Abstract
Prenatal exposure to elevated maternal glucocorticoids (dexamethasone (DEX) or cortisol (CORT)) for 2 days early in pregnancy can ‘programme’ alterations in adult offspring of sheep, including elevated arterial pressure. DEX treatment also results in greater angiotensin II type 1 (AT1) receptor expression in the medulla oblongata in late gestation fetuses than in saline (SAL)- or CORT-exposed animals. We hypothesized that this would result in functional changes in brainstem angiotensinergic control of cardiovascular function in DEX- but not CORT-exposed animals. To test this hypothesis, cardiovascular responses to intracerebroventricular (i.c.v.) angiotensin II were examined in adult male offspring exposed to DEX (0.48 mg h−1; n = 7), CORT (5 mg h−1, n = 6) or SAL (n = 9) from 26 to 28 days of gestation. Increases in mean arterial pressure during i.c.v. infusion of angiotensin II (1 or 10 μg h−1) were significantly greater in the DEX group (10 ± 1 mmHg at 1 μg h−1) compared with SAL (6 ± 1 mmHg) or CORT (6 ± 1 mmHg) animals (P < 0.05). i.c.v. infusion of the AT1 antagonist losartan significantly decreased cardiac output and heart rate in DEX animals, but not in SAL or CORT animals. Thus, increased expression of brainstem AT1 receptor mRNA after prenatal DEX is associated with increased responsiveness of cardiovascular control to activation of brain AT receptors by exogenous and endogenous angiotensin II. The altered role of the brain RAS in sheep exposed prenatally to DEX was not observed in sheep exposed prenatally to cortisol, suggesting these two glucocorticoids have distinct programming actions.
There is substantial epidemiological evidence suggesting that long-term adult health can be influenced by an adverse prenatal environment (Osmond & Barker, 2000; Barker, 2004). It has been hypothesized that exposure to a suboptimal intrauterine environment alters, or ‘programmes’, the development of fetal tissues, rendering the offspring more susceptible to diseases such as hypertension and diabetes (Roseboom et al. 2001).
Short-term maternal glucocorticoid exposure has been used to investigate the mechanisms underlying the consequences of a suboptimal intrauterine environment in rats (Ortiz et al. 2001) and sheep (Dodic et al. 1998). These studies have demonstrated that offspring exposed to high levels of the synthetic glucocorticoid, dexamethasone, at critical periods of development, become hypertensive in adulthood. Treatment of rats with carbenoxolone throughout pregnancy, which inhibits inactivation of endogenous glucocorticoids by 11β-hydroxy-steroid dehydrogenase 2, results in low birth weight and hypertension in the adult progeny (Langley-Evans, 2001). The development of hypertension in this model was prevented when the source of glucocorticoids was removed by maternal adrenalectomy (Gardner et al. 1997). It has also been suggested that elevated glucocorticoid exposure in utero may contribute to the mechanisms leading to adult disease as a result of maternal undernutrition (Langley-Evans, 2001).
The renin–angiotensin system (RAS) has been shown to be affected in models of prenatal programming, and this could potentially contribute to altered adult cardiovascular function in programmed offspring. This concept is supported by the finding that hypertension in the offspring of pregnant rats fed a low protein diet (9% w/w) could be prevented by postnatal inhibition of angiotensin converting enzyme (ACE) (Sherman & Langley-Evans, 1998). Furthermore, increased angiotensin II type 1 (AT1) receptors were found in the brain of adult offspring of Wistar rats fed a low protein (9% w/w) diet during gestation (Pladys et al. 2004). These rats also developed hypertension, which could be attenuated by intracerebroventricular (i.c.v.) infusions of an ACE inhibitor or AT1 antagonist. Collectively, these observations are consistent with the hypothesis that alterations in the central RAS contribute to the development of hypertension programmed in utero via maternal protein restriction.
In sheep, expression of mRNA for the AT1 receptor in the medulla oblongata of late gestational fetuses (male and female at 130 days) and also in a cohort of hypertensive female offspring at 7 years of age was significantly greater in animals exposed to dexamethasone (for 48 h, from day 26 to 28 of gestation where term is ∼150 days) compared to saline-exposed controls (Dodic et al. 2002a). Interestingly, these effects of prenatal dexamethasone were not mimicked by the natural glucocorticoid, cortisol, even though prenatal exposure to either glucocorticoid was associated with adult hypertension (Dodic et al. 2002b). Based on these observations, we hypothesized that brain angiotensinergic circulatory control mechanisms are up-regulated in adult sheep exposed to dexamethasone in utero, but not those exposed to cortisol in utero. This hypothesis was tested by comparing responses of adult sheep exposed briefly to dexamethasone, cortisol, or saline vehicle in utero, to intracerebroventricular (i.c.v.) infusions of angiotensin II and the AT1 receptor antagonist losartan. Our results provide the first direct evidence of critical differences in the underlying mechanisms through which exposure to synthetic or natural glucocorticoids programme changes in adult cardiovascular function.
Methods
Animals
All experiments were approved by the Animal Ethics Committee of the Department of Physiology, Monash University, in accordance with the National Health and Medical Research Council of Australia guidelines. Ewes of known mating date received an intravenous infusion of saline (0.19 ml h−1, SAL, n = 9), dexamethasone (0.48 mg h−1, DEX, n = 7) or cortisol (5 mg h−1, CORT, n = 6) for 48 h from day 26 to day 28 of gestation. Singleton male offspring (wethers) were studied at 50–52 months of age. Males had castration and tail docking performed at 2 months of age as required by the Ethics committee. The construction of carotid artery loops took place at 1 year of age (Dodic et al. 1998). We have previously reported the basal mean arterial pressure in these animals at approximately 2 years of age (Dodic et al. 2002a,b). During experimentation, sheep were kept in metabolic cages and provided with 0.8 kg of lucerne chaff per day and water ad libitum.
Surgical preparation
Under general anaesthesia, at approximately 4 years of age, animals were instrumented with a guide tube placed into each lateral ventricle of the brain. General anaesthesia was induced with an intravenous (i.v.) injection of 5% sodium thiopentone (1 g, Thiobarb, Jurox, Australia) via the jugular vein. Following intubation, anaesthesia was maintained with 1.5–2.5% halothane (Merial, Australia) in 100% oxygen. The head of the sheep was held in a stereotaxic frame to allow correct positioning of the guide tubes, as previously reported (Mathai et al. 1998).
Systemic haemodynamic measurements
After a minimum of 2 weeks recovery from surgery, animals acclimatized to the laboratory for 7 days before cardiovascular measurements were recorded. A Tygon cannula was inserted into the carotid arterial loop for measurement of arterial pressure and heart rate. On the day prior to an experiment, animals were instrumented with a Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA, USA), inserted via the jugular vein, under local anaesthesia (2 ml, lignocaine, Troy Laboratories, Smithfield, NSW, Australia) and positioned with the catheter tip in the pulmonary artery. Mean arterial pressure (MAP, mmHg) and heart rate (HR, beats min−1) were measured simultaneously as previously described (Dodic et al. 2001). Cardiac output (CO, ml min−1 kg−1) was measured using the thermodilution technique with a cardiac output computer (9520A American Edwards Laboratories) as previously described (Dodic et al. 2001). Total peripheral resistance (TPR, mmHg ml−1 min kg) and stroke volume (SV, ml kg−1) were calculated using the measured parameters.
Cardiovascular responses to i.c.v. artificial cerebrospinal fluid or angiotensin II
Cardiovascular parameters were recorded during a 1-h control period, followed by a 1-h i.c.v. infusion of artificial cerebrospinal fluid (CSF) or angiotensin II (human, Auspep; 1 or 10 μg h−1) dissolved in artificial CSF. The infusion rate was 0.5 ml h−1. Artificial CSF was prepared as previously described (Mathai et al. 1998). Over the 2 h experiment, CO was measured every 20 min, while MAP and HR were measured continuously. One 5 ml blood sample, for measurement of plasma Na+, K+, Cl− and osmolality, was taken at the end of the 60 min control period and upon completion of the 60 min infusion period and immediately placed on ice. A 5-ml sample for analysis of plasma cortisol concentration was taken at the end of the 20 min control period and then at 20, 40 and 60 min after commencement of the angiotensin II infusion. Blood samples were centrifuged at 4°C for 15 min and plasma collected and frozen at −18°C until analysis. Water intake and urine output (spontaneously voided from the bladder) were measured hourly. The three treatments (saline, and 1 and 10 μg h−1 angiotensin II) were administered in random order on different days, allowing at least 24 h between experiments.
Assessing degree of receptor blockade with i.c.v. losartan
In a subset of animals (n = 4) from the SAL group, i.v. and i.c.v. infusions of angiotensin II were administered before and during i.c.v. infusion of losartan (1 mg h−1) over 4 h, in order to assess the degree of central and peripheral AT1 receptor blockade. MAP was measured over a 1-h control period, after which angiotensin II (10 μg h−1) was infused i.v. for 20 min. MAP was allowed to return to baseline before commencement of the i.c.v. losartan. After 3 h of losartan infusion, the 20 min i.v. angiotensin II infusion was repeated. MAP was again allowed to return to baseline, after which angiotensin II (10 μg h−1) was infused i.c.v. for 20 min.
Cardiovascular responses to i.c.v. losartan
The AT1 antagonist, losartan (Merck Research Laboratories, USA), was diluted to 2 mg ml−1 in artificial CSF. Following a 1-h control period, losartan or artificial CSF was infused i.c.v. (0.5 ml h−1) for 4 h. Cardiovascular parameters were measured at 60, 120, 180, 200, 220 and 240 min. One 12 ml blood sample, for Na+, K+ and Cl−, was taken at the end of the control period and at 240 min of losartan infusion. Plasma was collected as per the i.c.v. angiotensin II protocol.
Analysis of plasma samples
Plasma osmolality was analysed by freezing point depression using an Advanced Osmometer (Advanced Instruments Inc., Pomona, CA, USA). Plasma concentrations of Na+, K+ and Cl− were measured using a synchronic CX5 Clinical System (Beckman, USA). Plasma cortisol concentrations were determined before and during the 10 μg h−1 infusion of i.c.v. angiotensin II using a radioimmunoassay as previously described (Bocking et al. 1986). The sensitivity of the assay was 0.3 nmol l−1. Intra- and interassay coefficients of variation were 8 and 16%, respectively.
Statistical analyses
Statistical analyses were performed using SigmaStat software (SigmaStat 2.0.3 for Windows, SPSS Inc., Chicago, IL, USA). During the 1 h infusion of angiotensin II, values for each haemodynamic parameter as well as cortisol concentrations were averaged over three 20 min intervals. During the 4 h infusion of losartan, values were averaged over one hourly intervals. To allow for differences in basal arterial pressures, results at 20, 40 and 60 min are shown as the change from values obtained during the control hour. A two-way repeated measures analysis of variance (ANOVA) with Tukey's post hoc analysis was employed to test whether haemodynamic responses to i.c.v. angiotensin II and losartan differed between the three groups of sheep. ANOVA was also employed to determine whether basal haemodynamic variables, water intake or urine output differed between the three groups. All results are presented as the mean ± s.e.m., with the level of significance set at P = 0.05.
Results
The age and weight of animals in the three groups were similar at the time of experimentation (SAL 52 ± 1 months of age, DEX 52 ± 2 months of age and CORT 50 ± 2 months of age; SAL 52 ± 3 kg, DEX 53 ± 2 kg and CORT 57 ± 3 kg).
Intracerebroventricular (i.c.v.) infusion of artificial CSF
MAP, CO, TPR, HR and SV, averaged over the 1 h control period and 1 h i.c.v. infusion of artificial CSF (0.5 ml h−1), are shown in Table 1. Values were relatively stable over the 2 h experiment in all three groups with no apparent effect of i.c.v. CSF being observed. MAP was significantly greater in the CORT group over this 2-h period compared to SAL and DEX animals. HR, CO and SV did not differ significantly across the three groups whilst TPR tended to be greater in the CORT group (P = 0.1) compared to the DEX and SAL groups. Plasma osmolality and plasma concentrations of Na+, K+ and Cl− were similar in the three groups and did not change over the infusion period (Table 2).
Table 1.
Cardiovascular parameters averaged over a 1-h control period and 1 h intracerebroventricular infusion of artificial cerebrospinal fluid
| SAL | DEX | CORT | |
|---|---|---|---|
| Mean arterial pressure (mmHg) | 81 ± 2 | 82 ± 3 | 89 ± 3*# |
| Heart rate (beats min−1) | 69 ± 3 | 70 ± 4 | 70 ± 5 |
| Cardiac output (ml kg−1min−1) | 97 ± 4 | 98 ± 4 | 99 ± 6 |
| Stroke volume (ml kg−1) | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| Total peripheral resistance (mmHg ml−1 kg min) | 0.88 ± 0.05 | 0.87 ± 0.03 | 0.95 ± 0.06 |
Data are means ± s.e.m. from sheep exposed prenatally to saline (SAL, n = 9), dexamethasone (DEX, n = 8) or cortisol (CORT, n = 7) between days 26 and 28 of gestation. In all three groups, these variables did not change systematically across the 2-h period from which these data were derived
P ≤ 0.05 compared to SAL group
P ≤ 0.05 compared with DEX group.
Table 2.
Plasma osmolality and electrolyte composition before and after intracerebroventricular CSF, angiotensin II (1 or 10 μg h−1) infusion for 60 min or losartan (1 mg h−1) infusion for 240 min
| Infusion/group | Time | Osmolality | Sodium | Potassium | Chloride |
|---|---|---|---|---|---|
| Artificial CSF | |||||
| SAL | 0 | 299 ± 2 | 144 ± 1 | 4.4 ± 0.1 | 113 ± 1 |
| 60 | 295 ± 2 | 142 ± 2 | 4.2 ± 0.1 | 112 ± 1 | |
| DEX | 0 | 300 ± 2 | 145 ± 1 | 4.4 ± 0.1 | 113 ± 1 |
| 60 | 300 ± 3 | 144 ± 1 | 4.3 ± 0.1 | 114 ± 1 | |
| CORT | 0 | 293 ± 2 | 143 ± 1 | 4.2 ± 0.1 | 111 ± 1 |
| 60 | 292 ± 2 | 143 ± 1 | 4.2 ± 0.1 | 112 ± 1 | |
| P (time) | NS | NS | NS | NS | |
| Angiotensin II (1 μg h−1) | |||||
| SAL | 0 | 298 ± 2 | 144 ± 1 | 4.2 ± 0.1 | 113 ± 1 |
| 60 | 295 ± 2 | 144 ± 1 | 3.8 ± 0.1 | 112 ± 1 | |
| DEX | 0 | 297 ± 3 | 144 ± 1 | 4.2 ± 0.1 | 114 ± 1 |
| 60 | 293 ± 2 | 142 ± 1 | 3.8 ± 0.1 | 111 ± 1 | |
| CORT | 0 | 292 ± 1 | 142 ± 1 | 4.1 ± 0.1 | 111 ± 1 |
| 60 | 286 ± 2 | 140 ± 1 | 4.0 ± 0.1 | 109 ± 1 | |
| P (time) | P < 0.05 | NS | P < 0.05 | NS | |
| Angiotensin II (10 μg h−1) | |||||
| SAL | 0 | 297 ± 2 | 144 ± 1 | 4.2 ± 0.1 | 111 ± 1 |
| 60 | 288 ± 2 | 140 ± 1 | 3.7 ± 0.1 | 108 ± 1 | |
| DEX | 0 | 298 ± 3 | 144 ± 1 | 4.2 ± 0.1 | 112 ± 1 |
| 60 | 291 ± 2 | 142 ± 1 | 3.6 ± 0.1 | 109 ± 1 | |
| CORT | 0 | 295 ± 1 | 146 ± 1 | 4.2 ± 0.2 | 112 ± 1 |
| 60 | 290 ± 1 | 143 ± 1 | 3.8 ± 0.1 | 110 ± 1 | |
| P (time) | P < 0.01 | P < 0.05 | P < 0.01 | P < 0.05 | |
| Losartan (1 mg h−1) | |||||
| SAL | 0 | 301 ± 2 | 144 ± 1 | 4.5 ± 0.1 | 113 ± 1 |
| 240 | 307 ± 2 | 146 ± 1 | 4.3 ± 0.1 | 118 ± 1 | |
| DEX | 0 | 299 ± 3 | 144 ± 1 | 4.6 ± 0.1 | 113 ± 1 |
| 240 | 305 ± 2 | 146 ± 1 | 4.2 ± 0.1 | 119 ± 1 | |
| CORT | 0 | 292 ± 2 | 143 ± 2 | 4.5 ± 0.2 | 110 ± 2 |
| 240 | 295 ± 2 | 143 ± 2 | 4.4 ± 0.1 | 116 ± 2 | |
| P (time) | P < 0.05 | NS | NS | P < 0.05 | |
Data are means ± s.e.m. from sheep exposed prenatally to saline (SAL, n = 9), dexamethasone (DEX, n = 8) or cortisol (CORT, n = 6) between days 26 and 28 of gestation. Data were analysed using two-way ANOVA. P (time) tests for changes in these parameters across the course of the experiment. There were no effects of treatment or significant interactions between time and treatment. NS, not significant (P > 0.05).
Intracerebroventricular infusion of angiotensin II (1 μg h−1)
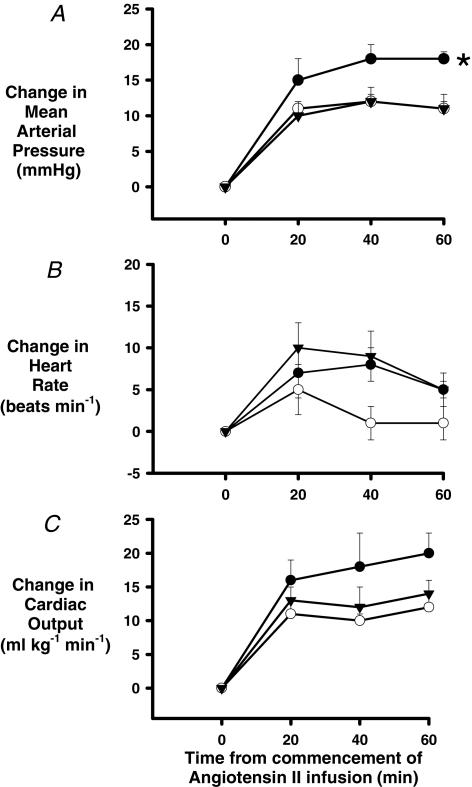
MAP was significantly increased in all groups (P < 0.001; Fig. 1A) during i.c.v. infusion of angiotensin at 1 μg h−1, but the increase was significantly greater in DEX animals than in SAL or CORT animals (P = 0.05). For example, 40 min after commencing the infusion MAP was increased by 5 ± 1, 6 ± 1 and 10 ± 1 mmHg, respectively, in SAL, CORT and DEX sheep. There was no consistent change in HR in the SAL group over the 1 h angiotensin infusion, but HR was significantly elevated by 6 ± 2 and 5 ± 2 beats min−1 40 and 60 min after commencing the infusion in the DEX group (P = 0.05). In the CORT group, the HR response was more variable and so did not differ significantly from that of either the DEX or SAL group. When all groups were considered collectively, CO demonstrated significant increases over time (P = 0.01). However, the increase in CO in the DEX group (10 ± 4 ml min−1 kg−1 at 40 min) tended to be greater than that in the SAL (3 ± 4 ml min−1 kg−1 at 40 min, P = 0.2) or CORT (4 ± 4 ml min−1 kg−1 at 40 min, P = 0.4) groups. However these apparent between-group differences did not reach statistical significance (Fig. 1C). SV and TPR did not change significantly over time (P = 0.1 and 0.4, respectively) when analysed over all three groups and there were differences between the treatment groups (data not shown).
Figure 1. Changes in systemic haemodynamic variables during a 1-h intracerebroventricular (i.c.v.) infusion of angiotensin II (1 μg h−1).
Responses were tested in male sheep prenatally exposed to saline (○, n = 9), dexamethasone (0.48 mg h−1, •, n = 7) or cortisol (5 mg h−1, ▾; n = 6) for 48 h from day 26–28 of gestation (term ∼150d). Results are shown as change (mean ± s.e.m.) of 20 min averages from the 1 h control period average of mean arterial pressure (A), heart rate (B) and cardiac output (C). Two-way repeated measures ANOVA with post hoc Tukey's test was used to detect significant differences between groups. *P ≤ 0.05 compared to SAL group, #P ≤ 0.05 compared to CORT group.
Only two animals drank during the 1 h control period (200 ml and 300 ml, respectively), despite the availability of water. Water intake during the 1 h i.c.v. infusion of angiotensin II (1 μg h−1) was similar between the groups (SAL = 1331 ± 287 ml, DEX = 1800 ± 175 ml, CORT = 1650 ± 386 ml). Urine output during angiotensin II infusion was also similar between the groups (SAL = 148 ± 39 ml, DEX = 113 ± 45 ml, CORT = 200 ± 78 ml). Plasma osmolality and concentrations of Na+, K+ and Cl−, before and after the infusion of angiotensin II, are shown in Table 2. Plasma osmolality was significantly reduced after the angiotensin II infusion in all groups, and this was associated with significant reductions in plasma concentrations of K+ but not Na+ or Cl−. The magnitudes of these changes were indistinguishable across the groups (Table 2).
Intracerebroventricular infusion of angiotensin II (10 μg h−1)
The increase in MAP in response to 10 μg h−1 angiotensin II was significantly greater in DEX animals compared with animals in the SAL and CORT groups (P = 0.05; Fig. 2A). Forty minutes after commencing the infusion, MAP was increased by 12 ± 1, 12 ± 2 and 18 ± 2 mmHg, respectively, in SAL, CORT and DEX sheep. CO increased in all groups (P < 0.001; Fig. 2C) but the increase in the DEX group tended to be greater than that in the SAL (P = 0.07) or CORT (P = 0.08) groups. HR responses to 10 μg h−1i.c.v. angiotensin II were variable and no significant differences between the groups were detected. TPR did not significantly alter throughout the infusion in all groups. SV tended to increase over time when all three groups were analysed collectively (P = 0.08). Water intake and urinary output during the 1 h i.c.v. angiotensin II infusion were similar between the groups of animals (water intake: SAL = 2563 ± 253 ml, DEX = 2713 ± 167 ml, CORT = 2700 ± 370 ml; urine output: SAL = 301 ± 84 ml, DEX = 277 ± 62 ml, CORT = 275 ± 138 ml). Plasma osmolality, Na+, K+ and Cl− were significantly reduced by the infusion and these effects were greater than those seen with the 1 μg h−1 dose. These responses were indistinguishable in the three groups of sheep (Table 2). Plasma cortisol concentrations were increased in all groups during the 10 μg h−1 infusion of angiotensin II (P < 0.001); however, the increase was similar in all groups (Fig. 3).
Figure 2. Changes in systemic haemodynamic variables during a 1-h intracerebroventricular (i.c.v.) infusion of angiotensin II (10 μg h−1).
Responses were tested in male sheep, prenatally exposed to saline (○, n = 9), dexamethasone (0.48 mg h−1, •, n = 7) or cortisol (5 mg h−1, ▾, n = 6) for 48 h from day 26 to 28 of gestation (term ∼150d). Results are shown as change (mean ± s.e.m.) of 20 min averages from the 1 h control period average in mean arterial pressure (A), heart rate (B) and cardiac output (C). Two-way repeated measures ANOVA with post hoc Tukey's test was used to detect significant differences between groups. *P ≤ 0.05 compared to SAL group, #P ≤ 0.05 compared to CORT group.
Figure 3. Plasma cortisol concentrations during a 1-h intracerebroventricular (i.c.v.) infusion of angiotensin II (10 μg h−1).
Responses were tested in male sheep, prenatally exposed to saline (○, n = 9), dexamethasone (0.48 mg h−1, •, n = 7) or cortisol (5 mg h−1, ▾, n = 6) for 48 h from day 26 to 28 of gestation (term ∼150d). Two-way repeated measures ANOVA was used to test for significant changes in cortisol concentrations over time, between treatments and between treatments over time. Plasma cortisol increased significantly over time (P < 0.01) but the responses were indistinguishable between groups.
Intracerebroventricular infusion of losartan
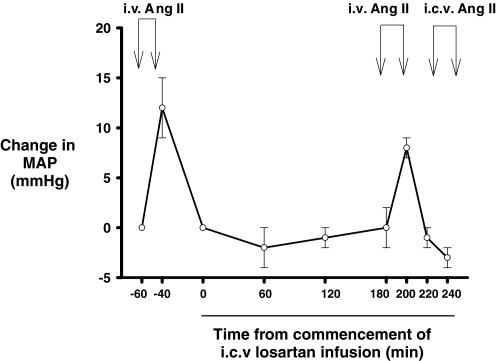
In the four SAL animals in which it was tested, the increase in MAP in response to i.v. angiotensin II (10 μg h−1) was similar prior to and during i.c.v. losartan. However, there was no significant increase in blood pressure in response to i.c.v. angiotensin II (10 μg h−1) during concomitant i.c.v. infusion of losartan, indicating blockade of central, but not peripheral AT1 receptors with the dose of 1 mg h−1 (Fig. 4).
Figure 4. Effects of intravenous (i.v.) and intracerebroventricular (i.c.v.) infusions of angiotensin II during the 4 h i.c.v. infusion of the AT1 antagonist losartan (1 mg h−1) in four sheep.
Angiotensin II (10 μg h−1) was first infused intravenously for 20 min (i.v. AII). MAP was then allowed to return to baseline before i.c.v. losartan infusion commenced. The i.v. angiotensin II infusion was repeated from 180 to 200 min after commencing the i.c.v. losartan infusion. MAP was again allowed to return to baseline, before responses to a 20-min i.c.v. infusion of angiotensin II (10 μg h−1) were tested (i.c.v. AII). Symbols and error bars represent the mean ± s.e.m. of changes in mean arterial pressure (ΔMAP) relative to a 1-h control period.
The 4 h infusion of losartan (1 mg h−1) did not significantly alter MAP in any group of animals (Fig. 5A). In the SAL group, HR initially increased (by 6 ± 1 beats min−1) at 60 min and then returned to baseline by 240 min of losartan infusion. HR in the DEX group was reduced at all time points, so that the overall response was significantly different from that of the SAL group (P = 0.05, Fig. 5B). There was a decrease in HR in the CORT group over the first 180 min of the losartan infusion, but HR returned to baseline by 240 min. Overall, the HR response in the CORT group did not differ significantly from that of either the SAL or the DEX group. CO tended to decrease during the losartan infusion in the SAL group. Responses of CO in the CORT group did not differ significantly from those of the SAL group. In contrast, in the DEX group i.c.v. losartan infusion was accompanied by profound reductions in CO, so that overall, this response differed significantly from those of both the SAL (P = 0.05) and CORT (P = 0.05) groups (Fig. 5C). SV in all groups decreased in the first hour and then returned to baseline over the next 3 h, while TPR tended to increase over the 4 h of losartan infusion. These responses were indistinguishable in the three groups. Plasma osmolality significantly increased during i.c.v. losartan in all groups. This was mainly due to increases in Cl− concentration (P < 0.05, Table 2). The magnitudes of changes in plasma osmolality and plasma concentration of Cl− were similar between the three groups.
Figure 5. Changes in systemic haemodynamic variables during a 4-h intracerebroventricular (i.c.v.) losartan infusion (1 mg h−1).
Responses were tested in male sheep, prenatally exposed to saline (○, n = 9), dexamethasone (0.48 mg h−1, •, n = 7) or cortisol (5 mg h−1, ▾, n = 6) from day 26 to 28 of gestation (term ∼150d). Results are shown as change (mean ± s.e.m.) of 60 min averages from the 1 h control period in mean arterial pressure (A), heart rate (B) and cardiac output (C). Two-way repeated measures ANOVA with post hoc Tukey's test was used to detect significant differences between groups. *P ≤ 0.05 compared to SAL group, #P ≤ 0.05 compared to CORT group.
Discussion
The major findings arising from the current study were that cardiovascular responses to central administration of angiotensin II, and to blockade of central nervous system AT1 receptors, were exaggerated in adult sheep that were briefly exposed to DEX in utero. These observations suggest that prenatal DEX exposure leads to increased responsiveness of central nervous system angiotensinergic mechanisms that influence circulatory control, to both exogenous and endogenous angiotensin II. This conclusion is consistent with our previous observation of greater AT1 receptor gene expression in the medulla oblongata of DEX-exposed fetuses (males and females at 130 days of gestation) and adults (7-year-old-females) compared with control animals (Dodic et al. 2002a). Indeed, these alterations in AT1 receptor gene expression after prenatal DEX exposure may directly underlie the alterations we observed in the current study, in responses to i.c.v. angiotensin II and losartan. This proposition is further supported by our observations in adult sheep exposed to CORT in utero. Unlike prenatal DEX exposure, prenatal CORT exposure does not cause altered brainstem AT1 receptor gene expression (Dodic et al. 2002b), and does not mimic the effects of prenatal DEX exposure on responses to i.c.v. angiotensin II and losartan.
As has been observed previously, i.c.v. angiotensin II dose-dependently increased MAP (Breuhaus & Chimoskey, 1990; May, 1996). In the current study, this pressor effect was associated with increased CO rather than TPR, and variable changes in HR and SV. This hyper-dynamic effect of i.c.v. infusion of angiotensin II is consistent with the recent demonstration that this treatment increases cardiac sympathetic nerve activity (Watson et al. 2004), which would be expected to promote increased CO. This could be mediated through increased cardiac contractility (and thus SV) and/or increased HR. We were able to detect significant increases in heart rate at least in the DEX group in response to 1 μg h−1 whilst SV showed a strong tendency to increase in response to 10 μg h−1. Thus both inotropic and chronotropic effects may be involved in the pressor response to i.c.v. angiotensin II that we observed. However, our observations contrast with those of a number of previous studies demonstrating pressor responses to i.c.v. angiotensin II associated with increased TPR but with no significant changes in CO or HR (Breuhaus & Chimoskey, 1990; May, 1996). This apparent contradiction may be explained, at least in part, by the availability of free access to water in the current study. When the thirst response was evoked by the i.c.v. angiotensin II, but not satiated in these previous studies, the mechanisms increasing blood pressure may have been different from those in the current study. Importantly, availability of water is unlikely to have confounded our major new finding, that pressor responses to i.c.v. angiotensin II are augmented in sheep prenatally exposed to DEX, but not CORT or SAL, since all groups of sheep drank similar amounts during angiotensin infusion. Thus, our results suggest that the sensitivity, to exogenous angiotensin II, of central nervous system mechanisms controlling CO, is increased in sheep prenatally exposed to DEX, but not those exposed to SAL or CORT.
Infusion of the AT1 receptor antagonist, losartan, decreased HR and CO in sheep prenatally exposed to DEX, but not those exposed to CORT or SAL. TPR was not significantly altered by losartan in any of the three groups, although the fact that MAP was not significantly reduced by losartan in the DEX group provides some evidence for a reflex increase in TPR. These results suggest that endogenous angiotensin II, acting at brain AT1 receptors, contributes to the tonic maintenance of HR and CO in adult sheep briefly exposed to DEX in utero. This does not appear to be the case in control sheep (SAL group), or in sheep briefly exposed to CORT in utero. We can be confident that the dose of losartan we used provided significant blockade of AT1 receptors in key cardiovascular control centres in the brain, without blockade of peripheral AT1 receptors, since it abolished pressor responses to i.c.v. but not i.v. angiotensin II. Taken collectively with our previous finding of increased AT1 receptor gene expression in the medulla oblongata of fetal and adult sheep exposed to DEX in utero, our observations with i.c.v. angiotensin II and losartan are consistent with the hypothesis that prenatal DEX exposure up-regulates central angiotensinergic control of arterial pressure. Because prenatal DEX exposure appears to predominantly affect control of CO rather than TPR, it seems likely that changes in angiotensinergic control of cardiac function, rather than control of sympathetic or neurohumoral vasomotor drive, dominate this effect (Dodic et al. 1999). The precise mechanisms underlying these effects of DEX exposure remain to be determined. One hypothesis that merits further investigation is that prenatal DEX exposure enhances central angiotensinergic control of cardiac sympathetic drive.
It is of interest that the differential responses to i.c.v. infusion of angiotensin II or losartan occurred despite there being no detectable differences in basal mean arterial pressure between the DEX and SAL groups. The blood pressure in the DEX animals was not found to be significantly elevated when measured over the control hour and CSF infusion. In contrast, the CORT group had significantly elevated arterial pressure compared to both other groups. This is despite both glucocorticoid treated groups having significantly elevated mean arterial pressure compared to SAL animals when measured previously over 3 days (Dodic et al. 2002a,b). Although the reasons for these differences are unclear, it does highlight the fact that short-term measurement of arterial pressure (over a couple of hours) may not be indicative of chronic basal arterial pressure.
Consistent with the results of previous studies (Breuhaus & Chimoskey, 1990), we found that i.c.v. angiotensin II dose-dependently increased water intake, and decreased plasma osmolality and electrolyte (Na+, K+ and Cl−) concentrations. Increased water intake may have contributed to the reduction in plasma osmolyte and electrolyte concentrations after angiotensin II infusion, since urinary fluid output during the 1 h infusion period was only ∼10% of water intake. However, our measurements of urinary fluid output are based on measurements of spontaneously voided volumes, so may not truly reflect total urine production. The fact that i.c.v. angiotensin II resulted in similar increases in water intake in DEX and CORT groups relative to the SAL group is consistent with our previous finding of little or no effect of prenatal glucocorticoid exposure on hypothalamic AT1 receptor gene expression (Dodic et al. 2002a,b), since angiotensin II-induced drinking is mediated via AT1 receptors in the hypothalamus (McKinley et al. 1996). The plasma cortisol response to i.c.v. angiotensin II, a second response dependent on hypothalamic AT1 receptors, was also similar between the groups, suggesting that hypothalamic angiotensinogeric mechanisms are largely unaffected by prenatal gluococorticoid treatment. The drinking response to angiotensin II has also been shown to be dependent upon different mechanisms from the pressor responses. Drinking responses involve activation of a protein kinase C and calcium/calmodulin-dependent protein kinase (Fleegal & Sumners, 2003) whilst pressor responses are mediated at least in part by increased superoxide production via a Rac-1-dependent NADPH oxidase (Zimmerman et al. 2004).
A potential limitation of our current studies relates to the fact that they were conducted in male offspring castrated within 2 weeks of birth. However, there are multiple lines of evidence indicating that the gonadal hormone status of these animals is unlikely to have confounded the major findings and conclusions derived from this study. Pressor responses to i.c.v. angiotensin II observed in the current study were of similar magnitude (10–12 mmHg for 1 μg h−1) to those observed previously in intact (Breuhaus & Chimoskey, 1990) or in ovariectomized female sheep (May (1996). It has also been shown that pulmonary pressor responsiveness to hypoxia is similar in intact male adult sheep compared with animals of a similar age that were castrated at birth (Wetzel & Sylvester, 1983). Stress responses of the hypothalamic–pituitary–adrenal axis vary by sex in Merino sheep, irrespective of whether the animals are intact or gonadectomised (Turner et al. 2002). Finally, whilst there are differences between MAP in adult male and female gonadectomised sheep (Dodic et al. 2002b), gonadal hormones have not been shown to affect mean arterial pressure in female sheep (Keller-Wood, 2000).
The reasons for the differential effects of DEX and CORT on brain angiotensinergic circulatory control are unclear, but may relate to differences in their profile of efficacy at the various receptors that are activated by steroids. For example, DEX exerts effects on the brain and peripheral circulation through pregnane-X receptors (PXR), particularly the PXR2 isoform, which does not recognize cortisol to any appreciable extent (Kliewer et al. 2002). In contrast, the mineralocorticoid receptor (MR) in the brain, which can be activated by CORT, can bind DEX but is not trans activated by the synthetic steroid (Rogerson et al. 2003). In adult sheep and rats, the MR is thought to be responsible for mediating the effects of glucocorticoids on neurogenesis (Montaron et al. 2003; Richards et al. 2003). Finally, in the human, there are multiple isoforms of the glucocorticoid receptors which may be differentially activated by various glucocorticoid ligands (Lu & Cidlowski, 2004), although the existence of multiple isoforms in the sheep remains to be explored. Importantly, consideration of these pharmacodynamic differences between DEX and CORT may well provide valuable insights into the mechanisms that control development of the brain RAS.
Conclusion
In conclusion, brief prenatal DEX exposure in sheep appears to programme up-regulation of brain angiotensinergic circulatory control mechanisms, particularly with respect to control of cardiac output. This may represent the functional correlate of increased brainstem AT1 receptor gene expression, we have previously demonstrated, after brief in utero DEX exposure. The precise mechanisms underlying this consequence of prenatal DEX exposure remain unknown. Our present observations also raise the possibility that the mechanisms underlying elevated arterial pressure in DEX- and CORT-exposed adults may differ. This proposition merits further investigation.
Acknowledgments
We would like to thank Debbie Bartal and Jan Loose for assistance with the cortisol assay. This work was supported by a project grant from the National Health and Medical Research Council (NH & MRC) of Australia 236851. M.D. is the recipient of a NH & MRC R. D. Wright Fellowship and K.M.M. a NH & RMC Peter Doherty Fellowship. C.N.M. and R.G.E. are Senior Research Fellows of the NH & MRC.
References
- Barker DJP. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–23. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Bocking AD, McMillen IC, Harding R, Thorburn GD. Effect of reduced uterine blood flow on fetal and maternal cortisol. J Dev Physiol. 1986;8:237–245. [PubMed] [Google Scholar]
- Breuhaus B, Chimoskey J. Hemodynamic and behavioural effects of angiotensin II in conscious sheep. Am J Physiol. 1990;258:R1230–R1237. doi: 10.1152/ajpregu.1990.258.5.R1230. [DOI] [PubMed] [Google Scholar]
- Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002a;40:729–734. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour E, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002b;16:1017–1026. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- Dodic M, Peers A, Coghlan J, May C, Lumbers E, Yu Z, Wintour E. Altered cardiovascular haemodynamics and baroreceptor-heart rate reflex in adult sheep after prenatal exposure to dexamethasone. Clin Sci. 1999;97:103–109. [PubMed] [Google Scholar]
- Dodic M, Samuel C, Moritz K, Wintour E, Morgan J, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res. 2001;89:623–629. doi: 10.1161/hh1901.097086. [DOI] [PubMed] [Google Scholar]
- Fleegal MA, Sumners C. Angiotensin II induction of AP-1 in neurons requires stimulation of PI3-K and JNK. Biochem Biophys Res Commun. 2003;310:470–477. doi: 10.1016/j.bbrc.2003.09.047. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Jackson AA, Langley-Evans SC. Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension. 1997;30:1525–1530. doi: 10.1161/01.hyp.30.6.1525. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M. Effects of a simulated estrous cycle on sodium, volume, ACTH, and AVP in sheep. Domest Anim Endocrinol. 2000;18:31–40. doi: 10.1016/s0739-7240(99)00061-2. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc. 2001;60:505–513. doi: 10.1079/pns2001111. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann N Y Acad Sci. 2004;1024:102–23. doi: 10.1196/annals.1321.008. [DOI] [PubMed] [Google Scholar]
- Mathai M, Evered M, McKinley M. Central losartan blocks natriuretic, vasopressin and pressor responses to central hypertonic NaCl in sheep. Am J Physiol. 1998;275:R548–R554. doi: 10.1152/ajpregu.1998.275.2.R548. [DOI] [PubMed] [Google Scholar]
- May C. Prolonged systemic and regional haemodynamic effects of intracerebroventricular angiotensin II in conscious sheep. Clin Exp Pharmacol Physiol. 1996;23:878–884. doi: 10.1111/j.1440-1681.1996.tb01137.x. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol. 1996;3:S99–S104. [PubMed] [Google Scholar]
- Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur J Neurosci. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108(Suppl. 3):545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1048. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- Richards EM, Hua Y, Keller-Wood M. Pharmacology and physiology of ovine corticosteroid receptors. Neuroendocrinology. 2003;77:2–14. doi: 10.1159/000068335. [DOI] [PubMed] [Google Scholar]
- Rogerson FM, Yao YZ, Smith BJ, Dimopoulos N, Fuller PJ. Determinants of spironolactone binding specificity in the mineralocorticoid receptor. J Mol Endocrinol. 2003;31:573–582. doi: 10.1677/jme.0.0310573. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- Sherman R, Langley-Evans S. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci. 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- Turner AI, Canny BJ, Hobbs RJ, Bond JD, Clarke IJ, Tilbrook AJ. Influence of sex and gonadal status of sheep on cortisol secretion in response to ACTH and on cortisol and LH secretion in response to stress: importance of different stressors. J Endocrinol. 2002;173:113–122. doi: 10.1677/joe.0.1730113. [DOI] [PubMed] [Google Scholar]
- Watson AM, Mogulkoc R, McAllen RM, May CN. Stimulation of cardiac sympathetic nerve activity by central angiotensinergic mechanisms in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1051–R1056. doi: 10.1152/ajpregu.00708.2003. [DOI] [PubMed] [Google Scholar]
- Wetzel RC, Sylvester JT. Gender differences in hypoxic vascular response of isolated sheep lungs. J Appl Physiol. 1983;55:100–104. doi: 10.1152/jappl.1983.55.1.100. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]