Abstract
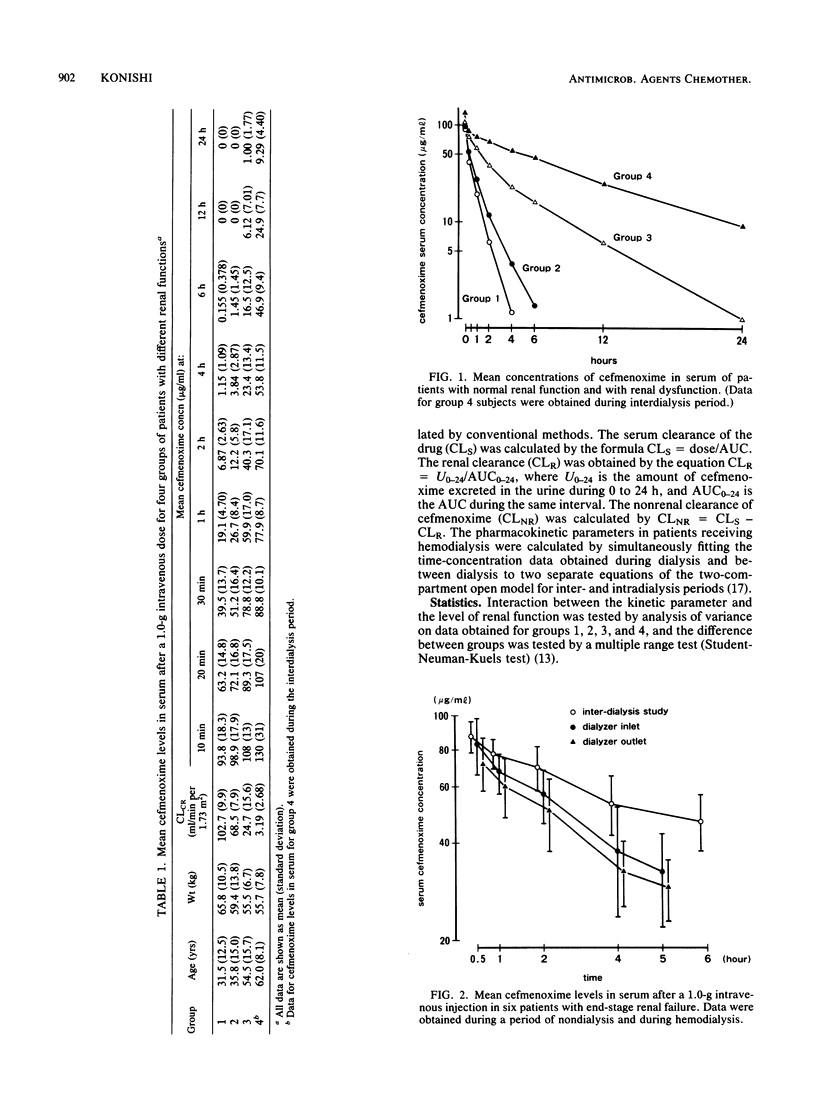
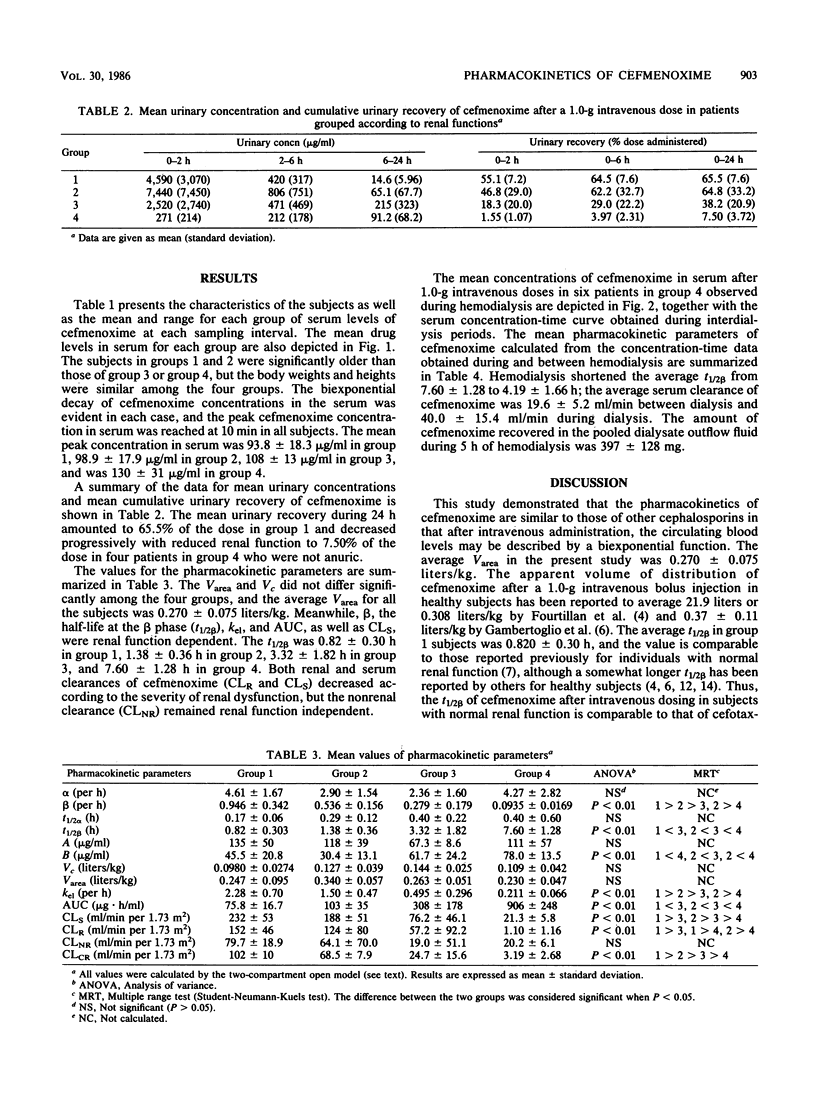
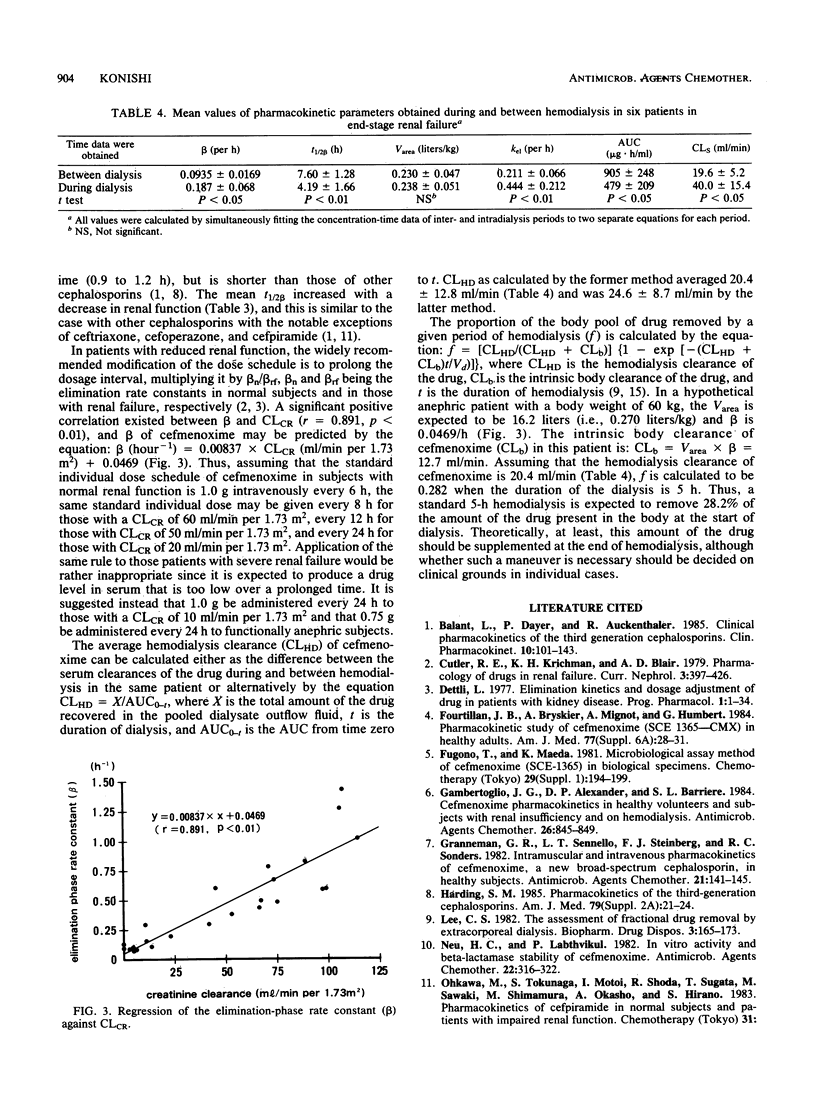
The pharmacokinetics of cefmenoxime were studied after a single intravenous 1.0-g dose to 24 subjects grouped according to their renal functions. Creatinine clearance was above 85, 50 to 85, 10 to 50, and below 10 ml/min per 1.73 m2 in groups 1, 2, 3, and 4, respectively. Cefmenoxime obeyed two-compartment-model kinetics in all four groups. The volume of distribution based on the area under the serum concentration-time curve was renal function independent, the average value being 0.270 +/- 0.075 liters/kg. The elimination-phase half-life (t1/2 beta) was 0.82 +/- 0.30 h in group 1, 1.38 +/- 0.36 h in group 2, 3.32 +/- 1.82 h in group 3, and 7.60 +/- 1.28 h in group 4. Cumulative 24-h urinary excretion accounted for 65.5 +/- 7.6% of the dose in group 1 and for 7.50 +/- 3.72% in group 4. Recommendations for dosage adjustment in patients with renal insufficiency are proposed based on the data obtained in this study. The effect of hemodialysis on cefmenoxime pharmacokinetics was studied in six patients in group 4; hemodialysis shortened the average t1/2 beta from 7.60 +/- 1.28 to 4.19 +/- 1.66 h. It was estimated that in a hypothetical anephric subject with a body weight of 60 kg, 5-h hemodialysis would remove 28.2% of the drug present in the body at the start of hemodialysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balant L., Dayer P., Auckenthaler R. Clinical pharmacokinetics of the third generation cephalosporins. Clin Pharmacokinet. 1985 Mar-Apr;10(2):101–143. doi: 10.2165/00003088-198510020-00001. [DOI] [PubMed] [Google Scholar]
- Fourtillan J. B., Bryskier A., Mignot A., Borsa F., Humbert G. Pharmacokinetic study of cefmenoxime (SCE 1365-CMX) in healthy adults. Am J Med. 1984 Dec 21;77(6A):28–31. doi: 10.1016/s0002-9343(84)80071-6. [DOI] [PubMed] [Google Scholar]
- Gambertoglio J. G., Alexander D. P., Barriere S. L. Cefmenoxime pharmacokinetics in healthy volunteers and subjects with renal insufficiency and on hemodialysis. Antimicrob Agents Chemother. 1984 Dec;26(6):845–849. doi: 10.1128/aac.26.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman G. R., Sennello L. T., Steinberg F. J., Sonders R. C. Intramuscular and intravenous pharmacokinetics of cefmenoxime, a new broad-spectrum cephalosporin, in healthy subjects. Antimicrob Agents Chemother. 1982 Jan;21(1):141–145. doi: 10.1128/aac.21.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M. Pharmacokinetics of the third-generation cephalosporins. Am J Med. 1985 Aug 9;79(2A):21–24. doi: 10.1016/0002-9343(85)90256-6. [DOI] [PubMed] [Google Scholar]
- Lee C. S. The assessment of fractional drug removal by extracorporeal dialysis. Biopharm Drug Dispos. 1982 Apr-Jun;3(2):165–173. doi: 10.1002/bdd.2510030207. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity and beta-lactamase stability of cefmenoxime. Antimicrob Agents Chemother. 1982 Aug;22(2):316–322. doi: 10.1128/aac.22.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk R. E., Sica D. A., Kerkering T. M., Kline B. J., Patterson P. M., Baggett J. W. Cefmenoxime pharmacokinetics in patients with renal insufficiency. Antimicrob Agents Chemother. 1984 Sep;26(3):322–327. doi: 10.1128/aac.26.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennello L. T., Quinn D., Rollins D. E., Tolman K. G., Sonders R. C. Effect of probenecid on the pharmacokinetics of cefmenoxime. Antimicrob Agents Chemother. 1983 Jun;23(6):803–807. doi: 10.1128/aac.23.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takki S., Gambertoglio J. G., Honda D. H., Tozer T. N. Pharmacokinetic evaluation of hemodialysis in acute drug overdose. J Pharmacokinet Biopharm. 1978 Oct;6(5):427–442. doi: 10.1007/BF01062724. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kida M., Nakao M., Iwahi T., Nishi T., Noji Y., Takeuchi M., Nozaki Y. Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56–65. doi: 10.1128/aac.19.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]



