to responses elicited at 30 deg s−1 (i.e. typically the smallest reflex response). Calculation of correlation coefficients revealed significant relationships between angular velocity versus torque (r = 0.62, P < 0.0001), SOL activity (r = 0.53, P < 0.001) and MG activity (r = 0.40; P < 0.01). A multiple linear regression used to determine the relative contribution of SOL and MG EMG activity to the joint torque revealed a significant relationship between combined SOL and MG activity versus peak torques at various velocities (r = 0.39, F = 4.31, P = 0.02). However, the standardized coefficients (β) were significant only for normalized SOL activity (β for SOL activity = 0.31, P = 0.03; β for MG activity = 0.15, P = 0.27). The combined data suggest the presence of velocity-dependent modulation of joint torque, SOL EMG and, to a lesser extent, MG activity. The stronger relation of SOL versus MG activity to joint torque is not completely unexpected due to the flexed knee posture of subjects during the testing procedures.
Responses to repeated stretch in control subjects
To evaluate whether the observed phenomena were related to SCI, we evaluated EMG and torque responses to repeated joint rotation in five subjects without neurological injury. The protocol for eliciting reflex responses was identical to that previously described, with subjects relaxed during all testing and no evidence of muscle activity prior to stretch. In all control subjects, there was no EMG activity above baseline levels during repeated joint rotation at all velocities. As expected, joint torque responses were low upon the first joint rotation, with the highest mean response at 1.2 ± 0.5 N m at 120 deg s−1 and remained small with no EMG observed above baseline. The very low peak torque responses are likely to be the result of an uncorrected movement artefact, rather than a reflex response. The results confirm that subjects without neurological injury do not generate reflex responses to imposed repeated stretch of plantarflexors at velocities ≤ 120 deg s−1.
Effects of repeated stretch at variable stretch reflex intervals
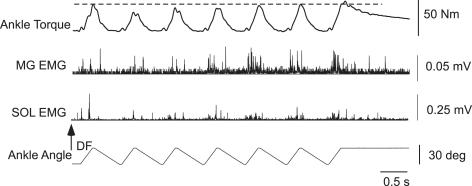
To determine the time course of reflex facilitation, responses to repeated 120 deg s−1 stretches were obtained at variable intervals in 13 subjects. Specifically, reflexes were generated at a constant 120 deg s−1 into DF, with the velocity of joint rotation into plantarflexion at 120, 60, 30, 15, 10.5 and 6 deg s−1 (intervals between stretches were 0.25, 0.5, 1, 2, 2.86 and 5 s, respectively, with the 120 deg s−1 data used from the first protocol). Figure 3 demonstrates an example of reflex responses to repeated stretch at 120 deg s−1 with 0.5 s intervals between DF perturbations. In this particular subject, reflex torque was reduced following the 2nd joint rotation, but rebounded following the 6th perturbation. Such changes were smaller than those observed with shorter intervals between stretches (Fig. 1). In addition, a large increase in EMG activity from the SOL and MG at the 5th versus 1st response with similar torque responses indicates the potential contributions of thixotropic (Hagbarth et al. 1985; Taylor et al. 1990) behaviours of passive mechanical structures to the small changes in joint torque responses.
Figure 3. Single subject responses to repeated 120 deg s−1 stretch at delayed intervals.
Single subject (subject 12) example of stretch reflex facilitation during repeated 120 deg s−1 joint rotations into DF, with 60 deg s−1 rotations into PF (0.5 s delay between stretches). Reduced reflex facilitation is observed during this protocol as compared to shorter duration intervals (Fig. 1).
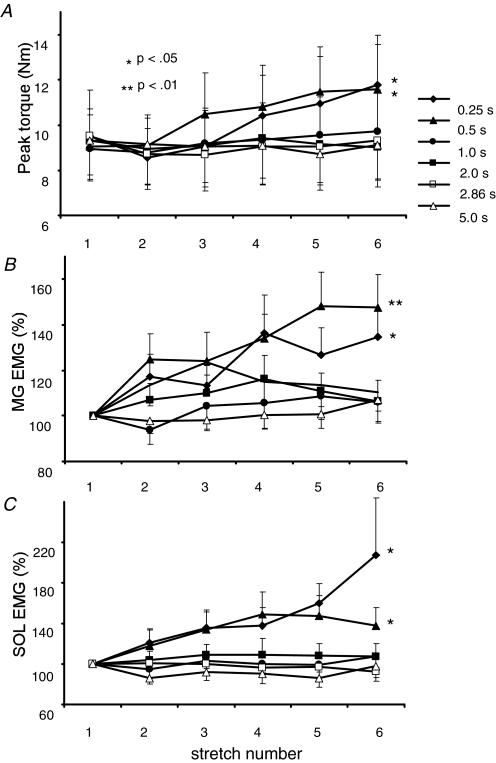
The small increment in net reflex responses with longer intervals was consistent across the subject population. In Fig. 4, averaged torque and EMG responses to repeated stretch reflexes at various intervals are presented. Significant increases in PF torques and MG and SOL EMGs were observed at 0.25 and 0.5 s intervals, while reflex responses with longer intervals between joint rotations did not elicit significant changes. In addition, EMG activity prior to repeated stretches was not elevated. Specifically, stretch intervals of 1 s duration did not elicit increases in reflex responses with DF perturbations at 120 deg s−1 (P >0.50 of all torque and EMG responses), although significant changes in reflex torques were observed during repeated, 30 deg s−1 constant-velocity perturbations (also 1 s stretch intervals; Fig. 2). Such differences may be related to the effects of stretch velocity on passive tissue changes (McNair et al. 2002) or to the differences in the temporal dynamics of the two stretch protocols. The combined data indicate that stretch reflex facilitation occurs only at intervals ≤ 1 s and following at least four consecutive stretches. These data are in contrast to the time course of flexion reflex facilitation in human SCI, in which elevated responses were observed upon the 2nd or 3rd responses at intervals up to 3 s (Hornby et al. 2003).
Figure 4. Mean reflex responses to repeated stretch at variable intervals.
A, means and s.e.m. of ankle torques in 13 subjects with repeated stretch at 120 deg s−1 using varying return velocities. The duration of the return phase into PF range of motion affected the reflex responses. Significance was shown at 0.25 s and 0.5 s intervals only, P < 0.01. MG EMG for 13 subjects with reliable recordings (B) and SOL EMG for 10 subjects with reliable recordings (C) also increased with return time up to 0.5 s only, with P < 0.01 for MG and P < 0.05 for SOL.
Prolonged stretch reflex responses to single versus repeated stretch reflexes
The hallmark of PIC-like behaviour is the presence of long-lasting activity following a brief excitatory stimulus (Hounsgaard et al. 1988). Such activity is at least partly responsible for the velocity-dependent, prolonged motor activity from the triceps surae observed during tonic stretch reflexes in decerebrate feline preparations (Crone et al. 1988). The experimental protocol of repeated, continuous muscle stretch described above could minimize the observation of long-lasting tonic stretch reflexes, and its potential facilitation with repeated stretch. In both subjects in Fig. 1, for example, long-lasting torque and SOL EMG responses were evident when the ankle was held in dorsiflexion following consecutive rotations.
To establish whether tonic stretch reflexes are facilitated with repeated prior stretches, we compared the reflex responses of single ‘ramp-and-hold’ joint perturbations at 120 deg s−1 to those elicited following repeated triangular-wave stretches in a subset of 9 subjects (6 incomplete). The peak and integrated area of ankle torque and EMG activity from the SOL and MG were measured following averaged trials of single perturbations during the final ramp-and-hold phase (3 s), and compared to ramp-and-hold stretches elicited following 1 or 2 repeated, triangular wave perturbations. (Note: MG and SOL EMG were obtained from only 7 subjects (5 incomplete) with reliable recordings during the ramp-and-hold trials.) An example of the reflex responses is demonstrated in Fig. 5. In response to a single ramp-and-hold stretch, elevated MG and SOL EMG and PF torques associated with a stretch reflex response were observed. Following two (Fig. 5B) or three (Fig. 5C) stretches, an increase in the amplitude and duration of stretch reflex responses (torque and EMG) was present (Fig. 5D).
Figure 5. Single subjects responses to single vs repeated ramp-and-hold perturbations.
A, example of MG and SOL EMG, and PF torque following a single ramp-and-hold perturbation or preceded by one (B) or two (C) repeated triangular wave inputs. D, ankle PF torque responses overlaid following the final ramp-and-hold perturbations (subject 7). Longer-lasting joint torque and EMG responses were evident during the final hold phase with repeated stretch.
To quantify changes in tonic stretch reflexes, mean changes in joint torque and EMG responses following single ramp-and-hold perturbations versus those elicited following 1 or 2 additional triangular wave stretches were obtained. Peak torques and integrated torque area (over 3 s from the start of the last stretch) increased by up to 27% following ramp-and-hold perturbations with repeated joint rotation, although the changes were not significant. In contrast, increases in integrated EMG from MG (P = 0.02) and SOL (P = 0.01) muscles were significant following repeated stretch reflexes (differences detected between the 1st and 3rd stretches; again measured during a 3 s epoch from the start of the last stretch). MG and SOL EMG analysed 100 ms prior to the imposed 1st to 3rd joint rotations were again not significantly different. With a presumed similar synaptic input during the final ‘ramp-and hold’ perturbations, prior joint rotation facilitated the net reflex activity.
Effects of baclofen on stretch reflex wind-up
The effects of baclofen on stretch reflex facilitation have not been assessed in human SCI. In our study, six subjects were prescribed baclofen at variable doses to control spastic behaviours (Table 1). Figure 6 demonstrates the reflex responses with the subject population separated on the basis of baclofen use. In subjects not using baclofen (Fig. 6A), initial reflex responses following the first perturbation were not significantly greater than observed in subjects receiving baclofen (P >0.30; Fig. 6B). However, repeated stretch responses of subjects not prescribed baclofen increased significantly at all angular velocities (increase of 34–71%, P < 0.02), while non-significant changes were observed in those taking baclofen (−2 to 29% change, P >0.30 at all velocities). In addition, integrated SOL activity at 60–120 deg s−1 in subjects not taking baclofen was increased significantly (P < 0.05, Fig. 6C), while changes in subjects using baclofen was not significant (Fig. 6D). In one subject, however, a large (>600%) increase in SOL EMG activity was observed during the last stretch at 120 deg s−1, thereby resulting in a large mean increase in SOL activity that was not significant. Changes in MG activity were not significantly different at any angular velocity for either group, except at 90 deg s−1 for those taking baclofen (P < 0.01; Fig. 6E and F). Analysis of all subjects' data revealed that the extent of facilitation (determined by the peak percentage increase in joint torques or EMG activity from initial value) was not correlated with the initial reflex torque or EMG responses (all correlation coefficients < 0.30, P >0.50). The results indicated that use of baclofen may have contributed to reduced reflex facilitation.
Figure 6. Comparison of PF torque responses for 6 repeated stretches at 120 deg s−1 in subjects not using anti-spastic medications (A) and subjects taking anti-spastic medications (B).
Significance was demonstrated only in the group not taking anti-spastic medications for all velocities. EMG responses from the MG (C and D) and SOL (E and F) of subjects not using anti-spastic medications versus those who required baclofen. For MG activity, significant increases were observed at only 90 deg s−1 in subjects using baclofen. In contrast, significant increases in SOL EMG was observed at 60–120 deg s−1 for subjects not using baclofen (E), whereas only one subject at 120 deg s−1 demonstrated a large increase in SOL activity when using baclofen (note large increase in SOL EMG at final stretches).
Discussion
Repeated plantarflexor stretch resulted in reflex facilitation in a manner consistent with other reflex behaviours that implicate a role for PICs in spastic reflexes. In particular stretch reflex facilitation is similar to wind-up of flexor reflexes observed with repeated electrocutaneous stimulation in human SCI (Hornby et al. 2003). Both behaviours are consistent with PIC-like activity, in which brief afferent inputs produce long-lasting outputs (Schmit & Benz, 2002; Gorassini et al. 2004). Together, these studies suggest that repeated or prolonged afferent stimuli in human SCI might result in uncontrolled facilitation of neural pathways that could disrupt motor function.
Temporal facilitation of spastic stretch reflexes contrasts with previous studies that describe a decline of stretch reflex responses to repeated joint rotation. Specifically, in subjects with stroke (Schmit et al. 2000; Nuyens et al. 2002) and SCI (Burke et al. 1971) repeated stretch of spastic extremities generated responses that decreased initially and remained depressed with continued perturbations. Primarily mechanical (i.e. thixotropic; Taylor et al. 1990; Bressel & McNair, 2002), but also afferent (Hagbarth et al. 1985; Haftel et al. 2004), and spinal (Thompson et al. 1992; Sawczuk et al. 1995) mechanisms may account for this depression. For example, in the study by Burke et al. (1971), sinusoidal stretches were applied at intervals similar to those applied here, although a larger excursion was applied (up to 100 deg) at the knee, which may have elicited greater thixotropic changes. In studies in subjects with stroke (Schmit et al. 2000; Nuyens et al. 2002), the duration between repeated perturbations were up to 5 s, providing ample time for mechanical creep of connective or muscle tissues.
In the present study, adaptive processes probably contributed to some of the changes in the reflex responses, particularly at higher velocities. However, repeated stretch produced torque responses that rebounded to levels exceeding the initial response, while EMG activity increased progressively. The mechanisms underlying stretch reflex facilitation must therefore counteract and overcome the neuromechanical factors underlying habituation. This finding is of particular interest and may provide insight into the mechanisms underlying spasticity in human SCI.
Potential mechanisms underlying stretch reflex wind-up
Augmented muscle activity in response to prolonged or repeated electrical stimuli in humans has been observed previously under different experimental conditions. In intact subjects (Collins et al. 2001, 2002; see also Nozaki et al. 2003) and individuals with SCI (Nickolls et al. 2004), low threshold, high frequency (> 50 Hz) electrical stimulation applied directly over shank muscles elicits long-lasting muscle contractions that appear to be unrelated to descending or muscular factors. These findings are attributed to PICs in spinal neurones, although the time course of the reflex potentiation with repeated stimulation in subjects with SCI is prolonged (1–3 min) and observed inconsistently (Nickolls et al. 2004). Facilitation of flexor spasms following human SCI is also observed (Hornby et al. 2003), and the time course (up to 3 s) is similar to that observed in spinal neurones demonstrating PICs (Svirskis & Hounsgaard, 1997; Bennett et al. 1998), suggesting that PICs may mediate long-lasting reflex behaviours.
Although PIC-like activity in spinal neurones may account for the stretch reflex facilitation observed here, other mechanisms may also contribute to the prolonged excitability. For example, while flexor reflex facilitation is thought to be mediated by PIC-like activity in spinal neurones, specific neuroactive agents have also been implicated. Substance P, for example, probably contributes to flexor reflex wind-up (Herrero et al. 2000), although it may be pain-pathway specific and contribute little to stretch reflex excitability. NMDA-mediated inputs could also contribute to the stretch reflexes, although these currents decay rapidly (< 300 ms) (Dale & Roberts, 1985; Dale & Grillner, 1986). It is therefore unlikely that sub- or supra-threshold depolarization due to ionotropic synaptic inputs alone could account for reflex facilitation at longer intervals.
Another potential mechanism of the observed facilitation of stretch reflex pathways could be an augmentation of afferent input with repeated joint rotations. However, in conditions of both repeated triangular wave and ‘ramp-and-hold’ perturbations, increased and/or longer-lasting responses observed with prior stretch activation occurred with similar or, more likely, depressed afferent input (e.g. see Hagbarth et al. 1985; Haftel et al. 2004). Only in conditions of gradually increasing γ-MN drive with repeated stretch should the afferent input increase from the initial stretch. However, γ-MN drive does not play a major role in spasticity (Wilson et al. 1999) and is unlikely to contribute substantially to the observed behaviours.
The primary cause of the enhanced and prolonged reflex activity associated with repeated stretch appears to be the history of prior joint rotation. This temporal facilitation cannot be accounted for by changes in passive electrical properties of spinal MNs or interneurones (i.e. alteration in resting potential or input resistance), which may occur even in ‘resting’ conditions in subjects with SCI versus uninjured controls (Katz & Rymer, 1989; cf. however, Hochman & McCrea, 1994; Bennett et al. 2001). Rather, repeated joint rotation probably caused depolarization-induced facilitation via PICs, which caused long-lasting, subthreshold depolarization and/or lowering of the PIC threshold and progressively recruited previously quiescent MNs. These prolonged single-joint responses are akin to the long-lasting muscle activity associated with multijoint spasms observed following other stimuli (Schmit & Benz, 2002; Hornby et al. 2003; Gorassini et al. 2004).
PIC-like activity in spinal neurones may therefore be a plausible explanation for reflex facilitation in human SCI, as both stretch and flexor reflexes display temporal- and stimulus-dependent properties indicative of PICs. One prominent difference between stretch and flexor reflex facilitation is the brief time course of enhanced stretch reflexes, which may be due to the method of reflex activation. Stretch of passive tissues (Taylor et al. 1990) and extra- and intrafusal muscle fibres could contribute to Ia adaptation (Haftel et al. 2004) thereby reducing the magnitude of stretch reflex facilitation. In decerebrate cats, PIC wind-up with repeated stretch has been observed (Bennett et al. 1998), although the time course was not investigated. However, a gradual increase in EMG was elicited, similar to the present results. Unfortunately, stretch reflex facilitation in chronic models of spasticity (Bennett et al. 1999; Thompson et al. 2001) has not yet been demonstrated. Despite the lack of comparable animal data, our results demonstrate reflex facilitation similar to motor behaviours in preparations with established PIC activity.
Effects of anti-spasticity medications on stretch reflex facilitation
Facilitation of joint torques and SOL EMG with repeated stretch in subjects using baclofen was reduced as compared to subjects not using anti-spastic medications. The effects of baclofen on hyperexcitable reflexes in individuals with neurological injury are well established (Campbell et al. 1995; Gracies et al. 1997; Meythaler et al. 2001), although the precise locus of action (i.e. pre- versus postsynaptic) remains uncertain. In acute spinal preparations, baclofen reduces PICs associated with long-lasting discharge and depolarization-induced facilitation (Svirskis & Hounsgaard, 1997), consistent with previous reports of a postsynaptic action of baclofen in humans (Azouvi et al. 1993; Orsnes et al. 2000). In contrast, data from rat MNs following chronic, complete SCI suggests baclofen operates primarily through presynaptic mechanisms (< 1 μm) (Li et al. 2004), particularly at lower doses.
Reflex facilitation with repeated stimuli is thought to originate at postsynaptic sites in MNs or interneurones (Svirskis & Hounsgaard, 1997; Bennett et al. 1998), while the role of presynaptic facilitation on PIC-like activity in humans is rarely discussed (Gorassini et al. 2002, 2004; Nickolls et al. 2004). The observation of reduced reflex facilitation in subjects using baclofen would suggest a postsynaptic mechanism as well, although such data contrast with those of Li et al. (2004), indicating a presynaptic action. Further research is required on human SCI using additional electrophysiological methods to elucidate the precise mechanisms (Orsnes et al. 2000).
Summary and conclusions
The data suggest that temporal facilitation of stretch reflexes in human SCI may be indicative of alterations in intrinsic spinal PICs. Consistent with data in animal models of spasticity, this observation may provide further evidence that alterations in intrinsic neuronal properties are partly responsible for velocity-dependent spasticity. Despite these findings, it remains unclear whether the mechanisms underlying hyperexcitable stretch reflexes are similar in individuals with spasticity of cerebral origin. As described earlier, previous data in subjects with stroke (Schmit et al. 2000; Nuyens et al. 2002) suggest habituation of stretch reflexes with repeated perturbations, although the temporal dynamics of the perturbations were not similar. Using the same protocol as described above, the study of reflex responses to repeated perturbations is required to ascertain whether similar time-dependent behaviours are observed in individuals with similar clinical presentation but different loci of neural injury. Such observations may provide insight into the mechanisms underlying spasticity in a variety of neurological diagnoses.
Acknowledgments
This work was supported by National Institute of Health Grant R01 NS-40901-01.
References
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- Azouvi P, Roby-Brami A, Biraben A, Thiebaut JB, Thurel C, Bussel B. Effect of intrathecal baclofen on the monosynaptic reflex in humans: evidence for a postsynaptic action. J Neurol Neurosurg Psychiatr. 1993;56:515–519. doi: 10.1136/jnnp.56.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini MA, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini MA. Short-term plasticity in hindlimb motoneurones of decerebrate cats. J Neurophysiol. 1998;80:2038–2045. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Benz EN, Hornby TG, Bode RK, Scheidt RA, Schmit BD. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil. 2005;86:52–59. doi: 10.1016/j.apmr.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Bressel E, McNair PJ. The effect of prolonged static and cyclic stretching on ankle joint stiffness, torque relaxation, and gait in people with stroke. Phys Ther. 2002;82:880–887. [PubMed] [Google Scholar]
- Burke D, Andrews CJ, Gillies JD. The reflex response to sinusoidal stretching in spastic man. Brain. 1971;94:455–470. doi: 10.1093/brain/94.3.455. [DOI] [PubMed] [Google Scholar]
- Calancie B, Molano MR, Broton JG. Interlimb reflexes and synaptic plasticity become evident months after human spinal cord injury. Brain. 2002;125:1150–1161. doi: 10.1093/brain/awf114. [DOI] [PubMed] [Google Scholar]
- Campbell SK, Almeida GL, Penn RD, Corcos DM. The effects of intrathecally administered baclofen on function in patients with spasticity. Phys Ther. 1995;75:352–362. doi: 10.1093/ptj/75.5.352. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–4065. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behavior in human motoneurones. J Physiol. 2002;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Grillner S. Dual-component synaptic potentials in the lamprey mediated by excitatory amino acid receptors. J Neurosci. 1986;6:2653–2661. doi: 10.1523/JNEUROSCI.06-09-02653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Roberts A. Dual component amino-acid mediated synaptic potential: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985;363:35–39. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichson P. Tonic ankle reflex in parkinsonian rigidity and in spasticity. The role of the fusimotor system. Acta Neurol Scand. 1971a;47:163–182. doi: 10.1111/j.1600-0404.1971.tb07474.x. [DOI] [PubMed] [Google Scholar]
- Dietrichson P. The silent period in spastic, rigid, and normal subjects during isotonic and isometric muscle contractions. Acta Neurol Scand. 1971b;47:183–193. doi: 10.1111/j.1600-0404.1971.tb07475.x. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties contribute to spastic hypertonia. Brain. 1981;104:431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol. 2002;87:1859–1866. doi: 10.1152/jn.00025.2001. [DOI] [PubMed] [Google Scholar]
- Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl. 1997;6:S92–S120. [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. J Neurophysiol. 2004;91:2164–2171. doi: 10.1152/jn.01147.2003. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hagglund JV, Nordin M, Wallin EU. Thixotropic behaviour of human finger flexor muscles with accompanying changes in spindle and reflex responses to stretch. J Physiol. 1985;368:323–342. doi: 10.1113/jphysiol.1985.sp015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurons and pain sensation: much ado about something? Progr Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. II. Motoneuron electrical properties. J Neurophysiol. 1994;71:1468–1479. doi: 10.1152/jn.1994.71.4.1468. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurons in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Roberts RC. Presynaptic inhibition of monosynaptic references in the lower limbs of subjects with upper motoneuron disease. J Neurol Neurosurg Psychiatr. 1986;49:937–944. doi: 10.1136/jnnp.49.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Rev. 2002;40:19–28. doi: 10.1016/s0165-0173(02)00185-6. [DOI] [PubMed] [Google Scholar]
- Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve. 2003;28:309–318. doi: 10.1002/mus.10443. [DOI] [PubMed] [Google Scholar]
- Katz R, Rymer WZ. Spastic hypertonia: mechanisms and management. Arch Phys Med Rehabil. 1989;70:144–155. [PubMed] [Google Scholar]
- Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K, Brown PB, Mendell LM. Central sprouting and functional plasticity of regenerated primary afferents. J Neurosci. 1994;14:3655–3671. doi: 10.1523/JNEUROSCI.14-06-03655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: Disordered Motor Control. Chicago: Year Book Medical Publishers; 1980. pp. 485–494. [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol. 2004;92:2694–2703. doi: 10.1152/jn.00164.2004. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Steinman S, Barash IA, Chambers H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve. 2004;29:615–627. doi: 10.1002/mus.20059. [DOI] [PubMed] [Google Scholar]
- McNair PJ, Hewson DJ, Dombroski E, Stanley SN. Stiffness and passive peak force changes at the ankle joint: the effect of different joint angular velocities. Clin Biomech (Bristol, Avon) 2002;17:536–540. doi: 10.1016/s0268-0033(02)00062-1. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Meythaler JM. Concept of spastic hypertonia. Phys Med Rehabil Clin N Am. 2001;12:725–732. [PubMed] [Google Scholar]
- Meythaler JM, Guin-Renfroe S, Brunner RC, Hadley MN. Intrathecal baclofen for spastic hypertonia from stroke. Stroke. 2001;32:2099–2109. doi: 10.1161/hs0901.095682. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–670. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Nozaki D, Kawashima N, Aramaki Y, Akai M, Nakazawa K, Nakajima Y, Yano H. Sustained muscle contractions maintained by autonomous neuronal activity within the human spinal cord. J Neurophysiol. 2003;90:2090–2097. doi: 10.1152/jn.00200.2003. [DOI] [PubMed] [Google Scholar]
- Nuyens GE, De Weerdt WJ, Spaepen AJ, Jr, Kiekens C, Feys HM. Reduction of spastic hypertonia during repeated passive knee movements in stroke patients. Arch Phys Med Rehabil. 2002;83:930–935. doi: 10.1053/apmr.2002.33233. [DOI] [PubMed] [Google Scholar]
- Orsnes G, Crone C, Krarup C, Petersen N, Nielsen J. The effect of baclofen on the transmission in spinal pathways in spastic multiple sclerosis patients. Clin Neurophysiol. 2000;111:1372–1379. doi: 10.1016/s1388-2457(00)00352-7. [DOI] [PubMed] [Google Scholar]
- Priebe MM, Sherwood AM, Thornby JI, Kharas NF, Markowski J. Clinical assessment of spasticity in spinal cord injury: a multidimensional problem. Arch Phys Med Rehabil. 1996;77:713–716. doi: 10.1016/s0003-9993(96)90014-3. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Schmit BD, Benz EN. Extensor reflexes in human spinal cord injury: activation by hip proprioceptors. Exp Brain Res. 2002;145:520–527. doi: 10.1007/s00221-002-1134-5. [DOI] [PubMed] [Google Scholar]
- Schmit BD, Dewald JP, Rymer WZ. Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch Phys Med Rehabil. 2000;81:269–278. doi: 10.1016/s0003-9993(00)90070-4. [DOI] [PubMed] [Google Scholar]
- Schmit BD, Hornby TG, Tysseling-Mattiace VM, Benz EN. Absence of local sign withdrawal in chronic human spinal cord injury. J Neurophysiol. 2003;90:3232–3241. doi: 10.1152/jn.00924.2002. [DOI] [PubMed] [Google Scholar]
- Sheean G. The pathophysiology of spasticity. Eur J Neurol. 2002;9(Suppl. 1):3–9. doi: 10.1046/j.1468-1331.2002.0090s1003.x. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Depolarization-induced facilitation of a plateau-generating current in ventral horn neurons in the turtle spinal cord. J Neurophysiol. 1997;78:1740–1742. doi: 10.1152/jn.1997.78.3.1740. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Dalton JD, Seaber AV, Garrett WE. Viscoelastic properties of muscle–tendon units. The biomechanical effects of stretching. Am J Sports Med. 1990;18:300–309. doi: 10.1177/036354659001800314. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Parmer R, Reier PJ, Wang DC, Bose P. Scientific basis of spasticity: insights from a laboratory model. J Child Neurol. 2001;16:2–9. doi: 10.1177/088307380101600102. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68:1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- Wilson LR, Gracies JM, Burke D, Gandevia SC. Evidence for fusimotor drive in stroke patients based on muscle spindle thixotropy. Neurosci Lett. 1999;264:109–112. doi: 10.1016/s0304-3940(99)00181-0. [DOI] [PubMed] [Google Scholar]