Abstract
Previous In vivo studies revealed that the mixed agonist-antagonist buprenorphine can down-regulate μ and up-regulate δ2 and κ1, opioid receptors in rat brain. In this report brain regional differences in opioid receptor adaptation were addressed. Rats received i.p. injections with buprenorphine (0.5–2.5 mg/kg) and were killed 20 h later. Membranes from 7 brain regions were analyzed for μ (3H-[D-Ala2,N-mephe4,Gly-ol5] enkephalin), κ1, (3H-U-69593), δ1(3H-[D-Pen2,D-Pen5] enkephalin) and δ2 (3H-deltorphin II) receptor binding parameters. Buprenorphine induced down-regulation of μ receptors in frontal cortex, occipital cortex, thalamus, hippocampus, striatum and brain stem. Kd values for 3H-[D-Ala2,N-mephe4,Gly-ol5] enkephalin were unchanged from controls. Up-regulation of κ1, receptors was observed in frontal, parietal, occipital cortexes and striatum. Binding to δ2 sites was elevated in frontal and parietal cortexes. Buprenorphine did not alter δ1 binding in any of the regions examined. Changes in opioid receptor adaptation induced by buprenorphine were further supported by data from cross-linking of 125l-β-endorphin to cortical membrane preparations. A reduction in a 60- to 65-kDa band was detected in frontal and occipital cortices in which binding assays revealed down-regulation of μ receptors. In parietal cortex neither the 60- to 65-kDa product nor Bmax changes were observed. These results indicate that buprenorphine is a useful tool to study brain opioid receptor adaptation in vivo and the information accrued may be relevant to the mode of action of this drug in the treatment of heroin and cocaine abuse.
Antagonist- and agonist-induced changes in opioid receptor levels have been more readily demonstrated in cell cultures and in developing rat brain than in adult brain (Chang et al., 1982; Bhargava and Gulati, 1990; Tempel et al., 1990; Zadina and Kastin, 1986; Belcheva et al., 1992; Belcheva et al., 1993). Agonist-mediated brain opioid receptor down-regulation in vivo has been more difficult to substantiate. Earlier in vivo down-regulation studies were performed with whole brain membranes. Therefore, opioid binding changes in discrete brain regions, possibly involving specific receptor types or subtypes, may have gone undetected.
Recently, the importance of brain regional specificity for opioid receptor adaptation was discovered. Opioid receptor up- and down-regulation appears to occur only in discrete brain regions and for certain receptor types and subtypes (Belcheva et al., 1993; Belcheva et al., 1994; Dingledine et al., 1983; Loh et al., 1988; Steece et al., 1989; Werling et al., 1989). Receptor autoradiography studies revealed 2-fold increases in opioid binding in several subregions of brain with no change in adjacent subregions after chronic antagonist administration to rats (Tempel et al., 1984; Paden et al., 1987). Similarly, chronic agonist exposure resulted in down-regulation of μ and δ receptors in selective rat brain regions (Bhargava and Gulati, 1990; Tao et al., 1988). The opioid antagonist, naltrexone, elicited a transient down-regulation of δ2 receptors preceding up-regulation in both brain and NG108-15 cells (Belcheva et al., 1992; Belcheva et al., 1994). Treatment of rats with naltrexone resulted in lowered 3H-DSLET Bmax values in hindbrain, but not in striatum, hippocampus or cortex and subsequently 2-fold up-regulation of δ opioid binding in all four regions (Belcheva et al., 1994).
Cross-linking of opioid receptors with 125I-β-endorphin has proven useful for the estimation of their molecular sizes (Howard et al., 1985; Keren et al., 1988; McLean et al., 1989; Schoffelmeer et al., 1990; Ko et al., 1992). In β-endorphin competition binding experiments with unlabeled μ- and δ-selective ligands or by using cell lines known to express only certain classes of opioid receptor, it has been possible to associate a 60- to 65-kDa band with μ and a 50- to 55-kDa band with δ sites. NG108-15 cell membranes, which appear to contain only δ receptors, undergo 125I-β-endorphin cross-linking to generate 50- to 55-, 38- to 43- and 25- to 27-kDa bands. These and other results suggest the occurrence of limited proteolysis of both μ, and δ opioid receptors. Ko et al. 1992 proposed that only the 25-kDa band fulfilled the requirements for a δ opioid binding site, in part because its intensity alone was reduced under opioid receptor down-regulation conditions in NG108-15 cells. Inasmuch as this 25-kDa band is the predominant species cross-linked in NG108-15 cells but undetectable in brain (Howard et al., 1985; McLean et al., 1989), it was of interest to determine the size of the cross-linked product(s) down-regulated in brain.
When the mixed agonist-antagonist buprenorphine was administered to both adult and neonatal rats, it induced down-regulation of μ and up-regulation of κ1 and δ2 brain receptors (Belcheva et al., 1992). We report that buprenorphine administration to rats induces a brain-region-specific down- and up-regulation of the opioid sites. These conclusions are based on homologous competition studies with μ-, δ1-, δ2- and κ1-selective ligands and on 125I-β-endorphin cross-linking experiments performed with membrane preparations from rat brain regions.
Materials and Methods
Chemicals
DAMGE, DPDPE and β-endorphin were obtained from Chiron (San Diego, CA), whereas buprenorphine and U69593 were from NIDA Drug Supply (Research Triangle, NC) and deltorphin II was from BACHEM Bioscience Inc. (King of Prussia, PA). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) or Pierce (Rockford, IL).
Buprenorphine administration to rats
Adult male Sprague-Dawley rats received i.p. injections with either buprenorphine (0.5–2.5 mg/kg) or saline, and 20 hr later the animals were killed. In preliminary experiments a 6-day regimen of daily buprenorphine injections gave the same results for rat forebrain membranes (3H-DAMGE Bmax =54.7 ± 4.2 and 19.9 ± 1.1 fmol/mg prot. P < .05 for controls vs. 2.5 mg/kg buprenorphine-treated, respectively) as the single 20-hr treatment (3H-DAMGE BmM =70.1 ± 5.6 and 16 ± 2.4 fmol/mg prot. P < .05, for controls vs. 2.5 mg/kg buprenorphine-treated, respectively). Adult brains were collected, immediately dissected into different regions (brain stem, hippocampus, frontal cortex, occipital cortex, parietal cortex, striatum and thalamus), frozen on dry ice and stored at −70°C until binding assays were performed. For brain stem, frontal cortex, occipital cortex and parietal cortex, the tissue from a single brain provided sufficient protein to generate one binding curve. For binding assays using hippocampus, striatum and thalamus, regions from two to three brains were pooled to provide sufficient substrate. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Washing procedure to remove residual buprenorphine
After homogenization of rat brain regions using a Polytron, membrane preparations were collected by centrifugation at 20,000 × g and were washed at least five times with 50 mM Tris HCl, pH 7.4. This procedure proved sufficient to remove most of the residual buprenorphine as shown by several lines of evidence (Belcheva et al., 1993).
Opioid receptor binding assays
Rat membrane preparations were assayed for opioid binding activity as described (Belcheva et al., 1993). Membrane preparations (300–800 μg of protein) were incubated in duplicate with 1 nM 3H-U69593 (64 Ci/mmol, Amersham, Arlington Heights, IL) or 1 nM 3H-DAMGE (24–38 Ci/mmol, Chiron) at 25°C for 1 hr, or 2 nM 3H-DPDPE (30 Ci/mmol, Chiron) at 25°C for 3 h. 3H-Deltorphin II (0.5 nM, 55 Ci/mmol, Dupont NEN, Boston MA) binding was performed at 35°C for 45 min. In the 3H-DPDPE experiments, filters were presoaked in incubation buffer containing 0.3% polyethylenimine. Bmax and Kd values were estimated from homologous competition binding assays performed in the presence of 10 to 12 different concentrations of the corresponding unlabeled ligand using the LIGAND program (Munson and Rodbard, 1980). In all experiments shown, a single site model fit the data better than a two or more site fit. Statistical analyses of binding data were achieved using the Student’s t test. Protein concentrations were determined by the method of Lowry (Lowry et al., 1951) with bovine serum albumin as a standard.
Cross-linking with 125I-β-endorphin
The method developed by Howard et al. 1985 was used with some modifications. Membrane preparations from brain regions of control and buprenorphine treated rats were washed five times with 50 mM Tris-HCl buffer to remove the residual drug. Then the pellets were suspended in 50 mM phosphate buffer, pH 7.4 (50 mM K2HPO4, 1 mM EDTA, 10 μM leupeptin and 0.01% bacitracin) and incubated with 125I-β-endorphin (2 nM, 2000 Ci/mmol, Chiron) in the presence and absence of 1 μM unlabeled β-endorphin for 1 hr at room temperature. The incubation was halted by centrifugation for 5 min in a centrifuge (Beckman microfuge) at the highest speed. After resuspension of the pellets in phosphate buffer, they were incubated with the cross-linker BSO-COES (1 mM bis[2-(succinimidooxycarbonyloxy)ethyl]sulfone, Pierce) for 15 min on ice. The reaction was terminated by addition of 50 mM Tris-HCl, pH 7.4, and centrifugation. To remove labeled β-endorphin that was not cross-linked, samples were resuspended in 50 mM NaCl and incubated for 30 min at room temperature. Samples were adjusted to equal protein content (150–200 μg) and electrophoresed on SDS-PAG (10%). After staining with Coomassie blue to ascertain uniform protein loading, gels were dried and exposed to diagnostic films (Kodak X-Omat, XRP) for a period of 6 to 11 days. The intensity of the bands was quantified with a Phosphorimager (Molecular Dynamics, Sunnyvale, CA) and ImageQuant software. In each experiment samples of total and nonspecific binding for controls and treated animals were run on the same gel. Background was estimated for each lane and subtracted out before determining specific density. Statistical analyses were performed with the JMP program from SAS Institute Inc. (Gary, NC). In representative experiments optical transmission readings of the autoradiograms were also analyzed using a computerized video imaging system (Biological Detection Systems, Inc., Pittsburgh, PA). Background optical transmission as well as nonspecific binding were determined and subtracted from values obtained from various brain regions. Results gained were in good agreement with those obtained using the Phosphorimager.
Results
Effect of buprenorphine on μ opioid density in rat brain regions
Buprenorphine induced down-regulation (30–77%) of μ opioid receptor binding in six of the seven brain regions studied (figs. 1 and 2). In regions such as frontal cortex, thalamus, hippocampus and brain stem, a dose of 0.5 mg/kg buprenorphine was sufficient to elicit down-regulation. Occipital cortex and striatum required a higher concentration of the drug (2.5 mg/kg) to generate similar changes in receptor densities. Parietal cortex μ binding was affected by neither of the two doses of buprenorphine. In frontal cortex and brain stem, 0.5 and 2.5 mg/kg buprenorphine caused a similar reduction in DAMGE binding, although in thalamus down-regulation increased with the higher concentration of the drug. The observed brain-region-specific μ receptor down-regulation was induced by buprenorphine without statistically significant changes in DAMGE affinity.
Fig. 1.
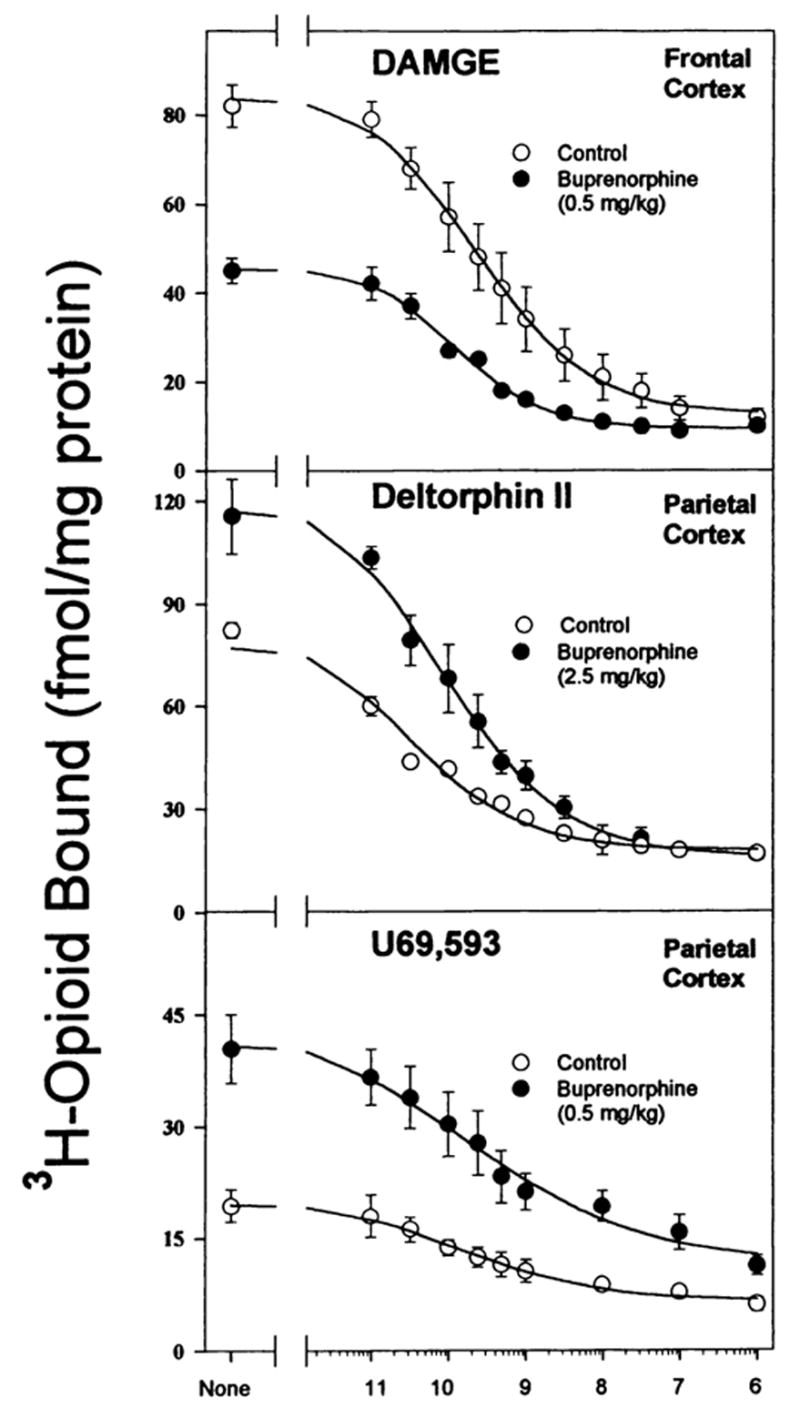
Homologous competition binding curves demonstrating buprenorphine-induced down- and up-regulation of opioid binding in brain regions. In all experiments adult male rats were injected ip with buprenorphine (0.5 or 2.5 mg/kg) or saline 20 hr before they were killed. Homologous competition binding assays were performed on membranes from brain regions and parameters (Kd and Bmax) were generated from curves such as these. Data are the mean ± S.E.M. of three experiments.
Fig. 2.
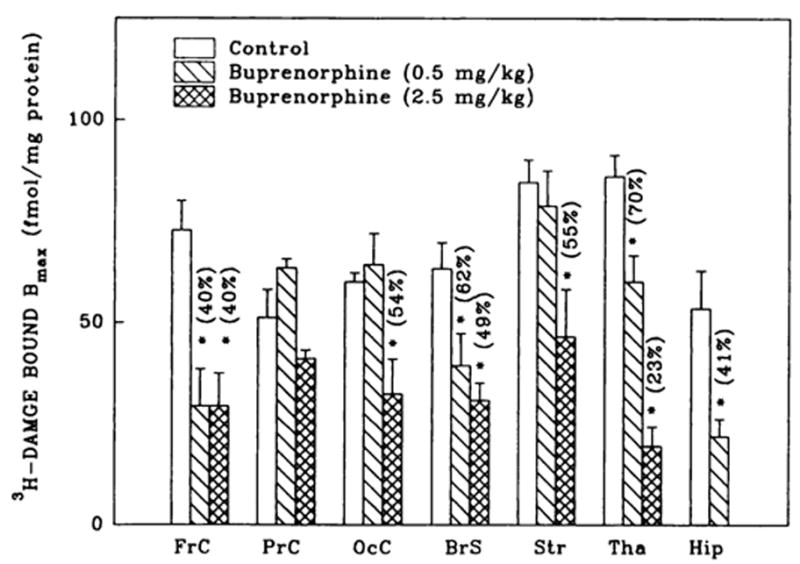
3H-DAMGE (μ) binding changes in brain regions from rats treated with buprenorphine. Corresponding Kd values ranged as follows: 1.1 ± 0.1–1.5 ± 0.1 (controls); 0.7 ± 0.1–2.4 ± 0.6 (0.5 mg/kg buprenorphine) and 0.9 ± 0.3–2.4 ± 0.2 nM (2.5 mg/kg buprenorphine). Numbers in parentheses represent residual binding as % of controls, *vs. controls, P < .05. Data are the mean ± S.E.M. of three to six experiments.
Differential effects of buprenorphine on δ1 and δ2 opioid binding
Buprenorphine (2.5 mg/kg) up-regulated δ2-selective deltorphin II binding in frontal and parietal cortices, (fig. 3). There were no δ2 binding density changes in parietal cortex and striatum after buprenorphine treatment. Consistent with receptor autoradiography studies (Mansour et al., 1987), there was little or no detectable δ binding in hippocampus or thalamus. DPDPE binding to the three cortical regions was unaffected by 0.5 mg/kg buprenorphine treatment (fig. 3). In contrast to deltorphin II binding, 2.5 mg/kg buprenorphine had no effect on DPDPE binding in frontal cortex (Bmax =10.2 ± 2.2 [n = 4] and 6.3 ± 0.8 [n = 5] fmol/mg prot. for controls vs. 2.5 mg/kg buprenorphine-treated, respectively). The observed changes in Bmax values were not accompanied by modifications in deltorphin II binding affinities.
Fig. 3.
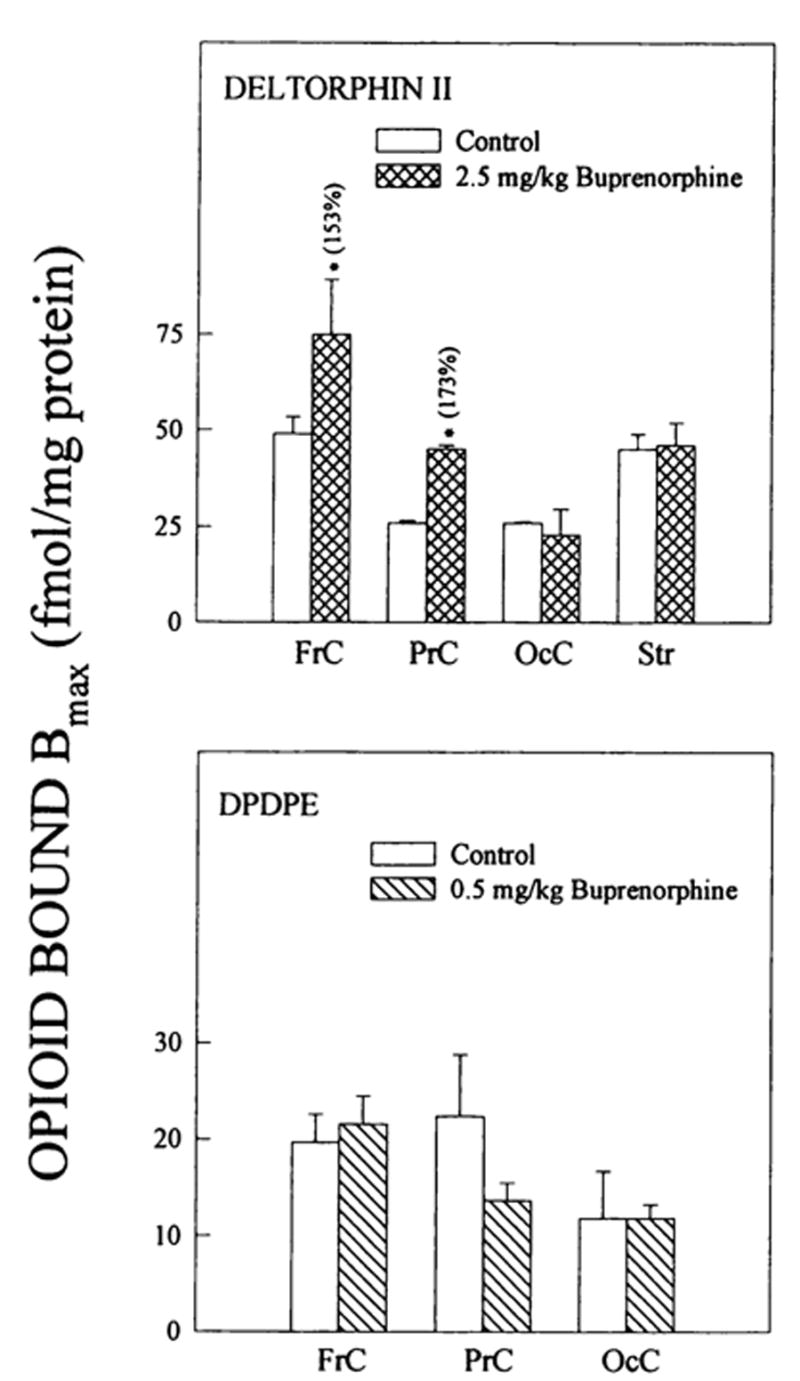
Changes in δ opioid binding to brain regions from buprenorphine-treated rats. δ1 binding was detected with DPDPE and δ2 densities with deltorphin II. Corresponding Kd values for deltorphin II ranged from: 1.0 ± 0.05–2.0 ± 0.1 (controls) and 1.0 ± 0.2–1.8 ± 0.3 nM (2.5 mg/kg buprenorphine) and for DPDPE: 1.9 ± 0.6–3.3 ± 1.4 (controls) and 1.6 ± 0.2–2.5 ± 0.6 nM (0.5 mg/kg buprenorphine). Numbers in parentheses represent binding as % of controls, *vs. controls, P < .05. Data are the mean ± S.E.M. of three to five experiments.
Up-regulation of κ1 binding sites in rat brain regions
Buprenorphine administration resulted in the up-regulation (2- to 4-fold) of κ1-opioid binding in frontal, parietal and occipital cortices and in striatum, but not in brain stem (fig. 4). Although 0.5 mg/kg buprenorphine elevated striatal κ1-sites, higher doses (≥1.0 mg/kg) of the drug had no effect. U69593 binding sites were not detected in hippocampal membrane preparations. Similar to μ and δ binding, buprenorphine treatment did not evoke changes in U69593 binding affinity in the brain regions in most cases examined.
Fig. 4.
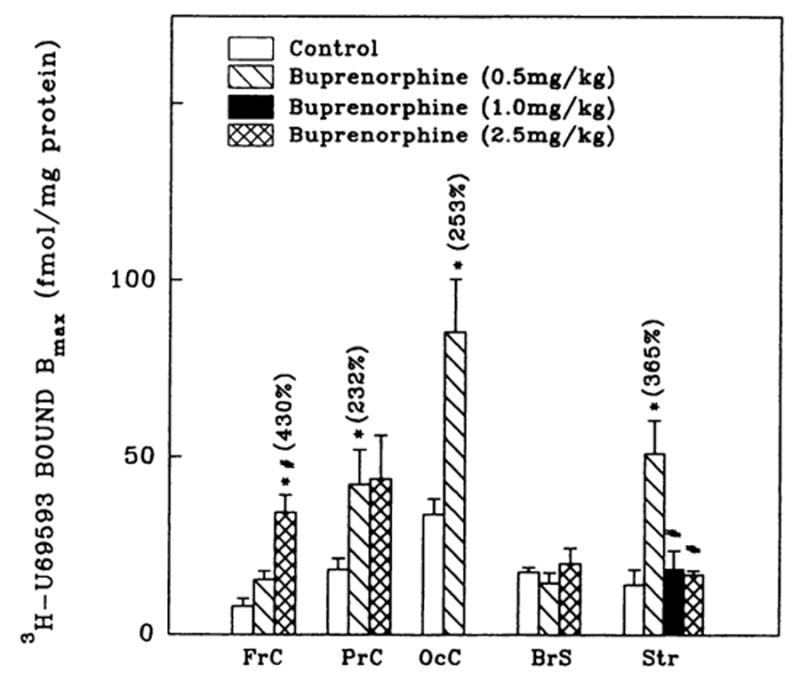
3H-U69593 (κ1) binding densities in brain regions from rats administered buprenorphine. Corresponding Kd values ranged as follows: 1.1 ± 0.2–4.8 ± 0.9 (controls); 2.2 ± 0.3–5.7 ± 1.2 (0.5 mg/kg buprenorphine); 2.2 ± 0.6 (1.0 mg/kg buprenorphine) and 2.2 ± 0.6–5.3 ± 0.4 nM (2.5 mg/kg buprenorphine). Numbers in parentheses represent binding as % of controls, *vs. controls, P < .05. #vs. treated (0.5 mg/kg), P < .05. Data are the mean ± S.E.M. of three to six experiments.
125I-β-endorphin cross-linking of membrane preparations from rat brain regions
Electrophoretic analysis of 125I-β-endorphin cross-linked membrane preparations from frontal, parietal and occipital cortices of control and buprenorphine (2.5 mg/kg) treated rats revealed the existence of two major bands at 60- to 65- and 43- to 46-kDa (which includes the contribution of β-endorphin). The reduction in intensity of the reaction products in the presence of 1μM β-endorphin suggests that they are related to opioid binding sites (data not shown). Buprenorphine treatment differentially affected the intensity of the reaction products (fig. 5). The quantification of the gels shows a decrease in the labeling of the 60- to 65-kDa band and no significant effect on the 43- to 46-kDa band in frontal cortex. A significant decrease in the intensity of both bands was seen for occipital cortex upon buprenorphine treatment. In contrast, no effect was observed for the 60- to 65-kDa reaction product in parietal cortex membranes but an increase in the 43- to 46-kDa band was found.
Fig. 5.
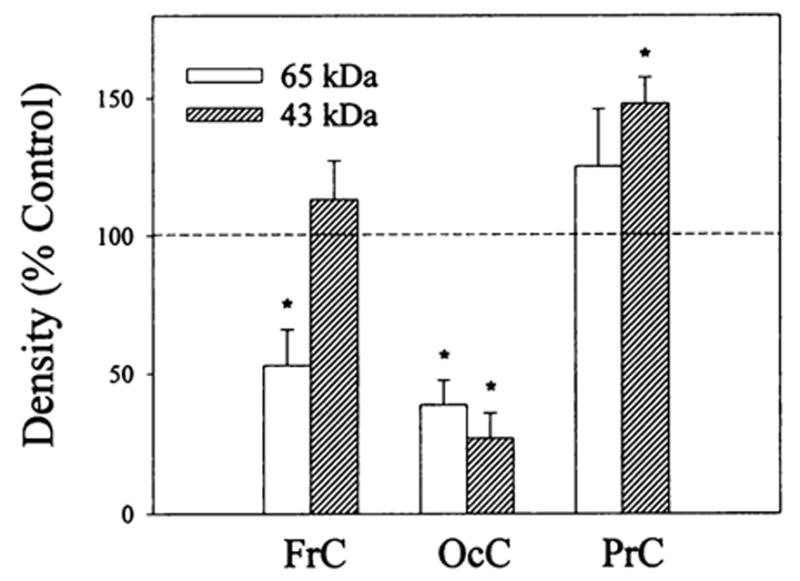
Densitometric analysis of 125I-β-endorphin cross-linked bands in frontal, parietal and occipital cortex membrane preparations from rats treated with buprenorphine. Rats were administered (i.p.) 2.5 mg/kg buprenorphine; 20 hr later they were sacrificed and their brains were removed and dissected. Membrane preparations (after five washes with 50 mM Tris-HCI, pH 7.4) were cross-linked with 2 nM 125I-β-endorphin in the presence (nonspecific binding) and absence (total binding) of 1 μM unlabeled β-endorphin as described in “Materials and Methods.” Equal amounts of protein (150–200 μg) were solubilized in sodium dodecyl sulfate (SDS) buffer and polyacrylamide gel ectrophoresis (PAGE) (10%) was performed. Specific densities of the 60- to 65- and 43- to 46-kDa bands in each brain region were determined as the difference between the density of the total and nonspecific binding for control and buprenorphine treated samples. Observed density changes induced by the buprenorphine treatment are expressed as percent of control values that were normalized to 100%. Data are the mean ± S.E.M. of three to four experiments, *vs. controls, P < .05.
Discussion
In a previous study, buprenorphine administration to rats was shown to induce down-regulation of μ. and up-regulation of κ1 and δ2 receptors in forebrain (Belcheva et al., 1993). Our present results indicate that the buprenorphine-engendered differential receptor adaptation is brain-region specific. Down-regulation of μ opioid binding was found in all brain regions analyzed except parietal cortex. Apparent loss in sites due to receptor blockade by residual buprenorphine was ruled out by several lines of evidence (Belcheva et al., 1993), including the absence of elevated 3H-DAMGE Kd values for membranes from treated animals. This was important to ascertain given the fact that buprenorphine dissociation from the opioid receptor is slow (for a review see Rothman et al., 1995). Previously 3H-DSLET was used to measure δ2 binding but this peptide also binds to μ, and possibly other receptors. In this study, we used the more δ2 selective ligand deltorphin II and an increase in binding densities was detected in two of four regions examined. In contrast, treatment with 0.5 to 2.5 mg/kg buprenorphine did not alter δ1 opioid binding in any brain regions tested. A 2- to 4-fold up-regulation of κ1 opioid binding in parietal and occipital cortices as well as striatum, with no change in brain stem, were observed after buprenorphine administration.
Earlier behavioral investigations suggested that buprenorphine acts as a partial agonist (Cowan et al., 1977; Martin et al., 1976). Subsequently, μ and possibly δ and κ agonist activity of buprenorphine were reported in a number of other pharmacological studies (Dum and Herz, 1981; Sadee et al., 1982; Kajiwara et al., 1986). However, with the availability of more κ-selective ligands as competitors, buprenorphine has proven to be a potent κ opioid receptor antagonist (Richards and Sadee, 1985; Leander, 1987; Negus et al., 1989). The up-regulation data generated in this and our previous publication on buprenorphine (Belcheva et al., 1993) support the notion that buprenorphine is also a δ2 antagonist. Moreover, independent evidence that buprenorphine is a δ2 antagonist has originated from recent studies with a cloned δ opioid receptor containing a point mutation at residue 95 (Kong et al., 1993). The aspartate at position 95, which is in the second transmembrane domain of this G protein coupled receptor, is known to be critical for high affinity δ binding. A mutant which has an asparagine in that position (D95N) binds with lowered affinity to six δ agonists tested. However, the Kd of buprenorphine for the D95N mutant did not vary from that of the wild-type as seen for antagonists such as naltrindole and its benzofuran analog. As a result of these properties some authors have referred to buprenorphine as a mixed agonist-antagonist (Rothman et al., 1995).
The 125I-β-endorphin cross-linking data (fig. 5) further support the evidence for buprenorphine-induced down-regulation obtained in homologous competition studies with 3H-DAMGE. For example, in frontal and occipital cortex membranes the labeling of the 60- to 65-kDa band was significantly reduced, although the intensity of this band was not changed in parietal cortex membranes. The labeling of the 43- to 46-kDa band is attenuated differentially by buprenorphine treatment. The identity of the peptide responsible for this band is unknown. It may be either a truncated form of opioid receptor (which could be derived from μ, or δ types as suggested by opioid receptor purification studies (Bidlack et al., 1981; Fujioka et al., 1985; Loukas et al., 1994) and/or a completely different molecule that is associated with the receptor and cross-linked to β-endorphin because of its proximity. Nevertheless, these data demonstrate that both 60- to 65- and 43- to 46-kDa bands can be diminished upon agonist-induced down-regulation of opioid receptors.
The differential receptor subtype and brain region-specific up- and down-regulation may be explained by several phenomena. Some brain regions may receive more drug secondary to increased blood flow and/or increased blood brain barrier permeability. Although this possibility cannot be ruled out, other data argue against it. For example, receptor autoradiography of naltrexone-induced opioid sites has revealed brain subregion-specific up-regulation of μ, opioid receptors in neocortex, septum, amygdala, substantia nigra and thalamus as discussed above (Tempel et al., 1984; Paden et al., 1987). Moreover, we observed extensive down-regulation of μ binding in brain stem but no κ1 up-regulation in response to buprenorphine. Similarly, parietal cortex κ1 receptors are up-regulated but μ receptors are unaffected by the buprenorphine regimen.
A second explanation for the regional specificity is the existence of time- and dose-dependency differences for receptor adaptation in various brain regions. Naltrexone induced up-regulation of δ sites in some rat brain regions in 6 hr, although in others >24 hr were required (Belcheva et al., 1994). In addition, chronic etorphine treatment of rats produced a time-dependent decrease in diprenorphine binding to membranes of different brain regions. In striatum and cortex, 1-day etorphine treatment did not alter opioid binding, although a significant decrease in binding was found in mid-brain membrane preparations. After 3 days of etorphine exposure, all three brain regions showed opioid receptor down-regulation (Tao et al., 1987). Moreover, μ opioid binding was increased in many rat brain regions after chronic (7 days) administration of a low dose of naloxone (0.5 mg/kg/h), although δ sites measured with DADLE and κ sites labeled with bremazocine were not significantly affected. In contrast, 3 mg/kg/hr naloxone caused an up-regulation of all three opioid receptor types, suggesting that this phenomenon takes place when a sufficient proportion of the receptors in vivo are occupied by the ligand (Morris et al., 1988).
Finally, we have shown that δ2 but not δ1 receptors undergo up-regulation. Interestingly, δ2 but not δ1 opioid receptor subtypes were implicated in the development of physical dependence on morphine in mice (Miyamoto et al., 1994). Thus, opioid receptor adaptation may be subtype-specific and dependent upon its physiological role, as has been proposed for β-adrenergic receptors (Liggett et al., 1993; Barak et al., 1994). Although β2 adrenergic receptors undergo agonist-induced down-regulation and sequestration, β3 does not; this was reported to be consistent with its function in adipocytes which is considered to be critical for a sustained, tonic output of energy substrates for extended elevated sympathetic activity. In addition, in contrast to β2, the β3 receptor lacks two determinants, tyrosine residues and a sequence (NPXXY) in its C-terminal close to the end of the seventh transmembrane domain, that are believed to be among the important signals for internalization and sequestration, respectively (Liggett et al., 1993; Barak et al., 1994). All opioid receptors cloned thus far have the (NPXXY) sequestration sequence, but not the internalization signal (Evans et al., 1992; Kieffer et al., 1992). However, we cannot exclude the possibility that opioid receptors may have a different signaling sequence for down-regulation or that yet-to-be-cloned opioid receptors contain C-terminal tyrosines.
Whether buprenorphine-induced differential up- and down-regulation in rat brain regions has any relevance to the utility of this drug in treating dual cocaine and heroin abuse (Mello and Mendelson, 1980; Kosten et al., 1989; Johnson et al., 1992) is contingent on a greater understanding of the underlying mechanisms of drug dependence and tolerance. Nevertheless, a recent study on differential regulation of brain opioid receptors after repeated cocaine administration to rats showed significant increases in 3H-DAMGE binding measured in some cortex regions, nucleus accumbens, caudate putamen and basolateral amygdaloid nucleus of cocaine-treated animals (Unterwald et al., 1992). Chronic cocaine exposure of rats also differentially altered 3H-naloxone binding to critical brain reward regions in investigations utilizing receptor autoradiography (Hammer, 1989). An increase in opioid receptor labeling was found for nucleus accumbens and the regions to which it projects, although the receptor density was reduced in regions containing neurons of ascending catecholamine systems. Based on these and our results on the differential effects of buprenorphine, an attractive hypothesis is that, by down-regulating μ and up-regulating some δ and κ opioid receptors in rat brain, buprenorphine may be capable of suppressing the effect of cocaine and other drugs of abuse.
ABBREVIATIONS
- BrS
brain stem
- DAMGE
[D-Ala2,N-mephe4Gly-ol5] enkephalin
- Deltorphin II
[Tyr-D-Ala-Phe-Glu-Val-Val-Gly-NH2]
- DPDPE
[D-Pen2D-Pen5] enkephalin
- DSLET
[D-Ser2[scaP]L-Leu5] enkephalyl-Thr
- FrC
frontal cortex
- Hip
hippocampus
- OcC
occipital cortex
- PrC
parietal cortex
- Str
striatum
- Tha
thalamus
Footnotes
This work was supported in part by NIDA Grant DA 05412.
References
- Barak LS, Tiberi M, Freedman NJ, Kwatra MM, Lefkowitz RJ, Caron MG. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated (β2-adrenergic receptor sequestration. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- Belcheva MM, Barg J, Gloeckner C, Gao XM, Chuang DM, Coscia CJ. Antagonist-induced transient down-regulation of δ opioid receptors in NG108-15 cells. Mol Pharmacol. 1992;42:445–452. [PubMed] [Google Scholar]
- Belcheva MM, Barg J, McHale RJ, Dawn S, Ho M, Ignatova E, Coscia CJ. Differential down- and up-regulation of rat brain opioid receptor types and subtypes by buprenorphine. Mol Pharmacol. 1993;44:173–179. [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Barg J, McHale RJ, Coscia CJ. Naltrexone induces down- and up-regulation of δ opioid receptors in rat brain regions. Brain Res Bui. 1994;35:69–72. doi: 10.1016/0361-9230(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Bhargava HN, Gulati A. Down-regulation of brain and spinal cord μ-opiate receptors in morphine tolerant-dependent rats. Eur J Pharmacol. 1990;190:305–311. doi: 10.1016/0014-2999(90)94194-3. [DOI] [PubMed] [Google Scholar]
- Bidlack JM, Abood LG, Osei-Gyimah P, Archer S. Purification of the opiate receptor from rat brain. Proc Natl Acad Sci USA. 1981;78:636–639. doi: 10.1073/pnas.78.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KJ, Eckel RW, Blanchard SG. Opioid peptides induce reduction of enkephalin receptors in cultured neuroblastoma cells. Nature. 1982;296:446–448. doi: 10.1038/296446a0. [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, MacFarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Valentino RJ, Bostock E, King ME, Chang KJ. Down-regulation of δ but not μ opioid receptors in the hippocampal slice associated with loss of physiological response. Life Sci. 1983;33:333–336. doi: 10.1016/0024-3205(83)90510-6. [DOI] [PubMed] [Google Scholar]
- Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Inoue F, Kuriyama M. Purification of opioid-binding materials from rat brain. Biochem Biophys Res Commun. 1985;131:640–646. doi: 10.1016/0006-291x(85)91285-9. [DOI] [PubMed] [Google Scholar]
- Hammer RP. Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Howard AD, DeLa Baume S, Gioannini TL, Killer JM, Simon EJ. Covalent labeling of opioid receptors with radioiodinated human β-endorphin. J Biol Chem. 1985;260:10833–10839. [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–2755. [PubMed] [Google Scholar]
- Kajiwara M, Aoki K, Ishii K, Numata H, Matsumiya T, Oka T. Agonist and antagonist actions of buprenorphine on three types of opioid receptor in isolated preparations. Jpn J Pharmacol. 1986;40:95–101. doi: 10.1254/jjp.40.95. [DOI] [PubMed] [Google Scholar]
- Keren O, Gioannini TL, Hiller JM, Simon EJ. Affinity crosslinking of 125I-labeled human β-endorphin to cell lines possessing either μ- or δ-type opioid binding sites. Brain Res. 1988;440:280–284. doi: 10.1016/0006-8993(88)90996-1. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The δ-opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci USA. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JL, Lee NM, Loh HH. A 25-kilodalton protein, with properties of a delta-opioid-binding site, in NG108-15 cell membranes. J Biol Chem. 1992;267:12722–12727. [PubMed] [Google Scholar]
- Kong H, Raynor K, Yasuda K, Moe ST, Portoghese PS, Bell GI, Reisine T. A single residue, aspartic acid 95, in the δ opioid receptor specifies selective high affinity agonist binding. J Biol Chem. 1993;268:23055–23058. [PubMed] [Google Scholar]
- Kosten TR, Kleber HD, Morgan C. Treatment of cocaine abuse with buprenorphine. Biol Psychiatry. 1989;26:637–639. doi: 10.1016/0006-3223(89)90090-5. [DOI] [PubMed] [Google Scholar]
- Leander JD. Buprenorphine has potent kappa opioid receptor antagonist activity. Neuropharmacology. 1987;26:1445–1447. doi: 10.1016/0028-3908(87)90112-2. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Freedman NJ, Schwdoj DA, Lefkowttz RJ. Structural basis for receptor subtype-specific regulation revealed by a chimeric β3/β2 adrenergic receptor. Proc Natl Acad Sci USA. 1993;90:3665–3669. doi: 10.1073/pnas.90.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh HH, Tao P-L, Smith AP. Role of receptor regulation in opioid tolerance mechanisms. Synapse . 1988;2:457–462. doi: 10.1002/syn.890020414. [DOI] [PubMed] [Google Scholar]
- Loukas S, Mercouris M, Panetsos F, Zioudrou C. Purification to homogeneity of an active opioid receptor from rat brain by affinity chromatography. Proc Natl Acad Sci USA. 1994;91:4574–4578. doi: 10.1073/pnas.91.10.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of μ, δ, and κ opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine and nalorphine-like drugs in the non-dependent and morphine-dependent chronic spinal dog. J Pharmac Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- McLean S, Rothman RB, Chuang D-M, Rice KC, Spain JW, Coscia CJ, Roth BL. Cross-linking of 125l-β-endorphin to μ-opioid receptors during development. Dev Brain Res. 1989;45:283–290. doi: 10.1016/0165-3806(89)90046-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Bowen WD, Portoghese PS, Takemori AE. Lack of involvement of delta-1 opioid receptors in the development of physical dependence on morphine in mice. J Pharmacol Exp Ther. 1994;270:37–39. [PubMed] [Google Scholar]
- Morris BJ, Millan MJ, Herz A. Antagonist-induced opioid receptor up-regulation. II Regionally specific modulation of μ, δ and κ binding sites in rat brain revealed by quantitative autoradiography. J Pharmacol Exp Ther. 1988;240:729–736. [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. LIGAND:a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Negus SS, Picker MJ, Dykstra LA. Kappa antagonist properties of buprenorphine in non-tolerant and morphine-tolerant rats. Paychopharmacology. 1989;98:141–143. doi: 10.1007/BF00442021. [DOI] [PubMed] [Google Scholar]
- Paden CM, Krall S, Lynch WC. Heterogeneous distribution and up-regulation of μ, δ and κ opioid receptors in the amygdala. Brain Res. 1987;418:349–355. doi: 10.1016/0006-8993(87)90102-8. [DOI] [PubMed] [Google Scholar]
- Richards ML, Sadee W. Buprenorphine is an antagonist at the κ opioid receptor. Pharm Res. 1985;2:178. doi: 10.1023/A:1016340106299. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Ni Q, Xu H. Buprenorphine: A Review of the Binding Literature. In: Cowan A, Lewis JW, editors. Buprenorphine. Wiley-Liss; New York: 1995. pp. 19–30. [Google Scholar]
- Sadee W, Rosenbaum JS, Herz A. Buprenorphine: Differential interaction with opiate receptor subtypes in vivo. J Pharmacol Exp Ther. 1982;223:157–162. [PubMed] [Google Scholar]
- Schoffelmeer ANM, Yao Y-H, Gioanntoi TL, Hiller JM, Ofri D, Roques BP, Simon EJ. Cross-linking of human [I25I]| β -endorphin to opioid receptors in rat striatal membranes:biochemical evidence for the existence of a μ/β opioid receptor complex. J Pharmacol Exp Ther. 1990;253:419–426. [PubMed] [Google Scholar]
- Steece KA, Lee JM, Fields JZ, DeLeon-Jones FA, Rttzmann RF. Differential down-regulation of δ opioid binding sites during physical dependence on methionine enkephalin in the rat. Life Sci. 1989;44:1449–1455. doi: 10.1016/0024-3205(89)90323-8. [DOI] [PubMed] [Google Scholar]
- Tao P-L, Law P-Y, Loh HH. Decrease in delta and mu opioid receptor binding capacity in rat brain after chronic etorphine treatment. J Pharmacol Exp Ther. 1987;240:809–816. [PubMed] [Google Scholar]
- Tao PL, Lee HY, Chang LR, Loh HH. Decrease in δ-opioid receptor density in rat brain after chronic [D-Ala2, D-Leu5] enkephalin treatment. Brain Res. 1988;462:313–320. doi: 10.1016/0006-8993(88)90559-8. [DOI] [PubMed] [Google Scholar]
- Tempel AE, Gardner L, Zukin S. Visualization of opiate receptor upregulation by light microscopy autoradiography. Proc Natl Acad Sci USA. 1984;81:3893–3897. doi: 10.1073/pnas.81.12.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel A, Kessler JA, Zukin RS. Chronic naltrexone treatment increases expression of preproenkephalin and preprotachykinin mRNA in discrete brain regions. J Neurosci. 1990;10:741–747. doi: 10.1523/JNEUROSCI.10-03-00741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald EM, Horne-King J, Kreek MJ. Chronic cocaine alters brain μ opioid receptors. Brain Res. 1992;584:314–318. doi: 10.1016/0006-8993(92)90912-s. [DOI] [PubMed] [Google Scholar]
- Wering LL, McMahon PN, Cox BM. Selective changes in μ opioid receptor properties induced by chronic morphine exposure. Proc Natl Acad Sci USA. 1989;86:6393–6397. doi: 10.1073/pnas.86.16.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadina JE, Kastin AJ. Neonatal peptides affect developing rats: β- endorphin alters nociception and opiate receptors, corticotropin-releasing factor alters corticosterone. Dev Brain Res. 1986;29:21–29. doi: 10.1016/0165-3806(86)90078-7. [DOI] [PubMed] [Google Scholar]


