Abstract
Tin mesoporphyrin (SnMP), a competitive heme oxygenase (HO) inhibitor, also induces HO-1 mRNA and protein expression by a mechanism that is not fully understood. We examined whether the induction by SnMP is mediated by a de-repression of Bach1, a transcription factor that suppresses the HO-1 gene. Incubation of NIH3T3-HO-1-luc cells with SnMP attenuated HO activity with a concomitant increase in HO-1 mRNA and protein and a decrease in Bach1 and HO-2 proteins, which was not due to transcriptional down-regulation, but accelerated protein decay. Similarly, HO-1 protein degradation was increased by SnMP, despite of an elevation in HO-1 transcription. Transfection of Bach1 shRNA in Hepa cells raised basal HO-1 expression significantly, and SnMP treatment further increased HO-1 mRNA. In conclusion, SnMP induces HO-1 expression not only by de-repressing the HO-1 promoter by binding Bach1, but also by accelerating Bach1 degradation.
Keywords: Bach1, heme oxygenase, isozymes, metalloporphyrins, tin mesoporphyrin
Introduction
Heme oxygenase (HO) controls the rate limiting reaction in the degradation of heme to biliverdin. Biliverdin is then rapidly converted to bilirubin by biliverdin reductase. The enzyme exists in two isofoms: HO-1 and HO-2. HO-1 is highly inducible by conditions such as oxidative stress [1], or by agents like heme [2,3], UVA [4], lipopolysaccharide (LPS) [5,6], interleukin-1 (IL-1) [6], and tumor necrosis factor-alpha (TNF-α) [6] in vivo and in vitro. HO-2 is a housekeeping enzyme expressed constitutively in almost all tissues.
The induction of HO-1 by various stressors is mainly regulated at the transcriptional level. Several transcriptional activators, such as Nrf2 and NFκB, bind to the consensus sequences in the HO-1 promoter and enhancers located 15 kb upstream from the HO-1 coding sequence and upregulate HO-1 transcription [7]. Recently, Bach1, a leucine zipper protein, has been identified as a repressor of the HO-1 gene [8]. It binds to the MARE (Maf recognition element) regions within distal enhancers-1 and -2 (DE1 and DE2, respectively) of the HO-1 promoter by heterodimerizing to MafG or MafK proteins in the absence of heme. Heme is not only the substrate for HO enzyme, but also the natural inducer of HO-1 expression. Upon exposure to heme, Bach1 dissociates from its heterodimerization partners and is exported out of the nucleus by a Crm1-dependent mechanism [9]. Displacement of Bach1 leads to recruitment of Nrf2, also a leucine zipper protein that binds to HO-1 promoter by cooperating with MafG and MafK proteins, to activate gene expression [10]. In addition, heme can stabilize the Nrf2 protein and lead to the accumulation of heterodimers of Nrf2/MafG that bind to MARE to activate HO-1 transcription [11]. These mechanisms all lead to a higher expression of HO-1. In contrast, the induction of HO-1 transcription by cadmium chloride, a strong inducer of HO-1 with no structural similarities to heme, also involves the nuclear export of Bach1 via an ERK1/2 dependent pathway to activate HO-1 gene expression [12]. Therefore, the expression of HO-1 by different inducers involves both activation of positive inducers, such as Nrf2, and inactivation of gene repressors, like Bach1, to allow for gene transcription.
In our studies investigating the transcriptional regulation of HO-1, we have found that some heme analogs, metalloporphyrins, are potent competitive inhibitors of HO activity both in vitro and in vivo. However, although metalloporphyrins has been shown to suppress HO activity, some metalloporphyrins [e.g., tin mesoporphyrin (SnMP)] can also induce its transcription via direct effects on the HO-1 promoter [3,13,14].
To understand the mechanism by which SnMP induces the expression of HO-1 with a simultaneous inhibition of HO activity [2,3], we investigated the effects of SnMP on HO-1, HO-2, and Bach1 mRNA, protein, and protein stability. We also used shRNA to study the direct involvement of Bach-1 in HO-1 regulation. We hypothesized that SnMP binds to the heme-binding region of Bach1 and causes it to detach from the DNA-binding complex relieving the repression of the MARE site within the HO-1 promoter, and thus activating HO-1 gene expression.
Materials and Methods
Tissue culture
NIH3T3 cells, stably transfected with a transgene containing the full-length (15 kb) mouse HO-1 promoter driving expression of the reporter gene luciferase (NIH3T3-HO-1-luc), were obtained from Dr. Christopher H. Contag (Stanford University, Stanford, CA). Mouse Hepa cells were stably transfected with Bach1 shRNA expressing vector. The construction of this vector is described below. Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) in a humidified incubator with 95% air and 5% CO2.
HO enzyme activity assay
HO activity in harvested cells was determined through measurements of carbon monoxide (CO) as previously described [15]. Briefly, cell sonicates were incubated with equal (20 μl) volumes of NADPH (4.5 mM) and methemalbumin (50 μM) for 15 min at 37°C in 2-ml CO-purged septum-sealed vials. CO in the vial headspace was determined by gas chromatography, using a 60x0.53 cm (i.d.) stainless steel column packed with 5A molecular sieve, 60–80 mesh at a temperature of 125°C, and a reduction gas detector (RGA2, Peak Laboratories, Menlo Park, CA) operated at 270°C. HO activity was expressed as nmol CO/h/mg protein.
Construction of shRNA expressing vector
Oligonucleotides were designed using shRNA design software from Invitrogen (Carlsbad, CA) and synthesized by Operon Biotechnologies, Inc. (Huntsville, AL). Single-stranded oligonucleotides were first annealed to form double-stranded oligonucleotides and then ligated into a pENTR/U6 entry vector following the manufacturer’s instruction (Invitrogen). TOP10 competent cells were transformed with the recombinant DNA after ligation for amplification. Plasmids containing correct DNA inserts were used in a recombination reaction between pENTR/U6 entry clone and pLenti6/BLOCK-iT-DES vector using Invitrogen’s gateway system to produce a lentiviral construct. The obtained lentiviral vector carrying Bach1 shRNA was transduced into Hep1–6 cells using ViraPower Lentiviral technologies and selected in medium containing Blasticidin (50 μg/ml).
The following oligonucleotides were used to generate Bach1 shRNA double-stranded oligonucleotides: 5'-caccggaactgacaagatccgaactcgaaagttcggatcttgtcagttcc-3' (top strand) and 5'-aaaaggaactgacaagatccgaactttcgagttcggatcttgtcagttcc-3' (bottom strand).
Preparation of nuclear and cytosolic extracts
Cell pellets were immediately resuspended in cytosol extraction buffer containing protease inhibitor cocktail. Extraction of cytosol and nuclear fractions were isolated using the NE-PER Extraction Kit (Pierce Biotechnology, Rockford, IL) according to manufacturer’s instructions.
Western blot analysis
Protein content of nuclear and cytosolic extracts was quantified using the Bradford method. Protein samples were denatured by boiling in loading buffer and separated on a 12% discontinuous SDS-PAGE for Western blotting. Separated proteins were then transferred to a PVDF membrane (BioRad, Hercules, CA) using a semi-dry blotter. The membrane was probed for HO-1 protein with HO-1 polyclonal antibody (1:1000) that was raised against a 30-kDa soluble HO-1 protein expressed in Escherichia coli from rat liver cDNA (Berkeley Antibodies Inc., Berkeley, CA). Bach1 protein was detected in nuclear extracts using Bach1 anti-goat polyclonal antibody (1:100) obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). HO-2 protein was detected using rabbit HO-2 (1:5000) antibody obtained from Stressgene (San Diego, CA). Mouse monoclonal lamin A/C antibody was purchased from Upstate Cell Signaling Solutions (Charlottesville, VA). Immune complexes were detected with appropriate secondary antibodies conjugated with horseradish peroxidase (HRP, Santa Cruz Biotechnologies) and visualized by Western Blotting Detection Reagent (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were then exposed to Hyperfilm (Amersham Pharmacia Biotech) and band intensities were quantified by densitometry as previously described [16].
In vivo bioluminescence imaging (BLI)
NIH3T3-HO-1-luc cells, stably transfected with a 15-kb HO-1 gene upstream of transcription initiation site driving expression of the reporter gene, luciferase, were treated as described above. At different time points after the addition of SnMP (20 μM), luciferin (150 μg/ml) was added to the cells. Light emission, a measure of HO-1 promoter activity in living cells, was quantified using the In Vivo Imaging System (IVIS™, Xenogen Corp., Alameda, CA) and expressed as photons emitted/sec as previously described [17].
Quantitative real-time PCR
Cells were harvested and immediately lysed for total RNA isolation using RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Isolated RNA samples were treated with DNase I to remove any remnant genomic DNA contamination and stored at −80°C until analysis. Real-time PCR reactions were performed using the QuantiTect SYBR Green RT-PCR kit (Qiagen) in a 96-well plate using the Opticon MJ Research instrument (Waltham, MA). Parameters were set as follows: 50°C for 30 min, 95°C for 15 min, 40 cycles of 95°C for 15 sec, 60°C for 15 min, and then 72°C for 30 sec. The results were analyzed using Opticon software (MJ Research). The forward and reverse primers used for: Bach1: 5'-ggagcaggactgtgaggtgaa-3' (forward) and 5'-ggattggaaatcatttcgtgaga-3' (reverse), and for HO-1: 5'-ccttcccgaacatcgacagcc-3' (forward) and 5'-gcagctcctcaaacagctcaa-3' (reverse).
Protein stability assay
NIH3T3-HO-1-luc cells were incubated with the protein translation inhibitor cycloheximide (CHX: 10 or 15 μg/ml) or vehicle in the presence and absence of 20-μM SnMP. Cells were then harvested at different time points following the addition of SnMP and CHX for cytosol and nuclear extraction. HO-1, HO-2, and Bach1 proteins were detected by Western blot analysis as described above.
Statistical analysis
Data are presented as mean±SD. Differences were analyzed using Student’s unpaired t-test for all comparisons. A value of P≤0.05 was deemed statistically significant.
Results
Effect of SnMP on HO activity and HO-1 and HO-2 expression
NIH3T3-HO-1-luc cells were treated with different concentrations of SnMP (0, 5, 10, and 20 μM). 24h post-treatment, total HO activity and HO-1 and HO-2 protein and mRNA levels were measured. HO activity in control cells was 0.35±0.09 nmol CO/h/mg protein and was significantly inhibited 55% to 65% (P<0.05) by SnMP at all concentrations tested (Fig. 1A). When cell sonicates were analyzed for HO-1 and HO-2 protein levels, SnMP treatment led to a concentration-dependent increase in HO-1 protein of up to 2- to 3-fold over control levels at concentrations of 10 (P<0.05) and 20 μM (P<0.01), respectively (Fig. 1B). Similarly, HO-1 mRNA levels also significantly increased with increasing concentrations of SnMP over a 24-h time period up to 14-fold after treatment with 20-μM SnMP (Fig. 1C). Surprisingly, HO-2 protein levels decreased significantly (P<0.05) (Fig. 1B), while HO-2 mRNA levels remained unchanged after treatment with 20-μM SnMP (Fig. 1D).
Fig 1.
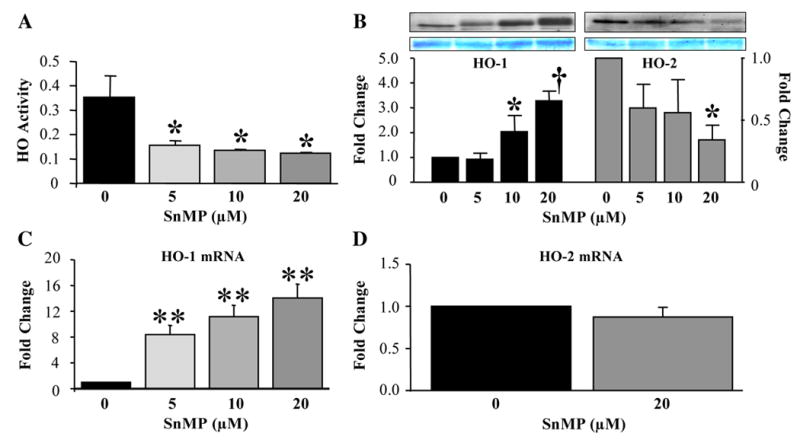
Effect of SnMP on HO activity and HO-1 and HO-2 expression. (A) HO activity was measured in NIH3T3-HO-1-luc cell sonicates after treatment with (A) SnMP (0, 5, 10, or 20 μM) or (B) 0, 20-μM SnMP alone, 20-μM hemin alone, or together for 24h. (B) HO-1 and HO-2 protein levels were measured in cell sonicates. Coomassie blue staining to confirm equal loading of protein in each lane). mRNA levels of HO-1 (C) and HO-2 (D) were measured by real-time PCR. Data are shown as mean±SD of 3–6 observations. *P<0.05, †P<0.01, **P<0.0001 vs. control.
Effect of SnMP HO-1 and HO-2 protein stability
Despite diminished HO-2 protein expression after treatment with SnMP, HO-2 mRNA levels were not affected. Therefore, we postulated that an increase in protein decay might be the underlying mechanism, which accounts for the low levels of HO-2 protein after SnMP treatment. We found that the very stable HO-2 protein is degraded faster in the presence of SnMP (data not shown). In control cells, HO-2 had a half-life of over 24h, while in cells treated with SnMP the half-life was shortened to 3h only. Similarly, SnMP also caused an acceleration of HO-1 protein decay. HO-1 protein was observed to have a half-life of 24h in cells treated only with CHX, but when SnMP was added, the half-life of HO-1 protein was significantly reduced to 3.5h. Lamin C levels remained unchanged by SnMP providing evidence that an overall protein destabilization was not responsible for the observed diminished levels of the HO isozymes.
Combined effects of hemin and SnMP on HO activity and HO-1 and HO-2 expression
Hemin (20 μM) treatment alone increased HO activity by 50% within 24h, but co-treatment of cells with an equimolar concentration of SnMP totally abolished this increase in HO activity (Fig. 2A). HO-1 protein levels increased 11-fold (P<0.01) following hemin treatment alone, and in the presence of SnMP, HO-1 protein was further induced to almost 30-fold (P<0.01) over control levels (Fig. 2B). In contrast, HO-2 levels decreased 60% only in cells treated with both hemin and SnMP (Fig. 2B).
Fig 2.
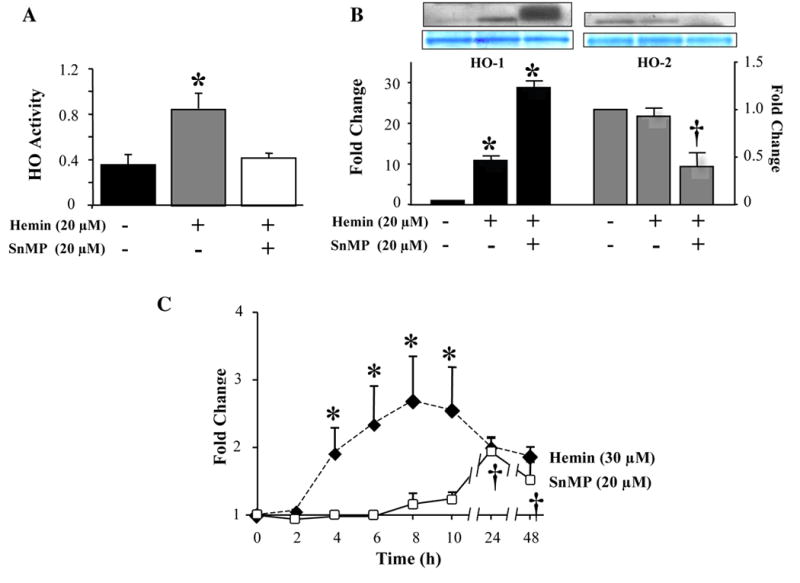
Combined effects of hemin and SnMP on HO activity and HO-1 and HO-2 expression. (A) HO activity was measured in NIH3T3-HO-1-luc cell sonicates after treatment with vehicle, 20-μM SnMP alone, 20-μM hemin alone, or together for 24h. (B) HO-1 and HO-2 protein levels were measured in cell sonicates. Coomassie blue staining confirmed equal loading of protein. (C) Time-dependent effects of SnMP and hemin on HO-1 promoter activity and HO-1 and HO-2 transcription. NIH3T3-HO-1-luc cells were treated with 20-μM SnMP or 30-μM hemin for the indicated time periods and HO-1 promoter activity was measured using the IVIS™. **P<0.01, †P<0.05. Data are shown as mean±SD of 3 observations.
Following treatment with hemin, HO-1 promoter activity was induced 4h after treatment, peaked to 2.5-fold at 8h, persisted for 2h, and progressively decreased from 24 to 48h (Fig. 2C). A significant increase (94% and 50%) in HO-1 promoter activity was found 24 and 48h after treatment with SnMP (Fig. 2C).
Effect of SnMP on Bach1 expression
To elucidate the possible involvement of Bach1 to the SnMP-mediated induction of HO-1 expression, we determined Bach1 protein levels following SnMP treatment. The abundance of Bach1 protein in the nucleus, measured by Western blot, showed a concentration-dependent decrease of 70% and 78% at 10- and 20-μM SnMP, respectively (P<0.0001) (Fig. 3A).
Fig 3.
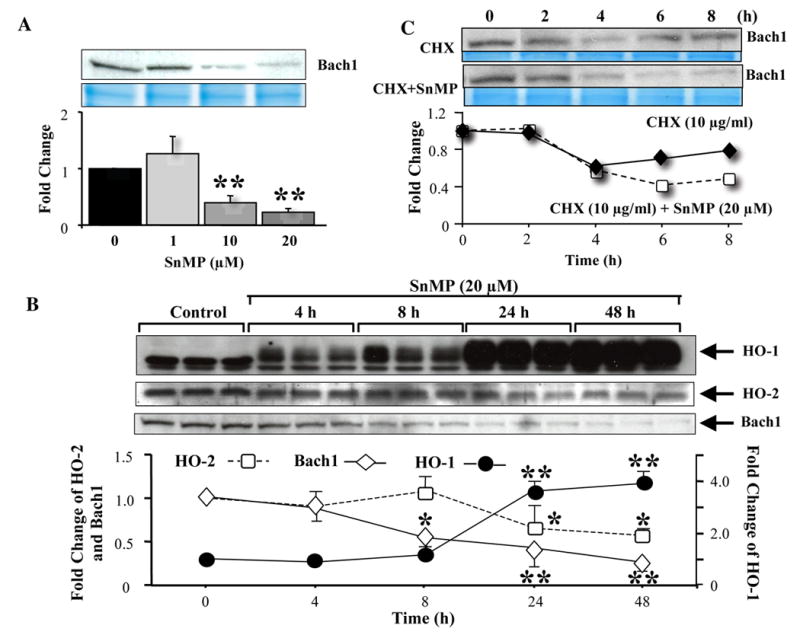
Effect of SnMP on Bach1 expression. (A) Cells were incubated with SnMP (0, 1, 10, and 20 μM) for 24h, harvested, and then cytosolic and nuclear fractions were extracted. Cytosolic and nuclear fractions were run on SDS/PAGE and immunoblotted with Bach1 antibodies. Coomassie blue staining confirmed equal loading of protein in each lane. (B) Time-dependent effect of SnMP on HO-1 (●), HO-2 (□), and Bach1 (◆) protein expression. Cells were treated with vehicle or 20-μM SnMP for indicated time periods. Cytosolic and nuclear fractions were extracted and then run on SDS/PAGE and immunoblotted with HO-1, HO-2, and Bach1 antibodies. *P<0.01, **P<0.0001 vs. control. Data are shown as mean±SD of 3 observations. (C) Transcriptional regulation of Bach1 and protein stability by SnMP. NIH3T3-HO-1-luc cells were treated with 10-μg CHX/ml (◆) alone or with 20-μM SnMP (□) and harvested at the indicated time points for nuclear and cytosolic protein isolation. Nuclear extracts were run on SDS/PAGE and immunoblotted with Bach1 antibody. Coomassie blue staining confirmed equal loading of samples. Data are representative of two independent experiments.
Attenuated expression of Bach1 protein in the nuclear extracts was also found to be time-dependent following SnMP treatment. Within 8 h after SnMP treatment, Bach1 protein decreased significantly, and at 24 and 48h, levels were further attenuated by 59% (P<0.01) and 76% (P<0.001), respectively (Fig. 3A). In contrast, HO-1 protein expression increased in a time-dependent manner in the presence of SnMP (Fig. 3B). Significant 3.6-fold upregulation of HO-1 protein was measured 24h after SnMP treatment, which persisted until 48h. HO-2 protein expression was significantly downregulated at 24 and 48h after start of incubation with SnMP (Fig. 3B). However, Bach1 mRNA levels were not affected in cells treated with SnMP (1.3±0.2, 1.0±0.1, and 1.3±5-fold for concentrations of 5, 10, and 20 μM, respectively) for 24h.
We also determined the protein stability of Bach1 following SnMP treatment. When translation was stopped by the addition of 10-μg CHX/ml to the incubation media in the presence of 20-μM SnMP, Bach1 protein levels decreased faster. This is indicative that this accelerated rate of Bach1 protein decay could be due to the action of SnMP (Fig. 3C).
Bach1 shRNA increases HO-1 expression
Stable transfection of Hepa1–6 cells with a lentiviral vector expressing Bach1 shRNA reduced Bach1 mRNA by 50%. Treatment of these cells with 20-μM SnMP did not significantly change the levels of Bach1 mRNA (Fig. 4A). Following SnMP treatment, HO-1 mRNA levels were significantly higher (~2-fold) in cells expressing Bach1 shRNA compared to vehicle-treated control cells (Fig. 4B).
Fig 4.
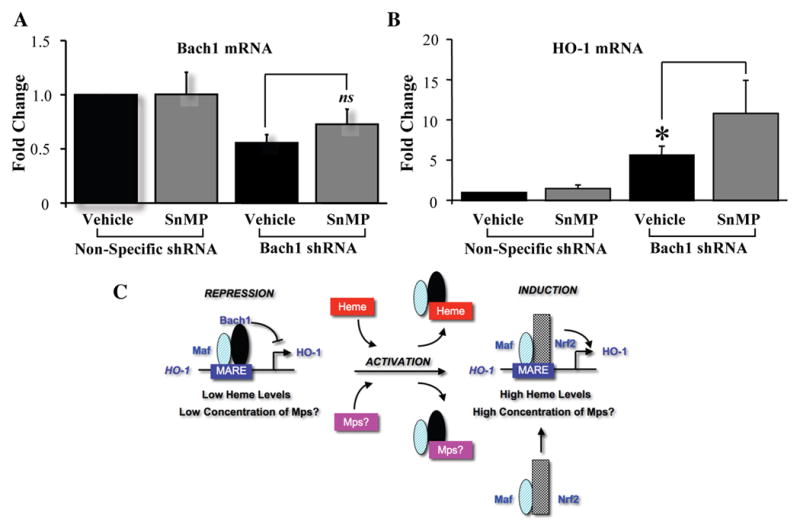
Bach1 shRNA increases HO-1 expression. Hepa cells were stably transfected with Bach1 shRNA plasmid, and then treated with vehicle or 20-μM SnMP for 24h. Bach1 (A) and HO-1 (B) mRNA levels were measured by real-time PCR and normalized by β-actin levels. *P<0.005 vs. control. Data are shown as mean±SD of 3 observations. (C) Proposed mechanism of action of the effect of SnMP on HO isozymes and Bach1 expression. HO-1, heme oxygenase-1; Mps, metalloporphyrins.
Discussion
Previous studies demonstrated that SnMP is a potent competitive inhibitor of HO activity, but can inversely activate the HO-1 gene [2,3]. In our studies, SnMP attenuated HO activity while increasing HO-1 gene expression at both the transcriptional and translational levels in support of previous observations [2,3]. In parallel to the increase in HO-1 protein, the decrease in Bach1 protein by SnMP strongly suggests that the effect is mediated through the de-repression of the HO-1 promoter.
The present study also demonstrates that SnMP accelerates the protein decay of Bach1. We speculate that a post-translational modification of Bach1 by SnMP-triggered events in the cell could cause a rapid degradation of Bach1 protein. Accelerated protein decay of Bach1 due to cobalt protoporphyrin (CoPP), a potent HO-1 inducer, has also recently been reported by Shan et al [18]. Transcription factors, like Nrf2 or NFκB, that regulate inducible genes are subject to a high degree of control in the cell through ubiquitination and proteosomal protein degradation [19,20]. Similarly, our findings strongly suggest the regulation of the abundance of Bach1 protein in the nucleus by proteosomal degradation to be a highly likely mechanism.
To get further insights into the role of Bach1 on the transcription of HO-1 gene and its role in SnMP-mediated HO-1 induction, we used a specially designed shRNA to target Bach1. Using this shRNA, we were able to attenuate Bach1 expression up to 50%, which resulted in 5-fold increase of HO-1 expression. Although the decrease in Bach1 gene expression attenuated the HO-1 upregulation by SnMP, it did not completely eliminate the induction. These data are in agreement with the findings reported by Shan et al. in human liver cells for hemin-induced HO-1 expression [21]. They suggest that other factors besides Bach1 degradation is involved in this process. In their recent work using CoPP to induce HO-1 expression [18], they also conclude that induction of HO-1 by CoPP involves the de-repression of Bach1 and the upregulation of Nrf2. In addition, transcriptional activation of HO-1 gene by SnMP was a late event (24h after treatment) as compared to the activation by hemin (4h after SnMP) in cell culture studies. Therefore, it is fair to assume that SnMP might indirectly promote the degradation of Bach1 by elevating intracellular heme levels in the cell. Theoretically, a 50% inhibition of HO activity might entail 50% more free heme in the cell available for interaction with Bach1 causing de-repression of the gene and allowing for transcriptional activation.
Our study also showed that inhibition of HO activity by SnMP could induce cellular oxidative stress (data not shown). This response was not due to the photoreactivity of SnMP since we carefully conducted all experiments under subdued lighting. Also, we have previously shown that treatment of NIH3T3-HO-1-luc cells with up to 30-μM SnMP does not cause cell death [13].
Contrary to the well-established dogma that HO-2 is a constitutively-expressed protein [22], we observed that HO-2 protein could be down-regulated by SnMP. Protein destabilization appears to be the underlying mechanism for the reduced levels of HO-2 protein by SnMP. Similar observation was made in our laboratory in the spleen of mice 24h after SnMP treatment where a significant attenuation of HO-2 protein was registered (unpublished data). As both HO-1 and HO-2 proteins were destabilized by SnMP, it is highly probable that the binding of SnMP to these proteins might mark them for faster degradation. Interestingly, HO-2 was more susceptible to SnMP-mediated protein decay than HO-1. The higher binding affinity of SnMP to HO-2 might be the reason for the differential effect of SnMP on the stability of these proteins (unpublished data).
In conclusion, the data presented here provide evidence that SnMP causes an increase in HO-1 transcript and protein with a concomitant decrease in nuclear abundance of Bach1 protein and HO-2 protein in the cytosol. This process of HO-1 induction by SnMP appears to involve both the de-repression of the HO-1 promoter by binding to Bach1 and acceleration of Bach1 degradation (Fig. 4C). These findings further suggest that the inhibition of HO activity by SnMP might be partly due to accelerated protein decay of both HO-1 and HO-2 isozymes, and not only via competitive enzyme inhibition.
Acknowledgments
This work was supported in part by National Institutes of Health grant #HD58013, the Mary L. Johnson Research Fund, and the Christopher Hess Research Fund. We thank Dr. Stacy Burns-Guydish for valuable discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dennery PA, Wong HE, Sridhar KJ, Rodgers PA, Sim JE, Spitz DR. Differences in basal and hyperoxia-associated HO expression in oxidant-resistant hamster fibroblasts. Am J Physiol. 1996;271:L672–679. doi: 10.1152/ajplung.1996.271.4.L672. [DOI] [PubMed] [Google Scholar]
- 2.DeSandre GH, Wong RJ, Morioka I, Contag CH, Stevenson DK. The effectiveness of oral tin mesoporphyrin prophylaxis in reducing bilirubin production after an oral heme load in a transgenic mouse model. Biol Neonate. 2005;89:139–146. doi: 10.1159/000088717. [DOI] [PubMed] [Google Scholar]
- 3.Morioka RJ, Wong A, Abate HJ, Vreman CH, Contag DK, Stevenson Systemic effects of orally-administered zinc and tin (IV) metalloporphyrins on heme oxygenase expression in mice. Pediatr Res. 2006;59:667–672. doi: 10.1203/01.pdr.0000215088.71481.a6. [DOI] [PubMed] [Google Scholar]
- 4.Tyrrell R. Redox regulation and oxidant activation of heme oxygenase-1. Free Radic Res. 1999;31:335–340. doi: 10.1080/10715769900300901. [DOI] [PubMed] [Google Scholar]
- 5.Rizzardini M, Carelli M, Cabello Porras MR, Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: Synergism by glutathione depletion and protection by N-acetylcysteine. Biochem J. 1994;304( Pt 2):477–483. doi: 10.1042/bj3040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Y, Shi Y, Ao LH, Harken AH, Meng XZ. TLR4 mediates LPS-induced HO-1 expression in mouse liver: Role of TNF-a and IL-1b. World J Gastroenterol. 2003;9:1799–1803. doi: 10.3748/wjg.v9.i8.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 8.Alam J, Igarashi K, Immenschuh S, Shibahara S, Tyrrell RM. Regulation of heme oxygenase-1 gene transcription: Recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase. Antioxid Redox Signal. 2004;6:924–933. doi: 10.1089/ars.2004.6.924. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Tashiro S, Hira S, Sun J, Yamazaki C, Zenke Y, Ikeda-Saito M, Yoshida M, Igarashi K. Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO J. 2004;23:2544–2553. doi: 10.1038/sj.emboj.7600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci USA. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Tashiro S, Sun J, Doi H, Satomi S, Igarashi K. Cadmium induces nuclear export of Bach1, a transcriptional repressor of heme oxygenase-1 gene. J Biol Chem. 2003;278:49246–49253. doi: 10.1074/jbc.M306764200. [DOI] [PubMed] [Google Scholar]
- 13.Hajdena-Dawson M, Zhang W, Contag PR, Wong RJ, Vreman HJ, Stevenson DK, Contag CH. Effects of metalloporphyrins on heme oxygenase-1 transcription: Correlative cell culture assays guide in vivo imaging. Mol Imaging. 2003;2:138–149. doi: 10.1162/15353500200303139. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Contag PR, Hardy J, Zhao H, Vreman HJ, Hajdena-Dawson M, Wong RJ, Stevenson DK, Contag CH. Selection of potential therapeutics based on in vivo spatiotemporal transcription patterns of heme oxygenase-1. J Mol Med. 2002;80:655–664. doi: 10.1007/s00109-002-0375-x. [DOI] [PubMed] [Google Scholar]
- 15.Vreman HJ, Stevenson DK. Heme oxygenase activity as measured by carbon monoxide production. Anal Biochem. 1988;168:31–38. doi: 10.1016/0003-2697(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 16.Abate A, Yang G, Wong RJ, Schroder H, Stevenson DK, Dennery PA. Apigenin decreases hemin-mediated heme oxygenase-1 induction. Free Radic Biol Med. 2005;39:711–718. doi: 10.1016/j.freeradbiomed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Feng JQ, Harris SE, Contag PR, Stevenson DK, Contag CH. Rapid in vivo functional analysis of transgenes in mice using whole body imaging of luciferase expression. Transgenic Res. 2001;10:423–434. doi: 10.1023/a:1012042506002. [DOI] [PubMed] [Google Scholar]
- 18.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach-1 and Nrf2 in up-regulation of heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20:E2258–E2267. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 19.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao W. Advances in NF-κB signaling transduction and transcription. Cell Mol Immunol. 2004;1:425–435. [PubMed] [Google Scholar]
- 21.Shan Y, Lambrecht RW, Ghaziani T, Donohue SE, Bonkovsky HL. Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: Insights from studies with small interfering RNAs. J Biol Chem. 2004;279:51769–51774. doi: 10.1074/jbc.M409463200. [DOI] [PubMed] [Google Scholar]
- 22.Maines MD. The heme oxygenase system: A regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]


