Abstract
Vascular oxidative stress is the key mechanism involved in the age-related decline in endothelium-dependent dilatation (EDD). We tested the hypothesis that xanthine oxidase (XO), a major vascular source of reactive oxygen species, contributes to the impairment in EDD with ageing. At baseline, brachial artery flow-mediated dilatation (FMD) was 55% lower in older (n = 9, 64 ± 2 years, 8M/1F, mean ± s.e.m.) versus young (n = 9, 26 ± 1 years, 8M/1F) healthy adults (3.41 ± 0.44 versus 7.53 ± 0.67%, P < 0.001), whereas endothelium-independent dilatation (EID; sublingual nitroglycerin) did not differ between groups. Plasma oxidized low-density lipoprotein (oxi-LDL), a measure of systemic oxidative stress, was greater at baseline in the older subjects (58.3 ± 5.9 versus 46.8 ± 2.4 U l−1, P < 0.05) and inversely correlated with baseline FMD (r = −0.54; P < 0.05). Acute administration of allopurinol, a competitive inhibitor of XO, reduced plasma uric acid concentrations similarly in both groups (P < 0.001), but did not affect FMD, EID, or oxi-LDL in either group. Vascular endothelial protein expression of XO (immunofluorescence) was not different in antecubital venous cells from the young and older subjects (0.56 ± 0.12 versus 0.68 ± 0.19 XO intensity/human umbilical vein endothelial cell intensity, P = 0.49). We conclude that XO does not contribute to oxidative stress-associated reductions in peripheral conduit artery EDD with ageing in humans, possibly due to an absence of age-associated up-regulation of endothelial XO.
Ageing is a major atherogenic risk factor (Lakatta & Levy, 2003). A central feature of vascular ageing is a reduction in endothelium-dependent dilatation (EDD) (Celermajer et al. 1994; DeSouza et al. 2000; Taddei et al. 2001). Impaired EDD, in turn, is an independent predictor of coronary artery disease risk in the elderly (Lind et al. 2005). As such, determining the mechanisms that cause impaired EDD with ageing has important clinical implications for the prevention of age-associated atherosclerotic diseases.
Vascular oxidative stress is the key mechanism mediating the age-related decline in EDD in sedentary healthy adults (Taddei et al. 2001; Eskurza et al. 2004). Oxidative stress suppresses EDD in older adults primarily by decreasing nitric oxide (NO) bioavailability (Taddei et al. 2001). Consistent with these observations in humans, in animal models vascular ageing is associated with an increased production of superoxide anions (van der Loo et al. 2000; Hamilton et al. 2001; Csiszar et al. 2002), which rapidly react with NO to form peroxynitrite, thus decreasing NO bioavailability (Milstien & Katusic, 1999). However, currently there is little or no information concerning the cellular and molecular mechanisms underlying vascular oxidative stress and impaired EDD with ageing in humans.
The xanthine oxidase (XO) system is an important sources of reactive oxygen species (ROS) in the vascular endothelium (Houston et al. 1999). XO-produced superoxide anions can react with NO to form peroxynitrite and reduce NO bioavailability (Godber et al. 2000). In agreement with this, XO inhibition with allopurinol (a competitive inhibitor) or oxypurinol (its active metabolite) improves EDD in certain states characterized by oxidative stress such as hypercholesterolaemia, congestive heart failure, chronic smoking and diabetes (Cardillo et al. 1997; Butler et al. 2000; Farquharson et al. 2002; Guthikonda et al. 2003, 2004).
In the present study, we tested the hypothesis that XO contributes to the impairment in peripheral conduit artery EDD with ageing in humans. To do so, we conducted a crossover study in which brachial artery flow-mediated dilatation (FMD), a measure of conduit artery EDD, and systemic oxidative stress, assessed by plasma oxidized low-density lipoprotein (oxi-LDL) concentrations, were measured at baseline (placebo control condition) and after acute oral administration of allopurinol in young and older adults. To gain insight into the molecular mechanisms involved, vascular endothelial cell protein expression of XO was measured using quantitative immunofluorescence (Feng et al. 1999; Colombo et al. 2002; Colombo et al. 2005).
Methods
Subjects
A total of 18 healthy sedentary adults were studied: nine young (8 men and 1 woman, aged 21–34 years) and nine older (8 men and 1 woman, aged 55–71 years). Subjects were normotensive (blood pressure (BP) < 140/90), non-smokers, non-obese and free of clinical diseases as assessed by medical history, physical examination, blood chemistry, and resting and exercise ECG (older group only). Subjects classified as having the metabolic syndrome by the criteria of the National Cholesterol Education Program Adult Treatment Panel III expert panel or a modification of the original World Health Organization definition were excluded (Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults, 2001; Lakka et al. 2002). Candidates who used antioxidants (e.g. vitamin C and E) within 6 weeks of the study or were taking other medications were excluded. Subjects gave their written informed consent to participate. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder and conformed to the Declaration of Helsinki.
Study design
We conducted a double-blind crossover study in which participants ingested a single dose of placebo or allopurinol (600 mg) in randomized order. The 600 mg dose of allopurinol was chosen because it previously has been shown to restore EDD in human subjects with impaired EDD (Guthikonda et al. 2003, 2004). The randomization of treatments was performed by the University of Colorado at Boulder General Clinical Research Center (GCRC) pharmacist. The investigators who performed data acquisition and analyses were blinded to group and treatment condition. Measurements were performed 4–5 h after the ingestion of allopurinol or placebo as previously described (Guthikonda et al. 2003).
Experimental procedures
All procedures were performed at the University of Colorado at Boulder GCRC. After completion of screening procedures, two main experimental sessions (i.e. placebo baseline and allopurinol treatment) were conducted within 7 days of each other (Guthikonda et al. 2004). Prior to the main sessions subjects fasted for 10–12 h. Subjects were studied in the supine position and instrumented with an intravenous catheter in one arm for acquisition of blood.
Brachial artery FMD and endothelium-independent dilatation (EID)
Brachial artery FMD and EID were measured using duplex ultrasonography as previously described by our laboratory (Eskurza et al. 2004, 2005). Reactive hyperaemia was produced by inflating a blood pressure cuff placed on the upper forearm for 5 min at 250 mmHg followed by a rapid deflation. Brachial artery baseline and peak (post-ischaemia) diameters were analysed off-line with an automatic wall tracking system (Vascular Analysis Tools, 4.0, Medical Imaging Analysis, LLC, Coralville, IA, USA). End-occlusion (i.e. end-ischaemia) diameters were obtained during the last 15–20 s of the occlusion period. Baseline and peak mean blood velocities were measured as previously described (Eskurza et al. 2004, 2005). Shear stress (SS) averaged over the entire cycle was calculated using the following formula: SS (dyne × cm−2) = 8μv/D, where μ, v and D represented viscosity (dyne × s × cm−2), velocity (cm × s−1) and diameter (cm), respectively (Mitchell et al. 2004; Eskurza et al. 2005). To calculate baseline SS (SSbase), baseline velocity and diameter were used (Eskurza et al. 2005). To calculate peak SS (SSpeak), peak velocity and the corresponding diameter obtained during the last 15–20 s of occlusion were used (Eskurza et al. 2005). Whole blood viscosity was measured using a viscometer, as previously described by our laboratory (Dinenno et al. 2001; Moreau et al. 2002). Brachial FMD was calculated as absolute and percentage change from baseline. In addition, FMD was normalized for local shear stress by the following formula:
as recently described (Eskurza et al. 2005). The coefficient of variation for trial-to-trial reliability for baseline diameter, peak diameter and FMD (Δ%) are 0.3, 0.6, and 8.1%, respectively (Eskurza et al. 2004, 2005).
Vascular endothelial cell protein expression
Protein expression was measured in vascular endothelial cells as previously described (Colombo et al. 2002). Following instrumentation, but prior to any experimental procedures, endothelial cells were collected from an antecubital vein using sterile J-wires briefly advanced through the left arm catheter. Endothelial cells were recovered from the wires, fixed with 3.7% formaldehyde, and plated on poly l-lysine coated slides (Sigma Chemical Co., St Louis, MO, USA). For immunofluorescence staining, cells were rehydrated with phosphate-buffered saline (PBS) and rendered permeable using 0.1% Triton X-100. After blocking non-specific binding sites with 5% donkey serum, cells were incubated with a primary antibody for XO (US Biological, Swampscott, MA, USA) followed by an antirabbit (Research Diagnostics, Inc., Concord, MA, USA) or antigoat (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) secondary CY3 antibody, respectively. For analysis, slides were viewed using a fluorescence microscope (Nikon Eclipse 600) and were digitally captured by a Photometrics CoolSNAPfx digital camera (Roper Scientific, Inc., Tucson, AZ, USA). Endothelial cells were identified by the presence of vWF staining and nuclear integrity was confirmed using 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI) staining. Once endothelial cells with intact nuclei were identified, images were captured and analysed using Metamorph Software (Universal Imaging Corp., Downingtown, PA, USA). The intensity of CY3 staining was used to quantify endothelial cell protein expression (ECPE). Values are reported as ratios of XO ECPE/human umbilical vein endothelial cell (HUVEC; control cells) ECPE (i.e. XO intensity/HUVEC intensity). Reporting ratios minimizes the possible confound of differences in intensity of staining among different staining sessions.
Arterial blood pressure
Resting blood pressure was measured over the brachial artery using a semiautomated device (Dynamap XL, Johnson and Johnson).
Plasma marker of oxidative stress and uric acid
Plasma samples were analysed for oxidized low-density lipoprotein (oxi-LDL) (ALPCO Diagnostics, Windham, NH, USA) and uric acid using the uricase reaction (Beckman Synchron LXI-20 System; Beckman Coulter Inc., Fullerton, CA, USA).
Body composition
Percentage body fat was measured with dual-energy X-ray absorptiometry (DXA-GE; Lunar Corporation, Madison, WI, software version 5.60.003), and waist and hip circumferences and body mass index (BMI) with anthropometry (Van Pelt et al. 1998).
Statistical analyses
Statistical analyses were performed with the SPSS statistical package (version 11.0; SPSS, Chicago, IL, USA). Differences in subject characteristics between the two groups were determined by Student's t test for independent group comparisons. Differences in brachial artery FMD between allopurinol and placebo conditions in young and older subjects were examined using repeated measures ANOVA with one within-subjects factor (allopurinol versus placebo) factor and one between-subjects factor (young versus older subjects). The critical test for the dependent variable was the interaction between the within-subjects and between-subjects factor; no within subject differences for young subjects, but large treatment differences for older subjects were expected. Differences in EID were examined as described above for FMD. No significant treatment by group interaction was expected for EID. Bivariate correlations were performed with Pearson product–moment correlations to examine relations of interest. Significance was set at P < 0.05. Values are means ± s.e.m.
Results
Subject characteristics
Clinical characteristics of the groups are shown in Table 1. Percentage body fat, waist circumference, waist-to-hip ratio, plasma total cholesterol and low-density lipoprotein–cholesterol, fasting glucose and diastolic blood pressure were higher in the older than in the young adults (P < 0.05). There were no other significant group differences.
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| Age (years) | 26 ± 1 | 64 ± 2* |
| Body mass (kg) | 77 ± 4 | 81 ± 4 |
| Body fat (%) | 19.5 ± 3.1 | 28.0 ± 2.9* |
| BMI (kg m−2) | 24.1 ± 1.3 | 26.3 ± 0.8 |
| Waist circumference (cm) | 80.8 ± 2.8 | 92.6 ± 4.0* |
| WHR | 0.84 ± 0.02 | 0.94 ± 0.03* |
| Total cholesterol (mmol l−1) | 4.1 ± 0.1 | 5.3 ± 0.3* |
| LDL cholesterol (mmol l−1) | 2.4 ± 0.1 | 3.3 ± 0.3* |
| HDL cholesterol (mmol l−1) | 1.1 ± 0.1 | 1.4 ± 0.1 |
| Triglycerides (mmol l−1) | 1.1 ± 0.1 | 1.3 ± 0.2 |
| Plasma glucose (mmol l−1) | 4.6 ± 0.1 | 5.5 ± 0.2* |
| Systolic BP (mmHg) | 110 ± 2 | 108 ± 3 |
| Diastolic BP (mmHg) | 60 ± 3 | 71 ± 3* |
| Heart rate (bpm) | 59 ± 3 | 59 ± 3 |
Data are means ± s.e.m. BMI, body mass index; WHR, waist to hip ratio; 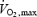 , maximal oxygen consumption; BP, blood pressure.
, maximal oxygen consumption; BP, blood pressure.
P < 0.05 versus young.
Allopurinol treatment effect
Plasma uric acid concentrations were not different in the young and older adults during baseline placebo control conditions, but were reduced (P < 0.001) in both groups after the administration of allopurinol (placebo control versus allopurinol, young: 356.1 ± 20.8 versus 307.8 ± 21.4 μmol l−1; older: 387.9 ± 27.5 versus 347.7 ± 34.8 μmol l−1).
Oxi-LDL was 25% higher (P < 0.05) in the older compared with the young adults during baseline placebo control conditions, but did not change in response to allopurinol (placebo control versus allopurinol, young: 46.8 ± 2.4 versus 48.1 ± 2.7 IU; older: 58.3 ± 5.9 versus 58.2 ± 5.5 IU).
Brachial artery dilatation
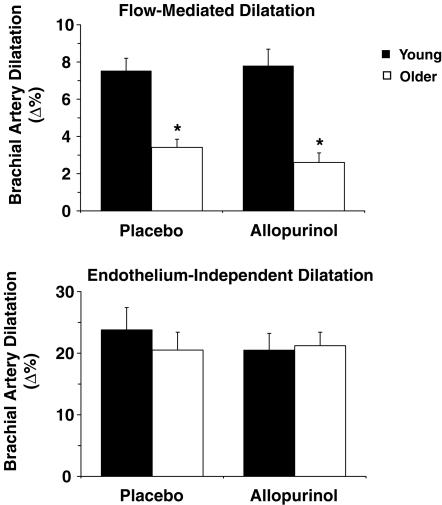
Brachial artery FMD (Δ%) was 55% lower in the older compared with the young adults (P < 0.001), whereas brachial artery EID (Δ%) was not different in the two groups (Fig. 1). Allopurinol administration did not affect brachial FMD or EID in either group (Fig. 1). Baseline group differences and responses to allopurinol were not affected when brachial FMD was expressed as absolute change (Δ mm) or normalized for peak shear stress (Table 2).
Figure 1.
Endothelium-dependent and -independent dilatation during baseline placebo conditions and in response to acute allopurinol administration Brachial artery flow-mediated dilatation (FMD) (top panel) and endothelium independent dilatation (EID) (bottom panel) in young (filled bars) and older (open bars) adults after acute oral placebo or allopurinol administration. FMD was lower in the older adults under both conditions, but EID was not different between groups in either condition. Allopurinol had no effect on FMD or EID in either group. Means ± s.e.m. are shown. *P < 0.001 versus young adults.
Table 2.
Brachial artery responses
| Young | Older | |||
|---|---|---|---|---|
| Parameter | Placebo | Allopurinol | Placebo | Allopurinol |
| Baseline D (mm) | 3.99 ± 0.19 | 3.97 ± 0.18 | 4.28 ± 0.22 | 4.34 ± 0.21 |
| Post-occlusion D (mm) | 3.90 ± 0.19 | 3.92 ± 0.20 | 4.25 ± 0.23 | 4.25 ± 0.23 |
| Baseline MBV (cm × s−1) | 5.0 ± 0.6 | 5.3 ± 0.5 | 4.5 ± 0.5 | 4.6 ± 0.4 |
| Peak MBV (cm × s−1) | 42.0 ± 3.7 | 42.3 ± 3.9 | 36.2 ± 2.5 | 33.4 ± 4.1 |
| SSbase (dyne × cm−2) | 3.6 ± 0.3 | 3.8 ± 0.2 | 2.9 ± 0.2 | 2.9 ± 0.3* |
| SSpeak (dyne × cm−2) | 31.9 ± 3.5 | 33.0 ± 3.5 | 22.5 ± 2.0* | 22.0 ± 1.9* |
| FMD (Δmm) | 0.29 ± 0.02 | 0.30 ± 0.03 | 0.14 ± 0.01* | 0.12 ± 0.02* |
| nFMD (Δmm/dyne × cm−2) × 103 | 9.8 ± 0.9 | 9.4 ± 1.4 | 5.9 ± 0.7* | 5.5 ± 1.4* |
| EID (Δ%) | 23.8 ± 3.6 | 21.9 ± 2.9 | 20.5 ± 2.7 | 21.2 ± 2.2 |
Data are mean ± s.e.m. D, diameter; MBV, mean blood velocity; FMD, flow-mediated dilatation; SSbase, baseline shear stress; SSpeak, peak shear stress; EID, endothelium-independent dilatation; nFMD = normalized FMD
P < 0.05 versus young.
Baseline and post-occlusion brachial artery diameters, and baseline and peak brachial artery mean blood velocities were not significantly different between groups, whereas baseline and peak shear stress were lower in the older adults (Table 2). Allopurinol administration did not affect any of these parameters.
Relations among systemic oxidative stress, age and brachial artery FMD
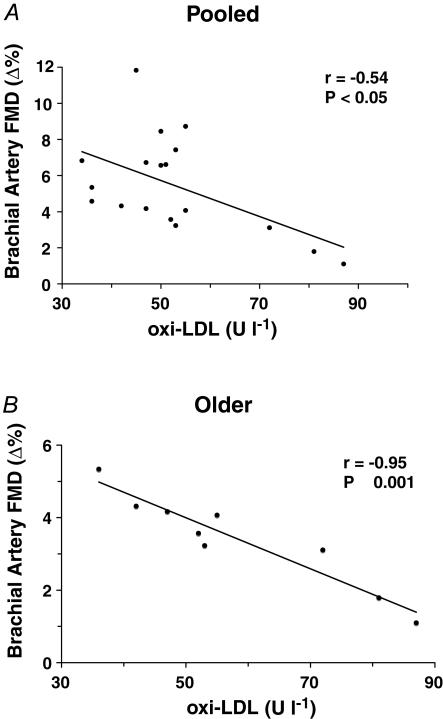
Plasma oxi-LDL was moderately positively related to age in the pooled sample (r = 0.53; P < 0.05) and strongly positively related to age within the older subjects (r = 0.77; P < 0.01). oxi-LDL was moderately inversely related to baseline FMD (Δ%) in the pooled sample (r = −0.54; P < 0.05) and highly inversely related to baseline FMD (Δ%) within the older adults (r = −0.95; P < 0.001) (Fig. 2). Baseline FMD (Δ%) was strongly inversely related to age within the pooled sample (r = −0.77; P < 0.001) and the older adults (r = −0.67; P < 0.05).
Figure 2. Relations between endothelium-dependent dilatation and systemic oxidative stress.
Brachial artery FMD under baseline placebo conditions was inversely related to plasma oxidized-LDL concentrations in the pooled sample (A) and among older adults (B).
Vascular endothelial cell protein expression
Vascular endothelial protein expression of XO was not different in the two groups (young: 0.56 ± 0.12 versus older: 0.68 ± 0.19 XO intensity/HUVEC intensity, P = 0.49).
Discussion
The results of the present study extend current insight into the mechanisms contributing to oxidative stress-associated impairment of EDD with ageing in humans. The key new finding was that inhibition of XO does not improve brachial artery FMD in older adults. Importantly, vascular endothelial protein expression of XO was not different in young and older adults. We also found that brachial FMD was inversely related to plasma oxi-LDL levels. Thus, our results suggest that XO does not contribute to oxidative stress-associated reductions in peripheral conduit artery EDD with ageing in humans, possibly due to an absence of age-associated up-regulation of endothelial XO.
EDD is markedly reduced with ageing, even in healthy adults (Celermajer et al. 1994; DeSouza et al. 2000; Taddei et al. 2001; Eskurza et al. 2004). Our laboratory and others recently demonstrated that oxidative stress is the key mechanism involved in the age-related impairment in EDD (Taddei et al. 2001; Eskurza et al. 2004). In agreement with these findings, in the present study brachial artery FMD was 55% lower in older compared with young healthy adults, whereas EID was not different. We also found that plasma oxi-LDL, a systemic marker of oxidative stress, was greater in the older adults. Importantly, oxi-LDL was inversely related to baseline brachial FMD within both the pooled sample and the group of older adults, whereas there was no relation between oxi-LDL and EID. Taken together, these observations suggest that oxidative stress was increased in our older subjects and was selectively associated with reductions in EDD.
In the present study, acute inhibition of XO, a major ROS producing enzyme within the vasculature (Houston et al. 1999), with allopurinol did not affect oxi-LDL in either group, suggesting that XO does not contribute significantly to age-associated increases in oxidative stress. Consistent with this, XO inhibition did not affect brachial artery FMD in either the young or the older subjects. This is in contrast to recent findings that XO inhibition with acute or chronic oral allopurinol administration or intravenous infusions of its active metabolite, oxypurinol, improves EDD in some other oxidative stress states (Cardillo et al. 1997; Butler et al. 2000; Farquharson et al. 2002; Guthikonda et al. 2003, 2004). Consistent with the present findings, however, inhibition of XO did not improve EDD in patients with essential hypertension (Cardillo et al. 1997), indicating that XO is not mechanistically involved in impaired EDD in all states associated with oxidative stress.
To gain insight into the molecular mechanisms involved, in the present study we measured vascular endothelial expression of XO protein. Although there are no data available in humans, several previous experimental observations supported the possibility that XO protein might be increased in older adults. First, inflammatory cytokines have been reported to increase with ageing in humans (Krabbe et al. 2004; Ungvari et al. 2004), and are known to up-regulate XO in vascular endothelial cells via increases in gene expression (Dupont et al. 1992). Second, NO inhibits XO activity in endothelial cells (Hassoun et al. 1995; Cote et al. 1996), and endothelial NO bioavailability decreases with ageing (Taddei et al. 2001; Singh et al. 2002). Third, peroxynitrite converts xanthine dehydrogenase to XO, the isoform responsible for the production of superoxide anions and hydrogen peroxide (Sakuma et al. 1997). Vascular peroxynitrite formation increases with ageing (van der Loo et al. 2000) and, thus, could stimulate an increase in XO protein expression in endothelial cells. Moreover, in hypercholesterolaemic patients baseline plasma XO activity is increased and allopurinol administration improves EDD (Cardillo et al. 1997; Spiekermann et al. 2003). However, in the present study we found no differences in vascular endothelial expression of XO protein in young and older sedentary healthy humans. These observations are in agreement with the lack of effect of allopurinol administration on EDD in the present study, as well as with previous findings of unchanged XO protein abundance in coronary arteries of older rats (Csiszar et al. 2002).
Collectively, these observations suggest that other vascular sources of superoxide anions such as NAD(P)H oxidase (Hamilton et al. 2001; Csiszar et al. 2002), uncoupled eNOS (Cosentino et al. 1998; Eskurza et al. 2005) and/or mitochondria (Beckman & Ames, 1998; van der Loo et al. 2000) may be involved in the increased superoxide anion production and development of vascular oxidative stress with ageing in healthy adult humans. This idea previously has been advanced based on findings in animal models (van der Loo et al. 2000; Hamilton et al. 2001; Csiszar et al. 2002; Bachschmid et al. 2004). Consistent with this possibility, the inhibition of NAD(P)H oxidase, and not inducible NO synthase, XO or cyclooxygenase, decreased vascular ROS generation in coronary vessels of older rats (Csiszar et al. 2002), suggesting that NAD(P)H oxidase is a major source of vascular ROS with ageing (Hamilton et al. 2001; Csiszar et al. 2002). In addition, we recently demonstrated that tetrahydrobioterin (BH4) administration restored brachial FMD in older sedentary humans, suggesting that BH4 bioactivity may be decreased with ageing. In the presence of a deficiency of this essential cofactor, eNOS is uncoupled and becomes a major source of ROS within the vasculature (Vasquez-Vivar et al. 1998). Finally, increased nitration of mitochondrial manganese superoxide dismutase in aortic tissue of older rats suggests that the mitrochondria also may be a major source of superoxide anions in vascular ageing (van der Loo et al. 2000).
Several limitations and alternative explanations related to our results should be mentioned. Because a single 600 mg oral dose of allopurinol was used, we cannot exclude the possibility that a more chronic regimen could have produced a greater inhibitory effect on XO that may have increased EDD in our older subjects. However, in the present study plasma uric acid concentrations decreased significantly in response to a single dose, indicating that cellular XO was inhibited. Furthermore, pharmacokinetic studies show that an oral dose of 600 mg achieves plasma concentrations that would inhibit XO activity by > 95% (Day et al. 1988). Thus, it is unlikely that inadequate XO inhibition explains the lack of effect of allopurinol on EDD in our older subjects.
As previously described (Colombo et al. 2002, 2005), we measured endothelial XO protein expression in cells obtained from veins because of the invasiveness and associated risk required to obtain arterial cells, particularly in older adults. However, we find significant positive correlations between endothelial expression of proteins in cells from veins compared with arteries in humans (authors' unpublished results). Thus, although absolute XO protein expression could differ between arteries and veins, the latter should provide an effective probe to determine age-associated differences, if present. Indeed, expected differences in vascular endothelial protein expression between healthy controls and patients with cardiovascular disease are observed in cells obtained from peripheral veins using the same procedures as those employed in the present study (Colombo et al. 2002).
We could not measure XO messenger RNA (mRNA) expression and/or enzyme activity because the number of endothelial cells that can be obtained with this technique is insufficient to perform these additional measurements. However, proteins are the key link between gene expression and physiological function, and thus determining XO protein expression in the vascular endothelium represented an appropriate first-step molecular assessment to add insight into our brachial artery responses. We recognize the possibility of age-associated differences in XO mRNA and/or post-translational modifications to XO.
It is possible that the reduced brachial FMD in the older subjects is mediated by greater vascular smooth muscle tone produced by increased sympathetic nerve activity or bioactivity of vasoconstrictors such as endothelin-1 or angiotensin II. However, sympathetic α-adrenergic vasoconstrictor tone is reduced, not increased, in the forearm of older adults (Dinenno et al. 2002), and blockade of the renin–angiotensin system has no effect on FMD in older humans (Rajagopalan et al. 2002). Thus, these mechanisms do not appear to be involved. We are unaware of any published information regarding the possible role of endothelin-1 on age-associated reductions in FMD. However, preliminary data indicate that forearm vasoconstrictor responsiveness to endothelin-1 is augmented in older humans, and that the forearm blood flow responses to acetylcholine may be increased during endothelin-1 receptor blockade in older adults (C. DeSouza, unpublished data). Thus, elevated endothelin-1 bioactivity could tonically suppress EDD with ageing in humans.
Finally, based on the available experimental evidence, FMD measured with the model used in the present study is believed to be dependent in part on the bioavailability of NO (Joannides et al. 1995; Mullen et al. 2001; Pyke & Tschakovsky, 2005). However, it is unknown if the contribution of NO to FMD differs with ageing or other physiological or pathophysiological states. As such, we cannot dismiss this possibility.
Conclusion
The results of the present study provide the first experimental evidence in humans that XO does not contribute to the oxidative stress-associated impairment in EDD with ageing. Our findings also suggest that the absence of a significant age-associated increase in XO protein expression in the vascular endothelium may be a key molecular mechanism underlying the lack of contribution of XO to reduced EDD in older adults.
Acknowledgments
This study was supported by NIH awards AG006537, AG013038, AG022241, and RR00051.
References
- Bachschmid M, van der Loo B, Schuler K, Labugger R, Thurau S, Eto M, Kilo J, Holz R, Luscher TF, Ullrich V. Oxidative stress-associated vascular aging is independent of the protein kinase C/NAD(P)H oxidase pathway. Arch Gerontol Geriatr. 2004;38:181–190. doi: 10.1016/j.archger.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. Mitochondrial aging: open questions. Ann N Y Acad Sci. 1998;854:118–127. doi: 10.1111/j.1749-6632.1998.tb09897.x. [DOI] [PubMed] [Google Scholar]
- Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Patton S, d'Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Luscher TF. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote CG, Yu FS, Zulueta JJ, Vosatka RJ, Hassoun PM. Regulation of intracellular xanthine oxidase by endothelial-derived nitric oxide. Am J Physiol. 1996;271:L869–L874. doi: 10.1152/ajplung.1996.271.5.L869. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Day RO, Miners J, Birkett DJ, Graham GG, Whitehead A. Relationship between plasma oxipurinol concentrations and xanthine oxidase activity in volunteers dosed with allopurinol. Br J Clin Pharmacol. 1988;26:429–434. doi: 10.1111/j.1365-2125.1988.tb03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534:287–295. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont GP, Huecksteadt TP, Marshall BC, Ryan US, Michael JR, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Invest. 1992;89:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adults Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- Feng L, Stern DM, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology. 1999;212:655–664. doi: 10.1148/radiology.212.3.r99au28655. [DOI] [PubMed] [Google Scholar]
- Godber BL, Doel JJ, Durgan J, Eisenthal R, Harrison R. A new route to peroxynitrite: a role for xanthine oxidoreductase. FEBS Lett. 2000;475:93–96. doi: 10.1016/s0014-5793(00)01639-2. [DOI] [PubMed] [Google Scholar]
- Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- Guthikonda S, Woods K, Sinkey CA, Haynes WG. Role of xanthine oxidase in conduit artery endothelial dysfunction in cigarette smokers. Am J Cardiol. 2004;93:664–668. doi: 10.1016/j.amjcard.2003.11.046. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Hassoun PM, Yu FS, Zulueta JJ, White AC, Lanzillo JJ. Effect of nitric oxide and cell redox status on the regulation of endothelial cell xanthine dehydrogenase. Am J Physiol. 1995;268:L809–L817. doi: 10.1152/ajplung.1995.268.5.L809. [DOI] [PubMed] [Google Scholar]
- Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly. The prospective investigation of the vasculature in uppsala seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25:2368–2375. doi: 10.1161/01.ATV.0000184769.22061.da. [DOI] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, Dinenno FA, Blackett SD, Hoetzer GL, Desouza CA, Tanaka H. Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol. 2002;283:H1409–H1417. doi: 10.1152/ajpheart.00035.2002. [DOI] [PubMed] [Google Scholar]
- Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Brook R, Mehta RH, Supiano M, Pitt B. Effect of losartan in aging-related endothelial impairment. Am J Cardiol. 2002;89:562–566. doi: 10.1016/s0002-9149(01)02297-4. [DOI] [PubMed] [Google Scholar]
- Sakuma S, Fujimoto Y, Sakamoto Y, Uchiyama T, Yoshioka K, Nishida H, Fujita T. Peroxynitrite induces the conversion of xanthine dehydrogenase to oxidase in rabbit liver. Biochem Biophys Res Commun. 1997;230:476–479. doi: 10.1006/bbrc.1996.5983. [DOI] [PubMed] [Google Scholar]
- Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond) 2002;102:595–600. [PubMed] [Google Scholar]
- Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107:1383–1389. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaley G. Vascular inflammation in aging. Herz. 2004;29:733–740. doi: 10.1007/s00059-004-2625-x. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt RE, Davy KP, Stevenson ET, Wilson TM, Jones PP, Desouza CA, Seals DR. Smaller differences in total and regional adiposity with age in women who regularly perform endurance exercise. Am J Physiol. 1998;275:E626–E634. doi: 10.1152/ajpendo.1998.275.4.E626. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]