Abstract
In autoimmune demyelinating diseases such as multiple sclerosis (MS), the degradation of myelin proteins results in destabilization of the myelin sheath. Thus, proteases have been implicated in myelin protein degradation, and recent studies have demonstrated increased expression and activity of a calcium-activated neutral proteinase (calpain) in experimental allergic encephalomyelitis, the corresponding animal model of MS. In the present study, calpain activity and expression (at translational and transcriptional levels) were evaluated in white matter from human patients with MS and Parkinson’s and Alzheimer’s diseases and compared with that of white matter from normal controls. Western blot analysis revealed that levels of the active form of calpain and calpain-specific degradation products (fodrin) were increased by 90.1% and 52.7%, respectively, in MS plaques compared with normal white matter. Calpain translational expression was up-regulated by 462.5% in MS plaques compared with controls, although levels of the specific endogenous inhibitor, calpastatin, were not altered significantly. At the transcriptional level, no significant changes in calpain or calpastatin expression were detected by reverse transcription–PCR. Using double immunofluorescent labeling, increased calpain expression was observed in reactive astrocytes, activated T cells, and activated mononuclear phagocytes in and adjacent to demyelinating lesions. Calpain activity and translational expression were not increased significantly in white matter from patients with Parkinson’s or Alzheimer’s diseases compared with that of normal controls. Because calpain degrades all major myelin proteins, the increased activity and expression of this proteinase may play a critical role in myelinolysis in autoimmune demyelinating diseases such as MS.
Keywords: calcium-activated neutral proteinase, experimental allergic encephalomyelitis, calpastatin, Parkinson’s disease, Alzheimer’s disease
Multiple sclerosis (MS), a common neurological disease of young adults, is characterized by plaques of demyelination in the central nervous system (CNS) white matter. The subsequent impairment of saltatory nerve conduction is responsible for symptoms (limb paralysis, loss of coordination, visual impairment, etc.) observed in patients with MS (1, 2). Demyelination is believed to be the result of a chronic T cell-mediated, autoimmune inflammatory response against myelin proteins (3). Thus, MS plaques often are distinguished by gliosis, inflammatory cell infiltration, and localized myelin/oligodendrocyte destruction. Because myelin proteins are degraded in MS (with resulting destabilization of the myelin sheath), proteases that degrade these proteins have been implicated in myelinolysis. Earlier studies found increased acidic and neutral proteinase activity in MS plaques whereas more recent investigations observed increased activity and expression of a calcium-activated neutral proteinase (calpain) in the corresponding animal model, experimental allergic encephalomyelitis (EAE) (4–7).
The calpain family is divided into ubiquitous and tissue-specific subclasses according to tissue distribution (8). Ubiquitous calpain, examined exclusively in this study, exists as proenzyme micro (μ)- and millicalpain (m-calpain) isoforms that require micromolar and millimolar calcium levels, respectively, for activation (9). The 110-kDa heterodimeric proenzyme, composed of 80-kDa catalytic and 30-kDa regulatory subunits, undergoes N-terminal autoproteolytic cleavage upon activation and can be distinguished from the active form (76 kDa) on this basis (10). Calpain normally is associated with a specific endogenous inhibitor, calpastatin, which also is degraded by calpain after activation (11). Calpain has been described as a biomodulator because the endopeptidase degrades (through limited proteolysis) a broad array of intra- and extracellular proteins including cytoskeletal, myofibrillar, histone, enzymatic, myelin, and receptor proteins (12–17). In addition to myelin protein degradation in autoimmune demyelinating diseases, calpain has been implicated in the degradation of lens proteins in cataract formation and neuronal degeneration in ischemia, Parkinson’s, and Alzheimer’s diseases (18–22).
To study the role of calpain in MS, calpain/calpastatin activity and expression were evaluated at both transcriptional and translational levels in tissue from patients with MS, Parkinson’s, and Alzheimer’s diseases. These results were compared with data observed in normal-appearing white matter (NAWM) from MS patients and white matter from patients who died from nonneurological diseases. Although calpain activity and translational expression were increased significantly in activated glial/inflammatory cells of MS plaques, increases observed in Parkinson’s and Alzheimer’s samples were not significant compared with normal controls.
MATERIALS AND METHODS
Activity and Translational Expression of Calpain and Calpastatin by Western Blotting.
Methods for Western blot analysis have been described (7). Briefly, human CNS white matter from normal controls, Parkinson’s, Alzheimer’s, NAWM, and MS plaque samples (n = 8–10 for each group) were obtained from the National Neurological Research Specimen Bank (Los Angeles). NAWM samples from MS patients were taken from white matter adjacent (within approximately 2 cm) to the plaque border. Samples were homogenized in 50 mM cold Tris⋅HCl buffer (pH 7.4) containing 0.32 M sucrose, 1.0 mM EDTA, and 0.1 mM PMSF (Bethesda Research Laboratories). Samples were normalized upon determination of total protein content (Lowry protein assay) before resolution (via PAGE) and transfer to a microporous membrane (23–25). Using polyclonal antibodies specific for 150-kDa calpain-proteolyzed fodrin fragments (1:500 dilution), 80-kDa proenzyme m-calpain (1:800), 76-kDa postautolytic μ-calpain (1:600), and calpastatin (1:400), the respective proteins were detected using an enhanced chemiluminescence system by Amersham Pharmacia (26–28). Proteins were quantified with quantity one densitometry software (PDI Imageware Systems, Huntington Station, NY), and results were expressed as percent change + SEM via one-way ANOVA with Fisher’s probable least-squares difference posthoc test. P values < 0.05 were considered significant.
Analysis of Calpain and Calpastatin Transcriptional Expression by Reverse Transcription–PCR.
Methods used to evaluate transcriptional expression of calpain and calpastatin also have been described (7). Briefly, after homogenization, total RNA was isolated from samples described above by using TRI Reagent (Molecular Research Center, Cincinnati). One microgram of heat-denatured RNA (as determined by spectrophotometric analysis) was aliquoted for cDNA synthesis with mouse murine leukemia virus reverse transcriptase (GIBCO/BRL) at 42°C for 1 hr. The cDNA then was aliquoted for PCR amplification in the presence of primers designed for human μ-calpain (sense, 5′-AGC CGG CCG TAC ACT TGA AGC GTG-3′, and antisense, 5′-CGG AAC ATG GTC TCT AGC CGC ACC-3′) (29), m-calpain (sense, 5′-CCC TCC CAA CCT GTT CAA G-3′, and antisense, 5′-GCC TCC AGT TCC CAT CCA-3′), calpastatin (sense, 5′-TCA CCT GTG GGT CGC CTA C-3′, and antisense, 5′-GCT CTG GCA ATA GTG GTT TTC C-3′), and β-actin (sense, 5′-GTG GGG CGC CCC AGG CAC CA-3′, and antisense, 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′) (30) genes. Primers for m-calpain, μ-calpain, and calpastatin each were used for coamplification with that of β-actin—an internal control. After evaluation of cycle curves for each primer set (coamplified with β-actin), PCR amplification with AmpliTaq Gold DNA polymerase (Perkin–Elmer) was performed using the following program: one cycle of denaturation at 94°C for 10 min; 35 cycles of template denaturation at 94°C for 1 min, template-primer annealing at 58°C for 1 min, and primer extension at 72°C for 2 min; one cycle of extension of all nascent DNA strands at 72°C for 6 min; and soaking at 4°C for at least 20 min. Reverse transcription–PCR products were electrophoresed at 3 V/cm on a 1.2% agarose gel and stained with ethidium bromide. The resolved bands were photographed and quantified with quantity one software (PDI Imageware Systems). The ratios of calpain or calpastatin mRNA expression to that of β-actin were calculated and analyzed statistically via one-way ANOVA + SEM by using statview 4.5 software as indicated above.
Calpain Localization by Double Immunofluorescent Labeling.
Tissue sections were snap-frozen in Tissue-Tek OCT compound (Miles) and sectioned using a Reichert–Jung cryostat. Cross-sections (5 μm) were blocked (30 min) with 2% horse and goat sera diluted in PBS. The sections were incubated (1 hr) with the previously described m-calpain antibody (1:200 dilution) and one of the following cell-specific mAbs: EBM 11 (Dako), specific for a 110-kDa glycoprotein in mononuclear phagocytes at 1:150 dilution; monoclonal glial fibrillary acidic protein MIG-G2 clone (BioSource International, Camarillo, CA) for astrocytic intermediate filament protein at 1:1,500; L243 (kindly donated by Henry McFarland, National Institute of Neurological Disorders and Stroke, National Institutes of Health) for MHC class II antigen at 1:200; and CD4 (OX38) for helper T cells at 1:100 (Accurate Chemicals). Tissue sections then were rinsed in PBS and incubated (30 min) with anti-rabbit FITC-conjugated and anti-mouse Texas red-conjugated secondary antibodies (Vector Laboratories) at 1:100 dilution. Sections were rinsed in PBS and distilled water and mounted with Vectashield mounting medium (Vector Laboratories).
RESULTS
Calpain Activity Evaluated by Substrate Degradation and Proenzyme Activation.
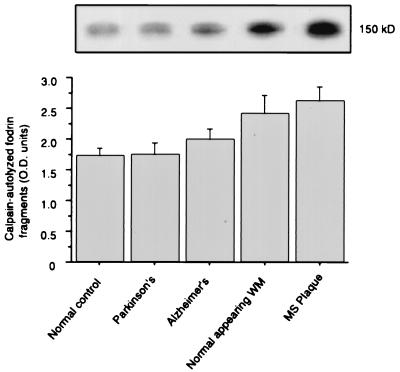
Western blotting techniques were used to examine calpain activity as measured by the production of calpain-specific degradation products. Calpain degrades the 230-kDa α-subunit of fodrin to produce a 150-kDa fragment that is recognized by the antibody used in this study. Although production of the 150-kDa fodrin fragment was increased marginally in white matter from Alzheimer’s patients compared with normal controls, only increases observed in NAWM [40.9% (P = 0.018)] and MS plaques [52.7% (P = 0.002)] were significant (Fig. 1).
Figure 1.
Calpain activity as measured by Western blot detection of 150-kDa, calpain-cleaved fodrin fragment production in CNS white matter from normal control, Parkinson’s, Alzheimer’s, NAWM, and MS plaque samples (n = 8–10). Western blots (Upper) were quantified via densitometry and analyzed by one-way ANOVA (+SEM).
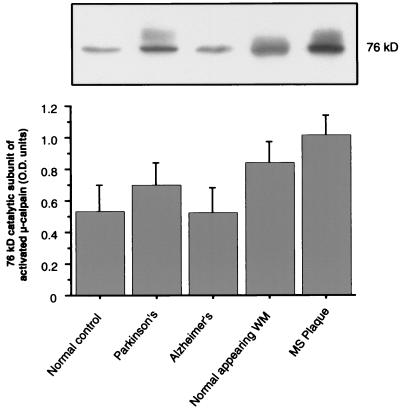
In addition to substrate degradation, calpain activity was evaluated by measuring the relative amount of active (76-kDa) enzyme. Activated μ-calpain levels were up-regulated in NAWM and Parkinson’s white matter samples, but the 90.1% (P = 0.028) increase in MS plaques alone was significant compared with normal controls (Fig. 2).
Figure 2.
Levels of 76-kDa activated μ-calpain in CNS white matter from normal control, Parkinson’s, Alzheimer’s, NAWM, and MS lesion samples (n = 8–10). Western blots (Upper) were quantified via densitometry and analyzed by one-way ANOVA (+SEM).
Calpain and Calpastatin Translational Expression.
Calpain and calpastatin translational expression levels also were measured via Western blotting. Using a polyclonal antibody specific for the 80-kDa catalytic subunit of proenzyme m-calpain, Western blotting revealed that calpain expression was increased significantly by 462.5% (P = 0.002) in MS plaque samples compared with normal controls (Fig. 3). Alterations of calpain expression in NAWM, Parkinson’s, and Alzheimer’s white matter samples were not significantly different from normal controls.
Figure 3.
Calpain translational expression measured by Western blot (Upper) detection of 80-kDa m-calpain proenzyme in CNS white matter from normal control, Parkinson’s, Alzheimer’s, NAWM, and MS plaque samples (n = 8–10). Western blots were quantified via densitometry and analyzed by one-way ANOVA (+SEM).
Calpastatin expression was measured with a polyclonal antibody specific for the central consensus sequence in domain 1 of human calpastatin (Fig. 4). The 110- and 68-kDa calpastatin isoforms were not significantly different in any sample set compared with normal controls. The 40-kDa calpastatin isoform was increased significantly by 40.4% (P = 0.027) in MS plaque samples compared with normal controls, but no significant alterations were observed in Parkinson’s, Alzheimer’s, or NAWM samples.
Figure 4.
Expression of 110-, 68-, and 40-kDa calpastatin isoforms in CNS white matter from normal control, Parkinson’s, Alzheimer’s, NAWM, and MS lesion samples (n = 8–10). Western blots (Upper) were quantified via densitometry and analyzed by one-way ANOVA (+SEM) as depicted graphically.
Transcriptional Expression of Calpain and Calpastatin.
Transcriptional expression of m-calpain (440 bp), μ-calpain (697 bp), and calpastatin (351 bp) was evaluated via reverse transcription–PCR. Products of each primer set were coamplified with that of a human β-actin primer set (630 bp). Because activated T cells and mononuclear phagocytes were examined in this study, β-actin was used as an internal control instead of glyceraldehyde-3-phosphate dehydrogenase because transcriptional levels of the latter can be altered upon cell activation (31). Also, to avoid excessive postmortem degradation of mRNA, samples were obtained from CNS tissue with a death-to-freezer time of less than 48 hr (32, 33). Transcriptional expression levels of m-calpain, μ-calpain, and calpastatin (as determined by the ratio to β-actin expression) were not significantly different from normal controls in any sample set (Fig. 5). Levels of μ-calpain gene expression appeared to be marginally lower on average than that of m-calpain or calpastatin.
Figure 5.
Transcriptional expression of μ-calpain (697 bp), m-calpain (440 bp), and calpastatin (351 bp) in CNS white matter from normal control, Parkinson’s, Alzheimer’s, NAWM, and MS plaque samples (n = 8–10). β-Actin (538 bp) served as an internal control for coamplification with each sample set via reverse transcription–PCR. The ratio of target optical density with that of β-actin (in each sample) was calculated after quantification by densitometry and analyzed by one-way ANOVA (+SEM).
Localization of Calpain Expression via Double Immunofluorescence Labeling.
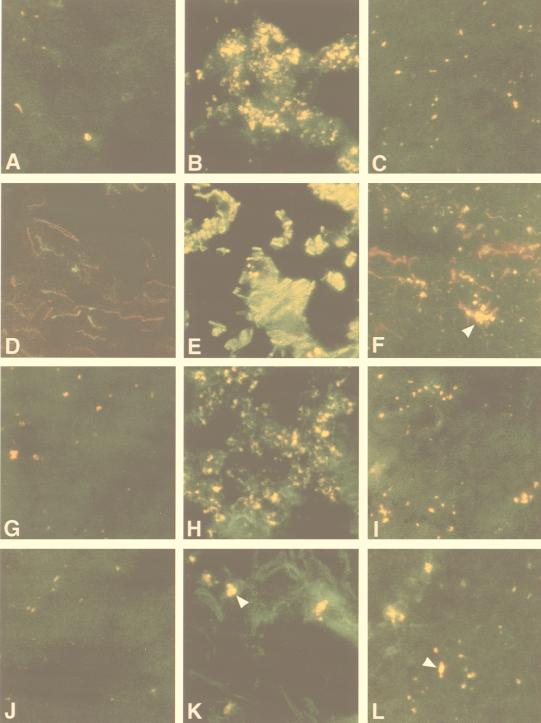
Double immunofluorescent labeling was used to identify calpain-positive cell phenotypes in normal controls, NAWM, and MS plaques (Fig. 6). Antibodies specific for the m-calpain proenzyme (FITC secondary; green) were added in combination with mAbs specific for astrocytes, T cells, and mononuclear phagocytes (Texas red secondary; red). When viewed with a dual-pass fluorescence filter, cells positive for calpain and the cell-specific marker appeared yellow.
Figure 6.
Localization of m-calpain expression in normal control white matter, NAWM, and MS lesions (×400). Using double immunofluorescent labeling, calpain immunoreactivity appears green in A–L. Antibodies specific for cells expressing MHC class II (L243) (A–C); astrocytes (glial fibrillary acidic protein) (D–F); mononuclear phagocytes (EBM 11) (G–I); and CD4-positive T cells (J–L) are labeled red. Cells positive for both calpain and the cell marker appear yellow. Normal control white matter is shown in the left column (A, D, G, and J) with MS lesion tissue (B, E, H, and K) and NAWM (C, F, I, and L) in the center and right columns, respectively.
Fig. 6 A–C depicts staining specific for calpain and MHC class II-positive cells. Labeling for calpain and astrocytes is shown in Fig. 6 D–F; that for calpain and mononuclear phagocytes is shown in Fig. 6 G–I; and that for calpain and T cells is shown in Fig. 6 J–L. The left column in Fig. 6 shows staining for each set in normal control white matter, whereas the center and right columns depict MS plaque and NAWM samples, respectively.
Although detectable levels of calpain were observed in T cells, astrocytes, and mononuclear phagocytes in normal controls, overall calpain expression was lower than that found in NAWM or MS plaques. In NAWM, calpain expression was increased in reactive astrocytes (Fig. 6F, arrowhead), CD4-positive T cells (Fig. 6L, arrowhead), and mononuclear phagocytes (Fig. 6I). Sections observed near the center of MS lesions (Fig. 6, center column) revealed extensive cellular destruction, gliosis, and inflammatory infiltrates. Compared with normal control and NAWM samples, calpain expression was markedly up-regulated in MHC class II-positive cells (Fig. 6B), Glial fibrillary acidic protein-positive cells/processes (Fig. 6E), mononuclear phagocytes (Fig. 6H), and CD4-positive T cells in MS plaques (Fig. 6K, arrowhead).
DISCUSSION
Recent studies have demonstrated increased calpain activity and translational expression in the optic nerves and spinal cords of Lewis rats with acute EAE, the corresponding MS animal model (7, 34–36). In EAE, calpain and calpastatin transcriptional expression were not altered significantly whereas calpain translational expression was up-regulated in glial/inflammatory cells upon activation.
These findings are consistent with data presented in the present study that suggest that calpain may play an important role in myelinolysis because all major myelin proteins are calpain substrates. By measuring the levels of active μ-calpain and calpain-autolyzed fodrin fragments, calpain activity was observed to be increased significantly in MS plaque samples whereas increases in Parkinson’s and Alzheimer’s samples were insignificant compared with normal controls. Although few, if any, studies have examined calpain activity and expression in white matter from Alzheimer’s and Parkinson’s patients, relative differences in calpain activity among the various neurological conditions may be caused by the number of calpain-positive glial/inflammatory cells present in the selected tissue sections. Previous immunohistochemical studies in our laboratory revealed that white matter from MS patients was characterized by markedly increased numbers of cells with elevated calpain expression compared with that from Alzheimer’s and normal control samples (N.L.B., unpublished data). Hence, increased calpain activity in MS plaques appears to be a result of both up-regulated calpain translational expression in glial/inflammatory cells and the migration of these cells to the lesion. Parkinson’s and Alzheimer’s diseases primarily are associated with neuronal damage in CNS gray matter and mesencephalic nuclei, wherein previous studies have observed increased calpain activation and expression (20–22). However, in MS, increased calpain activity does not appear to be confined to the lesion borders and is likely proportional to the number and activation state of glial and infiltrating inflammatory cells in the NAWM. Recent imaging studies also have demonstrated abnormalities in NAWM (37, 38).
Although calpain activation depends on increased calcium levels, the mechanism of calpain activation in disease remains poorly understood. Neither ubiquitous calpain isoform is appreciably activated at normal intracellular calcium concentrations (≈100 nM), whereas extracellular calcium levels (≈2 mM) can activate both isoforms (39). Thus, calpain localized in the myelin sheath may participate in intracellular myelin protein degradation if cytoplasmic calcium levels are increased in disease. In vitro studies have demonstrated vesicular disruption of the myelin sheath, similar to that in MS, when rat sciatic nerves were exposed to calcium ionophores at physiological pH (40). Complement fixation and perforin damage are possible calcium entry mechanisms in vivo (41, 42). Myelin proteins also may be degraded by calpain secreted from activated glial/inflammatory cells because calpain is activated at extracellular calcium levels. Previous in vitro and in vivo studies have observed calpain secretion from various cell types, including activated macrophages and T cells, with subsequent degradation of myelin proteins (26, 43–45).
Calpain translational expression as measured by levels of the 80-kDa m-calpain proenzyme was consistent with activity levels observed with the 76-kDa active form of μ-calpain. Thus, at least some of the increased calpain activity appears to depend on increased translational expression of the enzyme. Interestingly, levels of proenzyme m-calpain in NAWM were highly variable. This finding suggests that calpain levels are higher in NAWM adjacent to a lesion compared with white matter farther away. In addition to calpain translational expression, increased calpain activity also may result from insufficient calpastatin levels. Because the calpain/calpastatin ratio is critical for regulation of calpain activation, calpastatin expression also must be increased to properly control excessive calpain activation. The translational expression of both commonly observed calpastatin isoforms (110 and 68 kDa) remained largely unchanged in MS samples compared with normal controls (46). An increase in the 40-kDa isoform in MS plaque samples remains poorly understood, but may represent calpastatin fragments cleaved by activated calpain. Thus, calpain activity may be poorly regulated by calpastatin in pathological conditions such as MS.
As observed in previous animal model studies, calpain translational expression appears to be regulated posttranscriptionally (7, 36). This phenomenon has been observed in other rodent models and suggests that RNA-binding proteins may play a role in regulating calpain expression via alterations in mRNA stability, translational initiation, or mRNA masking, as demonstrated with other cysteine proteinases (e.g., cathepsin B) (47, 48).
In summary, because calpain activity and expression are up-regulated in glial/inflammatory cells involved in the autoimmune response, this enzyme, which degrades all major myelin proteins, may play a critical role in demyelination associated with MS. Thus, with the use of cell-permeable, calpain-specific inhibitors, the precise function(s) of calpain must be clarified further in animal models and human autoimmune demyelinating diseases.
Acknowledgments
Critical evaluation of this manuscript by William R. Tyor, M.D., and Swapan K. Ray, Ph.D., is greatly appreciated. Tissue specimens used in this study were obtained from the National Neurological Research Specimen Bank, Veterans Administration Medical Center, Los Angeles, CA, which is sponsored by the National Institute of Neurological Disorders and Stroke/National Institute of Mental Health, National MS Society, Hereditary Disease Foundation, Comprehensive Epilepsy Program, Tourette Syndrome Association, Dystonia Medical Research Foundation, and Veterans Health Services and Research Administration, Department of Veterans Affairs. This work was supported in part by grants from National Institute of Neurological Disorders and Stroke, National Institutes of Health (NS-31622 and NS-38146), Grant SCRF-1238 from the Paralyzed Veterans of America, Grant RG-2130B2 from the National MS Society, and the Medical University of South Carolina Medical Scientist Training Program with a scholarship provided by South Carolina Electric and Gas (D.C.S.).
ABBREVIATIONS
- MS
multiple sclerosis
- EAE
experimental allergic encephalomyelitis
- NAWM
normal-appearing white matter
- CNS
central nervous system
References
- 1.McFarlin D, McFarland H. N Eng J Med. 1982;307:1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- 2.McFarlin D, McFarland H. N Eng J Med. 1982;307:1246–1251. doi: 10.1056/NEJM198211113072005. [DOI] [PubMed] [Google Scholar]
- 3.Martin R, McFarland H. Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 4.Smith M. Neurochem Res. 1977;2:223–246. doi: 10.1007/BF00969354. [DOI] [PubMed] [Google Scholar]
- 5.Einstein E, Dalal K, Csejtey J. J Neurol Sci. 1970;11:109–121. doi: 10.1016/0022-510x(70)90121-8. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Quarles R, Brady R, Tourtellotte W. Ann Neurol. 1984;15:264–267. doi: 10.1002/ana.410150310. [DOI] [PubMed] [Google Scholar]
- 7.Shields D, Banik N. Brain Res. 1998;794:68–74. doi: 10.1016/s0006-8993(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Sorimachi H, Yoshizawa T, Kinbara K, Ishiura S. Biol Chem. 1995;376:523–529. doi: 10.1515/bchm3.1995.376.9.523. [DOI] [PubMed] [Google Scholar]
- 9.Mellgren R. FASEB J. 1987;1:110–115. doi: 10.1096/fasebj.1.2.2886390. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Tsuji S, Kubota S, Kimura Y, Imahori K, Imahori K. J Biochem. 1981;90:275–278. doi: 10.1093/oxfordjournals.jbchem.a133463. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Inomata M, Imajoh S, Suzuki K, Kawashima S. Biochemistry. 1989;28:449–455. doi: 10.1021/bi00428a007. [DOI] [PubMed] [Google Scholar]
- 12.Schlaepfer W, Zimmerman U. In: Intracellular Calcium-Dependent Proteolysis. Mellgren R L, Murachi T, editors. Boca Raton, FL: CRC; 1990. pp. 241–250. [Google Scholar]
- 13.Nelson W, Traub P. J Biol Chem. 1982;257:5544–5553. [PubMed] [Google Scholar]
- 14.Dayton W, Reville W, Goll D, Stromer M. Biochemistry. 1976;15:2159–2167. doi: 10.1021/bi00655a020. [DOI] [PubMed] [Google Scholar]
- 15.Sakai K, Akanuma H, Imahori K, Kawashima S. J Biochem. 1987;101:911–918. doi: 10.1093/oxfordjournals.jbchem.a121959. [DOI] [PubMed] [Google Scholar]
- 16.Tsubata T, Takahashi K. J Biochem. 1989;105:23–28. doi: 10.1093/oxfordjournals.jbchem.a122611. [DOI] [PubMed] [Google Scholar]
- 17.Banik N, Chakrabarti A, Hogan E. In: Myelin, Biology and Chemistry. Martenson R, editor. Caldwell, NJ: Telford Press; 1992. pp. 571–597. [Google Scholar]
- 18.Azuma M, Inoue E, Oka T, Shearer T. Curr Eye Res. 1995;14:27–34. doi: 10.3109/02713689508999911. [DOI] [PubMed] [Google Scholar]
- 19.Bartus R, Heyward N, Elliott P, Sawyer S, Dean R, Akiyuama A, Straub J, Harbeson S, Li Z. Stroke. 1995;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- 20.Mouatt-Prigent A, Karlsson J, Agid Y, Hirsch E. Neuroscience. 1996;73:979–987. doi: 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji T, Shimohama S, Kimura J, Shimizu K. Neurosci Lett. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, Elce J, Hamos J, Nixon R. Proc Nat Acad Sci USA. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmle U. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O, Rosebrough N, Farr A, Randall R. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Towbin H, Stachlin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saido T, Suzuki H, Yamazaki H, Tanoue K, Suzuki K. J Biol Chem. 1993;268:7422–7426. [PubMed] [Google Scholar]
- 27.Saido T, Yokota M, Nagao S, Yamaura I, Tani E, Tsuchiya T, Suzuki K, Kawashima S. J Biol Chem. 1993;268:25239–25243. [PubMed] [Google Scholar]
- 28.Shields D, Ray S, Wilford G, Banik N. J Neurosci Res. 1998;53:482–489. doi: 10.1002/(SICI)1097-4547(19980815)53:4<482::AID-JNR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Aoki K, Imajoh S, Ohno S, Emori Y, Koike M, Kosaki G, Suzuki K. FEBS Lett. 1986;205:313–317. doi: 10.1016/0014-5793(86)80919-x. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima-Ijima S, Hamada H, Reddy P, Kakunaga T. Proc Natl Acad Sci USA. 1985;82:6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brorson K, Brunswick M, Ezhevsky S, Wei D, Berg R, Scott D, Stein K. J Immunol. 1997;159:135–143. [PubMed] [Google Scholar]
- 32.Johnson S, Morgan D, Finch C. J Neurosci Res. 1986;16:267–280. doi: 10.1002/jnr.490160123. [DOI] [PubMed] [Google Scholar]
- 33.Harrison P, Heath P, Eastwood S, Burnet P, McDonald B, Pearson R. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- 34.Shields D, Tyor W, Deibler G, Banik N. Brain Res. 1998;784:299–304. doi: 10.1016/s0006-8993(97)01381-4. [DOI] [PubMed] [Google Scholar]
- 35.Shields D, Tyor W, Deibler G, Hogan E, Banik N. Proc Natl Acad Sci USA. 1998;95:5768–5772. doi: 10.1073/pnas.95.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shields D, Banik N. Exp Eye Res. 1998;67:403–410. doi: 10.1006/exer.1998.0537. [DOI] [PubMed] [Google Scholar]
- 37.Filippi M, Rocca M, Martino G, Horsfield M, Comi G. Ann Neurol. 1998;43:809–814. doi: 10.1002/ana.410430616. [DOI] [PubMed] [Google Scholar]
- 38.Lassmann H, Raine C, Antel J, Prineas J. J Immunol. 1998;86:213–217. doi: 10.1016/s0165-5728(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 39.Clapham D. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 40.Schlaepfer W. Nature (London) 1977;265:734–736. doi: 10.1038/265734a0. [DOI] [PubMed] [Google Scholar]
- 41.Scolding N, Jones J, Compston D, Morgan B. Immunology. 1990;70:6–10. [PMC free article] [PubMed] [Google Scholar]
- 42.Zajicek J, Wing M, Scolding N, Compston D. Brain. 1992;115:1611–1631. [PubMed] [Google Scholar]
- 43.Deshpande R, Goust J, Hogan E, Banik N. J Neurosci Res. 1995;42:259–265. doi: 10.1002/jnr.490420214. [DOI] [PubMed] [Google Scholar]
- 44.Adachi E, Mukaiyama T, Sasai K, Hayashi T, Kawashima S, Kasai Y, Hayashi M, Hashimoto P. Arch Histol Cytol. 1990;53:413–422. doi: 10.1679/aohc.53.413. [DOI] [PubMed] [Google Scholar]
- 45.Smith M, van der Maesen K, Somera F. J Neurosci Res. 1998;54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 46.Takano E, Ueda M, Tsunekawa S, Murakami T, Maki M, Hatanaka M, Murachi T. Biomed Biochem Acta. 1991;50:517–521. [PubMed] [Google Scholar]
- 47.Li J, Nixon R, Messer A, Berman S, Bursztajn S. Mol Brain Res. 1998;53:174–186. doi: 10.1016/s0169-328x(97)00295-7. [DOI] [PubMed] [Google Scholar]
- 48.Yano T, Kobayashi A, Kurata S, Natori S. Eur J Biochem. 1997;245:260–265. doi: 10.1111/j.1432-1033.1997.00260.x. [DOI] [PubMed] [Google Scholar]