Abstract
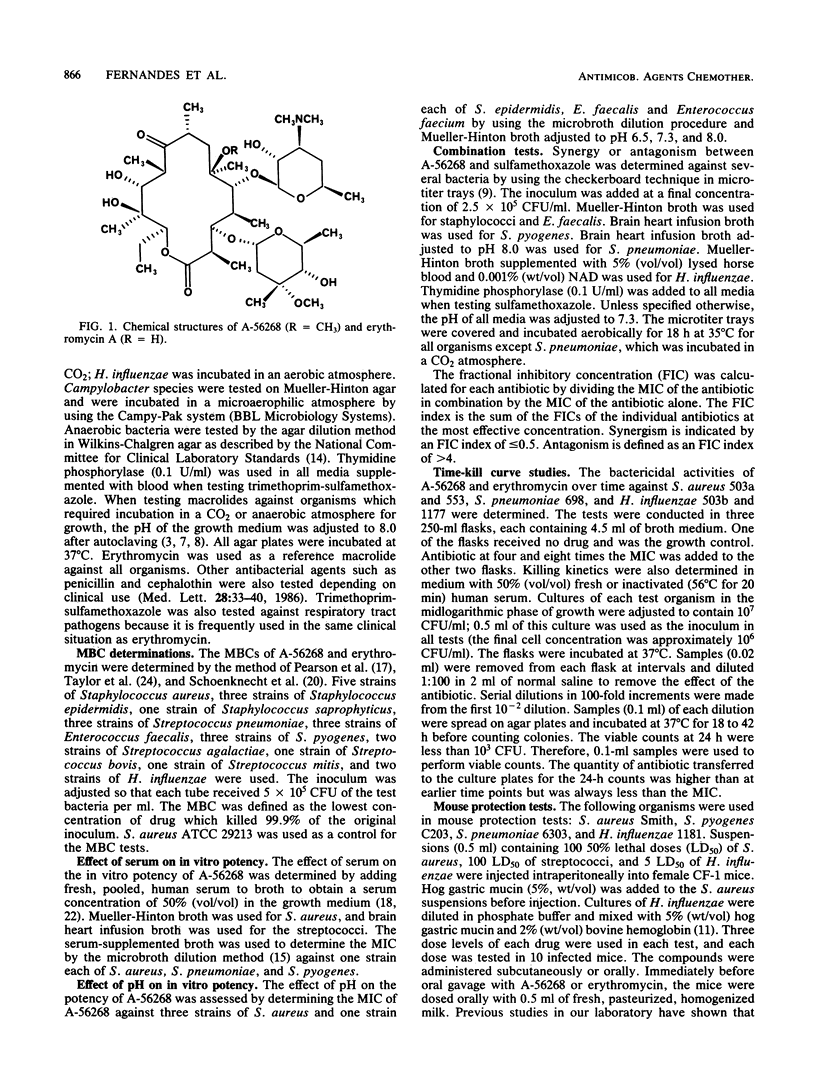
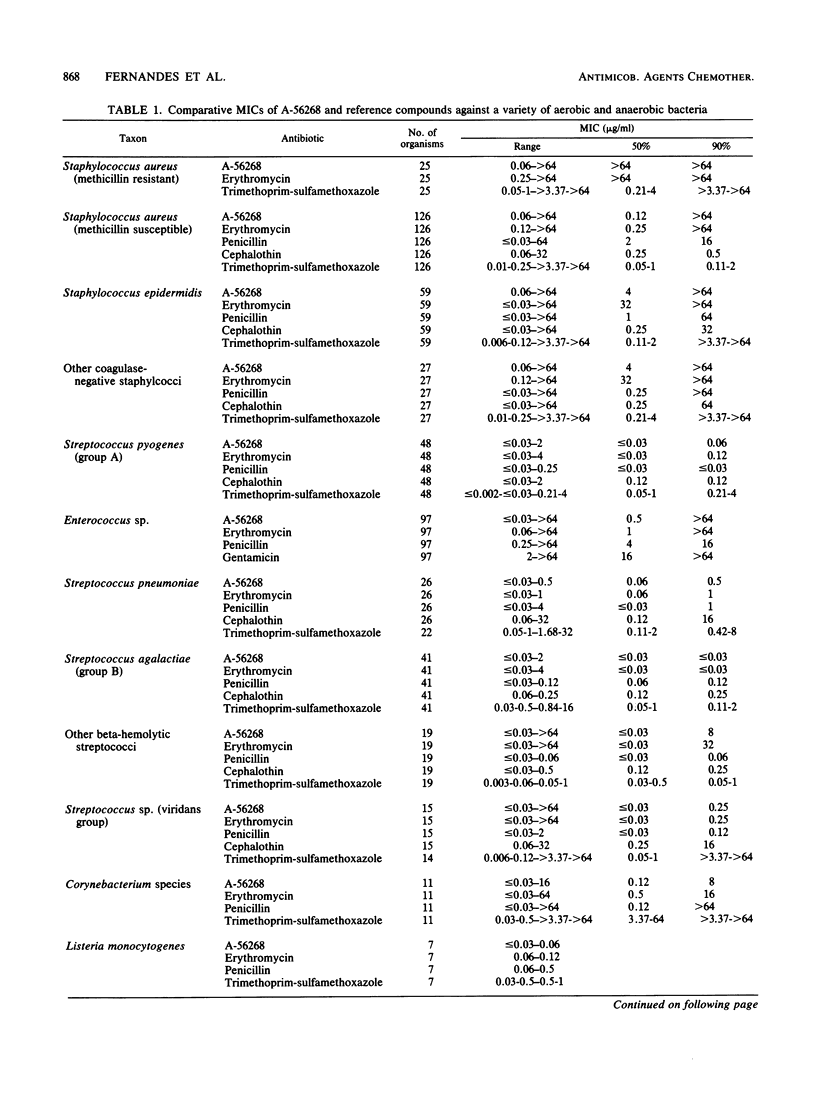
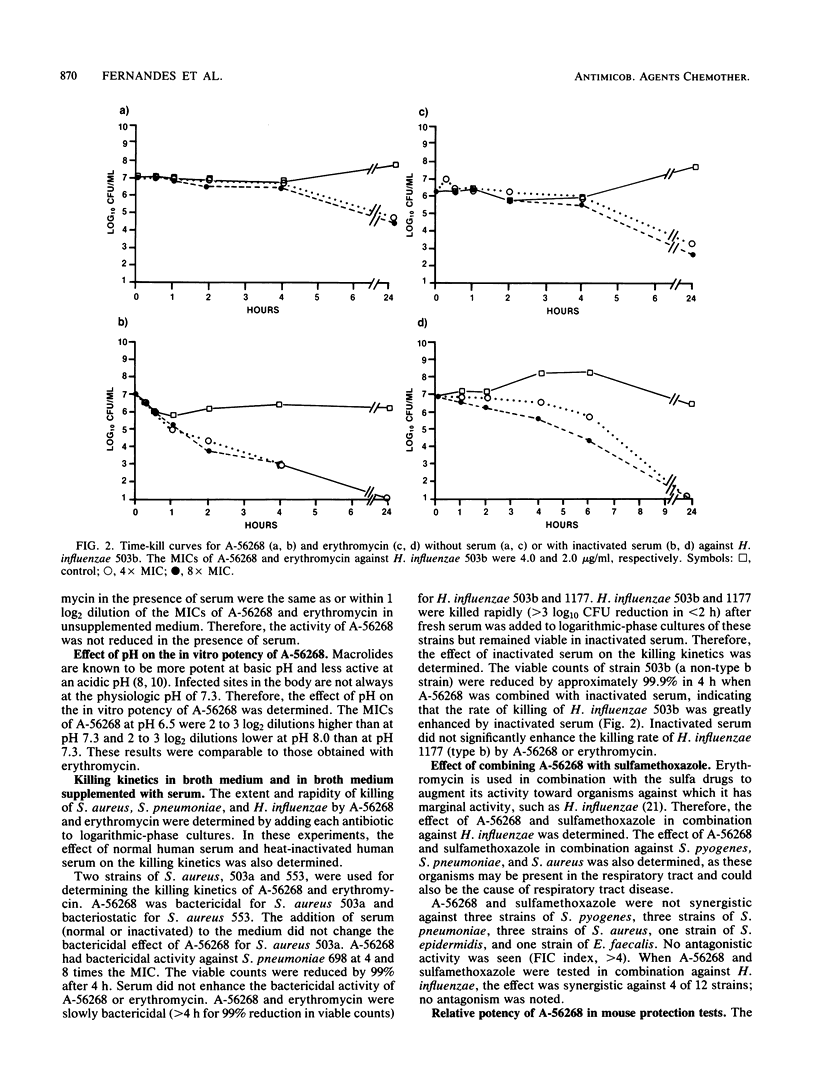
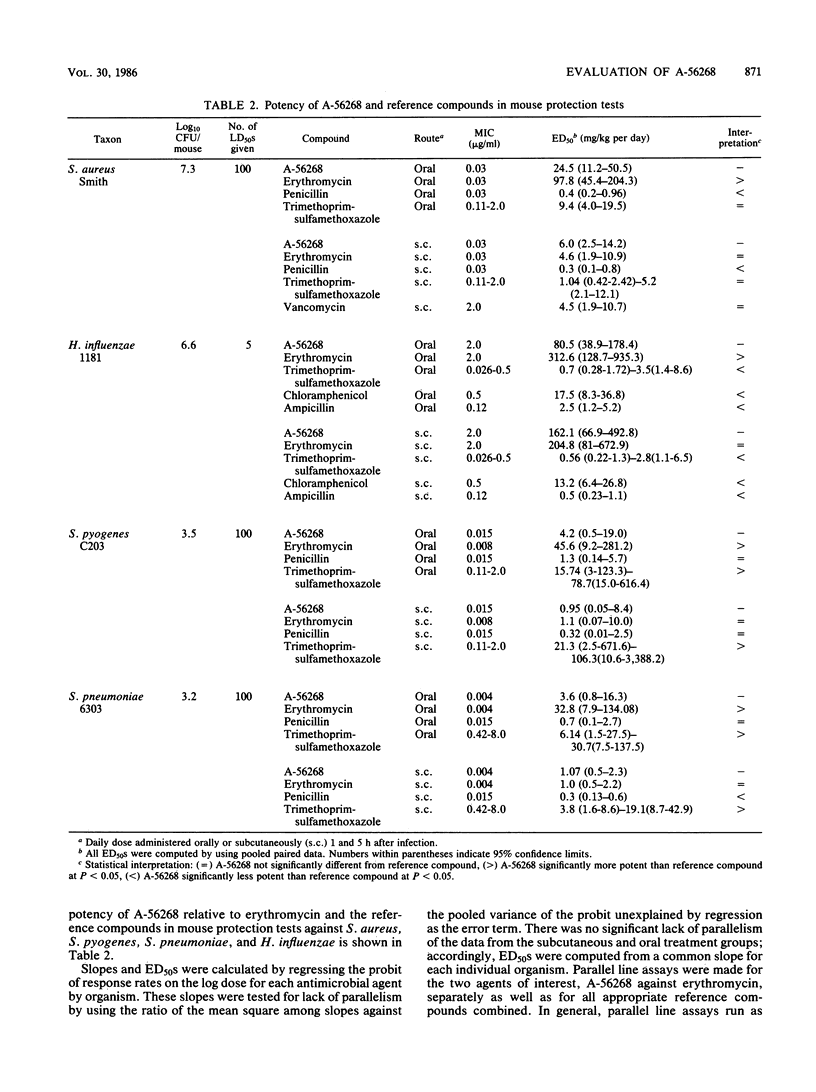
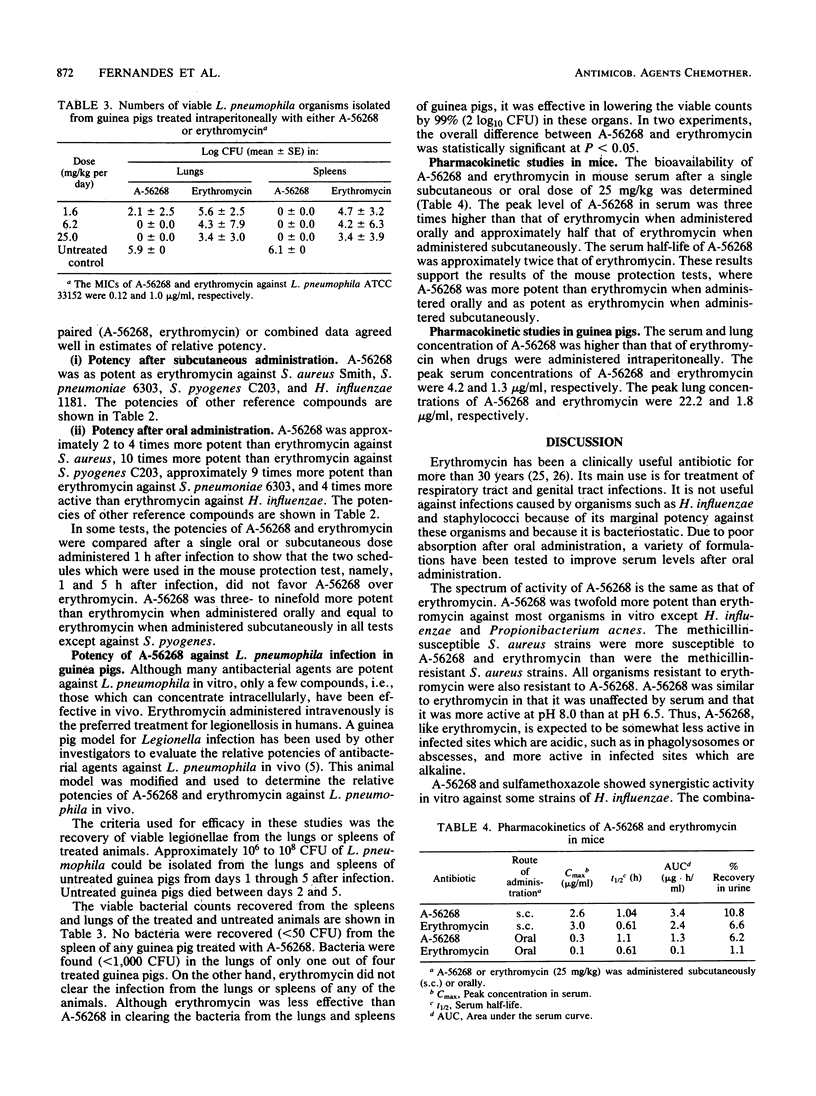
The in vitro and in vivo antibacterial activity of A-56268 (TE-031), the 6-O-methyl derivative of erythromycin, was compared with those of erythromycin and other reference drugs. A-56268 had the same spectrum of antibacterial activity as erythromycin. A-56268 was generally 1 log2 dilution more potent or equal to erythromycin against all organisms except haemophilus influenzae and Propionibacterium acnes, for which A-56268 was 1 log2 dilution and 3 log2 dilutions, respectively, less potent. The MBC of A-56268 and erythromycin was not significantly different from the MIC against Streptococcus pyogenes, Streptococcus pneumoniae, Staphylococcus epidermidis, and H. influenzae but was more than 2 log2 dilutions higher than the MICs for some Staphylococcus aureus strains. Human serum at a concentration of 50% did not change the in vitro potency of A-56268 or erythromycin. A-56268 was similar to erythromycin in being more active at pH 8.0 than at the physiologic pH of 7.3. The activity of A-56268 was synergistic with sulfamethoxazole against 4 of 12 strains of H. influenzae. In mouse protection tests, when administered orally A-56268 was more potent than erythromycin against H. influenzae, S. pyogenes, S. pneumoniae, and S. aureus. After subcutaneous administration the potencies of A-56268 and erythromycin were not statistically different from each other. A-56268 was more potent than erythromycin against Legionella infection in guinea pigs. The concentration of A-56268 in the serum and lung was higher than that of erythromycin after intraperitoneal administration. In A-56268 in the serum and lung was higher than that of erythromycin after intraperitoneal administration. In mice, the peak levels in serum of A-56268 and erythromycin were similar after subcutaneous administration and seven times higher for A-56268 after oral administration. The serum half-life of A-56268 was approximately twice that of erythromycin after administration by both routes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlam T., Neu H. C. In vitro comparison of the activity of RU 28965, a new macrolide, with that of erythromycin against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Apr;25(4):529–531. doi: 10.1128/aac.25.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Hamman J. W., Grundy W. E. Micromethod for assaying serum levels of erythromycin. Appl Microbiol. 1969 Jan;17(1):88–92. doi: 10.1128/am.17.1.88-92.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson J. E., Larsson P. pH and incubation atmosphere influence erythromycin activity against Branhamella catarrhalis. J Antimicrob Chemother. 1985 May;15(5):644–645. doi: 10.1093/jac/15.5.644. [DOI] [PubMed] [Google Scholar]
- Edelstein P. H., Calarco K., Yasui V. K. Antimicrobial therapy of experimentally induced Legionnaires' disease in guinea pigs. Am Rev Respir Dis. 1984 Nov;130(5):849–856. doi: 10.1164/arrd.1984.130.5.849. [DOI] [PubMed] [Google Scholar]
- Griffith R. S., Brier G. L., Wolny J. D. Synergistic action of erythromycin and cefamandole against Bacteroides fragilis subsp. fragilis. Antimicrob Agents Chemother. 1977 May;11(5):813–816. doi: 10.1128/aac.11.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. L., Swomley P., Drusano G. Effect of carbon dioxide and pH on susceptibility of Bacteroides fragilis group to erythromycin. Antimicrob Agents Chemother. 1981 Feb;19(2):335–336. doi: 10.1128/aac.19.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian V., Sabath L. D. Effect of pH on the activity of erythromycin against 500 isolates of gram-negative bacilli. Appl Microbiol. 1970 Nov;20(5):754–756. doi: 10.1128/am.20.5.754-756.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S., Takahashi Y., Watanabe Y., Omura S. Chemical modification of erythromycins. I. Synthesis and antibacterial activity of 6-O-methylerythromycins A. J Antibiot (Tokyo) 1984 Feb;37(2):187–189. doi: 10.7164/antibiotics.37.187. [DOI] [PubMed] [Google Scholar]
- Omura S., Sano H., Sunazuka T. Structure activity relationships of spiramycins. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):1–11. doi: 10.1093/jac/16.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. E., Schoenknecht F. Effect of several components of anaerobic incubation on antibiotic susceptibility test results. Antimicrob Agents Chemother. 1972 May;1(5):433–440. doi: 10.1128/aac.1.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. H., Wilson D. A., Stamm J. M., Chazen E. M. Treatment of otitis media caused by Hemophilus influenzae: evaluation of three antimicrobial regimens. South Med J. 1978 Dec;71(12):1493–1497. doi: 10.1097/00007611-197812000-00015. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Reller L. B. Serum dilution test for bactericidal activity. I. Selection of a physiologic diluent. J Infect Dis. 1977 Aug;136(2):187–195. doi: 10.1093/infdis/136.2.187. [DOI] [PubMed] [Google Scholar]
- Tadanier J., Martin J. R., Egan R. S., Goldstein A. W., Stanaszek R. S., Hirner E., Fischer F. Some chemical and stereochemical modifications of the erythromycin lactone rings. J Org Chem. 1974 Aug 23;39(17):2495–2501. doi: 10.1021/jo00931a006. [DOI] [PubMed] [Google Scholar]
- Taylor P. C., Schoenknecht F. D., Sherris J. C., Linner E. C. Determination of minimum bactericidal concentrations of oxacillin for Staphylococcus aureus: influence and significance of technical factors. Antimicrob Agents Chemother. 1983 Jan;23(1):142–150. doi: 10.1128/aac.23.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Wilson W. R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (1). Mayo Clin Proc. 1985 Mar;60(3):189–203. doi: 10.1016/s0025-6196(12)60219-5. [DOI] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Wilson W. R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (2). Mayo Clin Proc. 1985 Apr;60(4):271–278. doi: 10.1016/s0025-6196(12)60322-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Watanabe T., Shomura T., Someya S., Okamoto R., Ishihara S., Miyauchi K., Kazuno Y. Bacteriological evaluation of midecamycin acetate and its metabolites. Jpn J Antibiot. 1982 Jun;35(6):1462–1474. [PubMed] [Google Scholar]



