Abstract
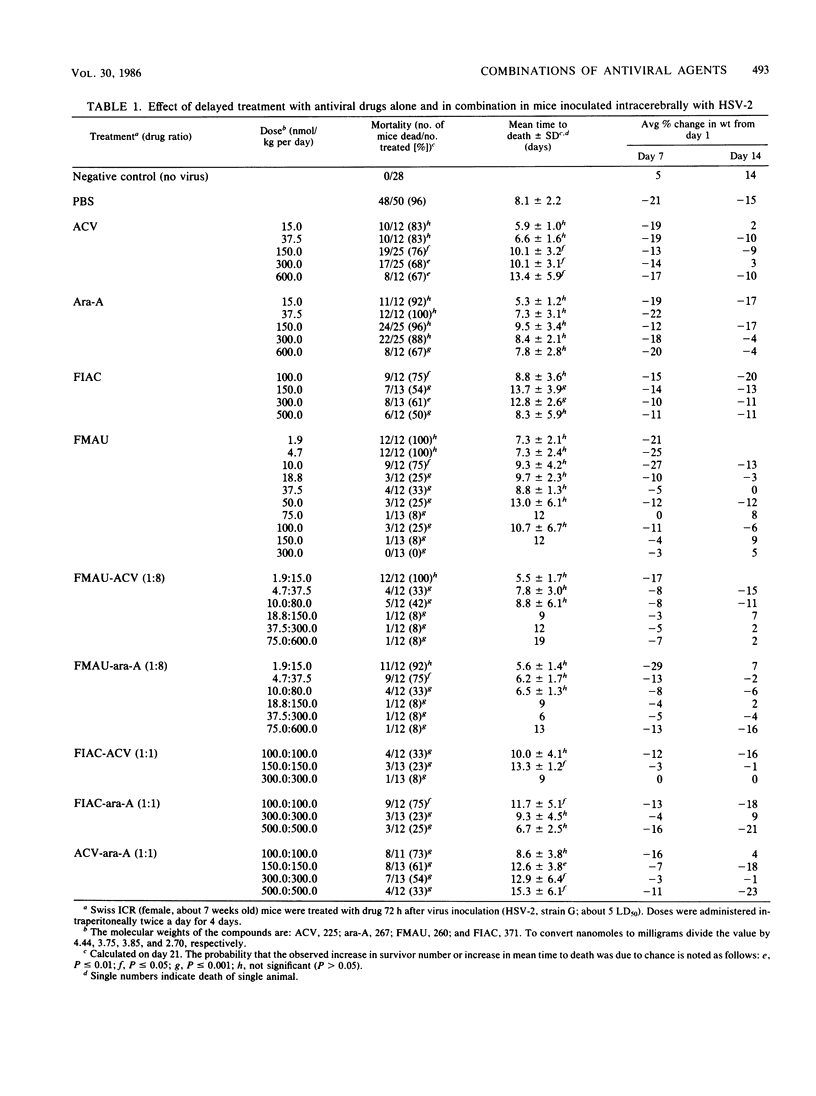
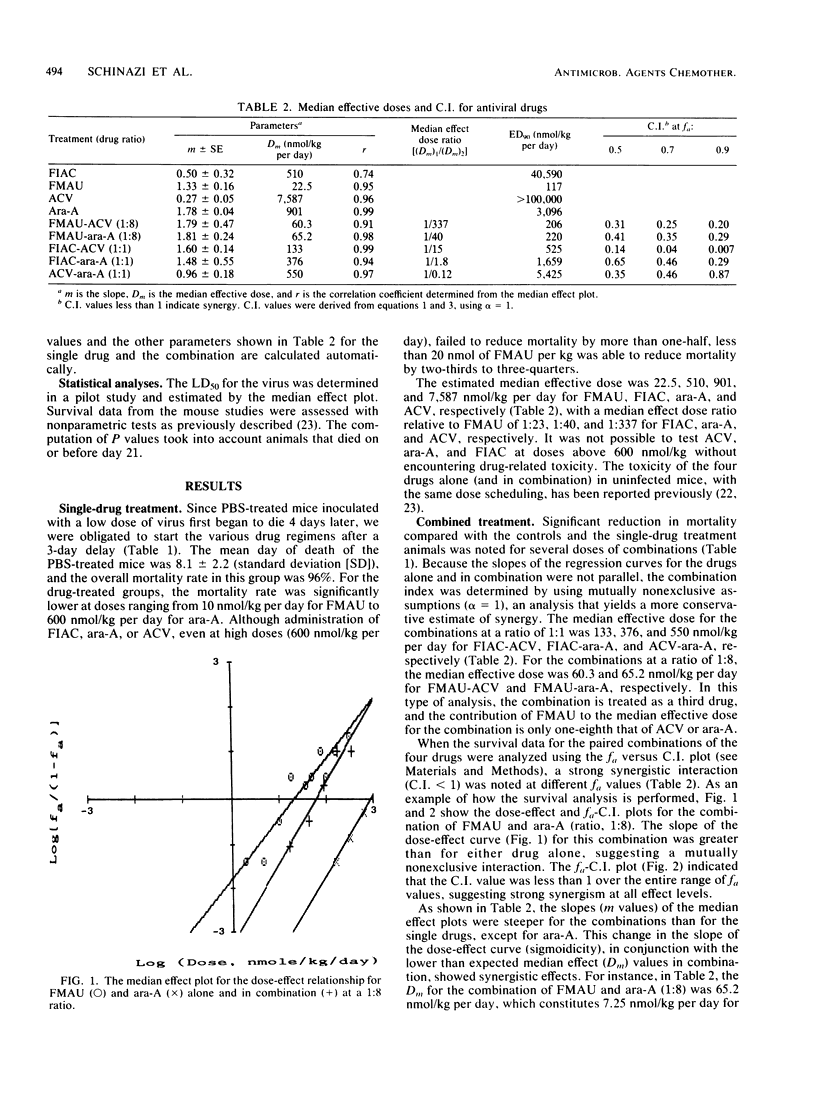
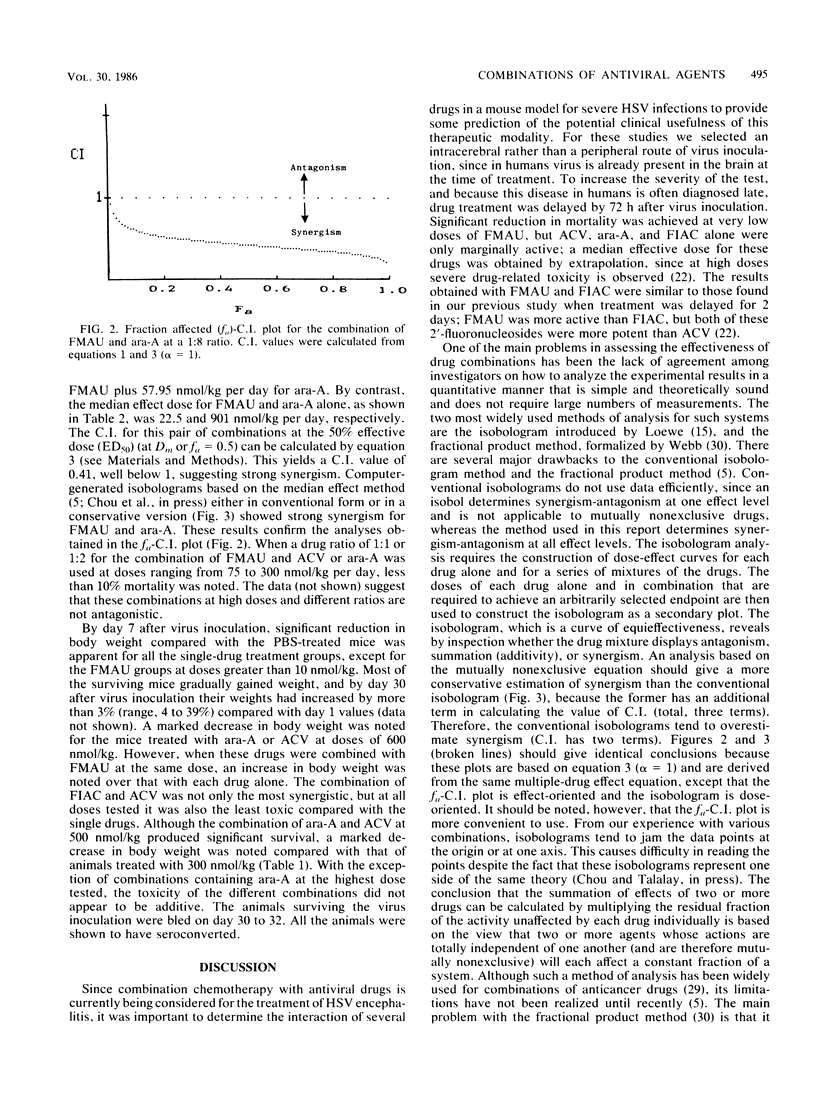
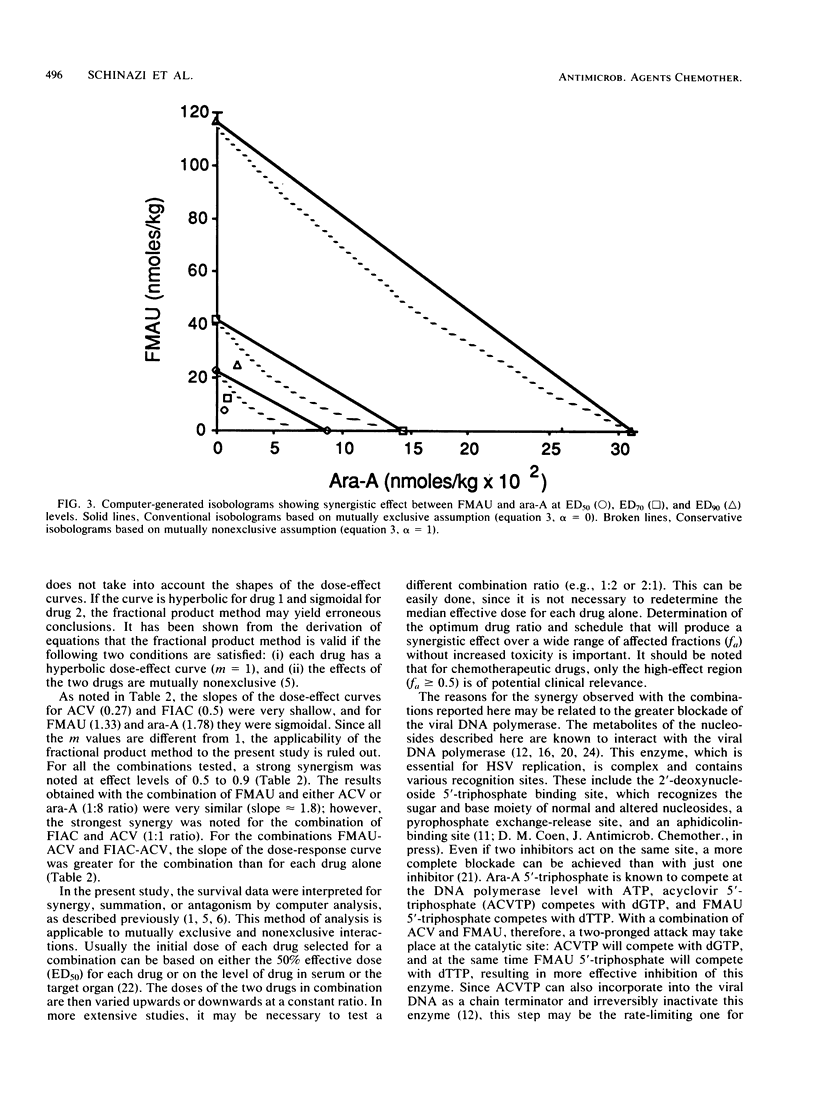
Mice were inoculated intracerebrally with a lethal dose of herpes simplex virus type 2. Three days later, the mice were treated intraperitoneally, twice daily for 4 days, with the following drugs alone or in combination: acyclovir (ACV), vidarabine (ara-A), 2'-fluoro-5-iodoaracytosine (FIAC), and 2'-fluoro-5-methylarauracil (FMAU). Despite delayed treatment, most of the animals receiving low doses of FMAU alone or in combination with ACV or ara-A survived. In contrast, significantly higher mortality rates were noted in mice receiving ara-A, ACV, or FIAC alone. The data were analyzed for quantitation of synergism, additivity, and antagonism of multiple drug effect by the median effect method. The median effective doses (in nanomoles per kilogram per day) calculated in this manner were: FMAU, 22.5; FIAC, 510; ara-A, 901; ACV, 7,587; ACV-ara-A (drug ratio, 1:1), 550; FIAC-ara-A (1:1), 376; FIAC-ACV (1:1), 133; FMAU-ACV (1:8), 60.3; and FMAU-ara-A (1:8), 65.2. Marked synergy was found throughout a wide range of effect levels with the five different combinations, with no increased toxicity over the single-drug treatments. Similar results were obtained when the data were analyzed by the isobologram method. Since many patients with severe herpetic infections, such as herpes encephalitis, have a poor prognosis despite single-drug therapy, the possible use of combinations including low doses of FMAU deserves further investigation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou T. C. Derivation and properties of Michaelis-Menten type and Hill type equations for reference ligands. J Theor Biol. 1976 Jul 7;59(2):253–276. doi: 10.1016/0022-5193(76)90169-7. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Feinberg A., Grant A. J., Vidal P., Reichman U., Watanabe K. A., Fox J. J., Philips F. S. Pharmacological disposition and metabolic fate of 2'-fluoro-5-iodo-1-beta-D-arabinofuranosylcytosine in mice and rats. Cancer Res. 1981 Sep;41(9 Pt 1):3336–3342. [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981 Mar 16;115(1):207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Crane L. R., Milne D. A., Sunstrum J. C., Lerner A. M. Comparative activities of selected combinations of acyclovir, vidarabine, arabinosyl hypoxanthine, interferon, and polyriboinosinic acid-polyribocytidylic acid complex against herpes simplex virus type 2 in tissue culture and intravaginally inoculated mice. Antimicrob Agents Chemother. 1984 Oct;26(4):557–562. doi: 10.1128/aac.26.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Ericson A. C., Larsson A., Aoki F. Y., Yisak W. A., Johansson N. G., Oberg B., Datema R. Antiherpes effects and pharmacokinetic properties of 9-(4-hydroxybutyl) guanine and the (R) and (S) enantiomers of 9-(3,4-dihydroxybutyl)guanine. Antimicrob Agents Chemother. 1985 May;27(5):753–759. doi: 10.1128/aac.27.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Cheng Y. C. Mutually exclusive inhibition of herpesvirus DNA polymerase by aphidicolin, phosphonoformate, and acyclic nucleoside triphosphates. Antimicrob Agents Chemother. 1985 Apr;27(4):445–448. doi: 10.1128/aac.27.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Spector T. Acyclovir triphosphate is a suicide inactivator of the herpes simplex virus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9575–9579. [PubMed] [Google Scholar]
- Karim M. R., Marks M. I., Benton D. C., Rollerson W. Synergistic antiviral effects of acyclovir and vidarabine on herpes simplex infection in newborn mice. Chemotherapy. 1985;31(4):310–317. doi: 10.1159/000238353. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Richards J. T., Overall J. C., Jr, Glasgow L. A. Alteration of mortality and pathogenesis of three experimental Herpesvirus hominis infections of mice with adenine arabinoside 5'-monophosphate, adenine arabinoside, and phosphonoacetic acid. Antimicrob Agents Chemother. 1978 Jan;13(1):53–60. doi: 10.1128/aac.13.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWE S. Antagonisms and antagonists. Pharmacol Rev. 1957 Jun;9(2):237–242. [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Aranucleosides and aranucleotides in viral chemotherapy. Pharmacol Ther. 1979;4(1):81–108. doi: 10.1016/0163-7258(79)90016-0. [DOI] [PubMed] [Google Scholar]
- Park N. H., Callahan J. G., Pavan-Langston D. Effect of combined acyclovir and vidarabine on infection with herpes simplex virus in vitro and in vivo. J Infect Dis. 1984 May;149(5):757–762. doi: 10.1093/infdis/149.5.757. [DOI] [PubMed] [Google Scholar]
- Philips F. S., Feinberg A., Chou T. C., Vidal P. M., Su T. L., Watanabe K. A., Fox J. J. Distribution, metabolism, and excretion of 1-(2-fluoro-2-deoxy-beta-D- arabinofuranosyl)thymine and 1-(2-fluoro-2-deoxy-beta-D-arabinofuranosyl)-5- iodocytosine. Cancer Res. 1983 Aug;43(8):3619–3627. [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Nahmias A. J. Different in vitro effects of dual combinations of anti-herpes simplex virus compounds. Am J Med. 1982 Jul 20;73(1A):40–48. doi: 10.1016/0002-9343(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Peters J., Sokol M. K., Nahmias A. J. Therapeutic activities of 1-(2-fluoro-2-deoxy-beta-D-arabinofuranosyl)-5-iodocytosine and -thymine alone and in combination with acyclovir and vidarabine in mice infected intracerebrally with herpes simplex virus. Antimicrob Agents Chemother. 1983 Jul;24(1):95–103. doi: 10.1128/aac.24.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., Peters J., Williams C. C., Chance D., Nahmias A. J. Effect of combinations of acyclovir with vidarabine or its 5'-monophosphate on herpes simplex viruses in cell culture and in mice. Antimicrob Agents Chemother. 1982 Sep;22(3):499–507. doi: 10.1128/aac.22.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., Prusoff W. H. Antiviral agents. Pediatr Clin North Am. 1983 Feb;30(1):77–92. doi: 10.1016/s0031-3955(16)34322-x. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Scott R. T., Peters J., Rice V., Nahmias A. J. Antiviral activity of 5-ethyl-2'-deoxyuridine against herpes simplex viruses in cell culture, mice, and guinea pigs. Antimicrob Agents Chemother. 1985 Oct;28(4):552–560. doi: 10.1128/aac.28.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköldenberg B., Forsgren M., Alestig K., Bergström T., Burman L., Dahlqvist E., Forkman A., Frydén A., Lövgren K., Norlin K. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet. 1984 Sep 29;2(8405):707–711. doi: 10.1016/s0140-6736(84)92623-0. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Martin J. C., Verheyden J. P., Matthews T. R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983 May;23(5):676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Averett D. R., Nelson D. J., Lambe C. U., Morrison R. W., Jr, St Clair M. H., Furman P. A. Potentiation of antiherpetic activity of acyclovir by ribonucleotide reductase inhibition. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4254–4257. doi: 10.1073/pnas.82.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriote F., Lin H. s. Synergistic interaction of anticancer agents: a cellular perspective. Cancer Chemother Rep. 1975 Sep-Oct;59(5):895–900. [PubMed] [Google Scholar]
- Whitley R. J., Alford C. A., Hirsch M. S., Schooley R. T., Luby J. P., Aoki F. Y., Hanley D., Nahmias A. J., Soong S. J. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986 Jan 16;314(3):144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Soong S. J., Hirsch M. S., Karchmer A. W., Dolin R., Galasso G., Dunnick J. K., Alford C. A. Herpes simplex encephalitis: vidarabine therapy and diagnostic problems. N Engl J Med. 1981 Feb 5;304(6):313–318. doi: 10.1056/NEJM198102053040602. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Yeager A., Kartus P., Bryson Y., Connor J. D., Alford C. A., Nahmias A., Soong S. J. Neonatal herpes simplex virus infection: follow-up evaluation of vidarabine therapy. Pediatrics. 1983 Dec;72(6):778–785. [PubMed] [Google Scholar]


