Abstract
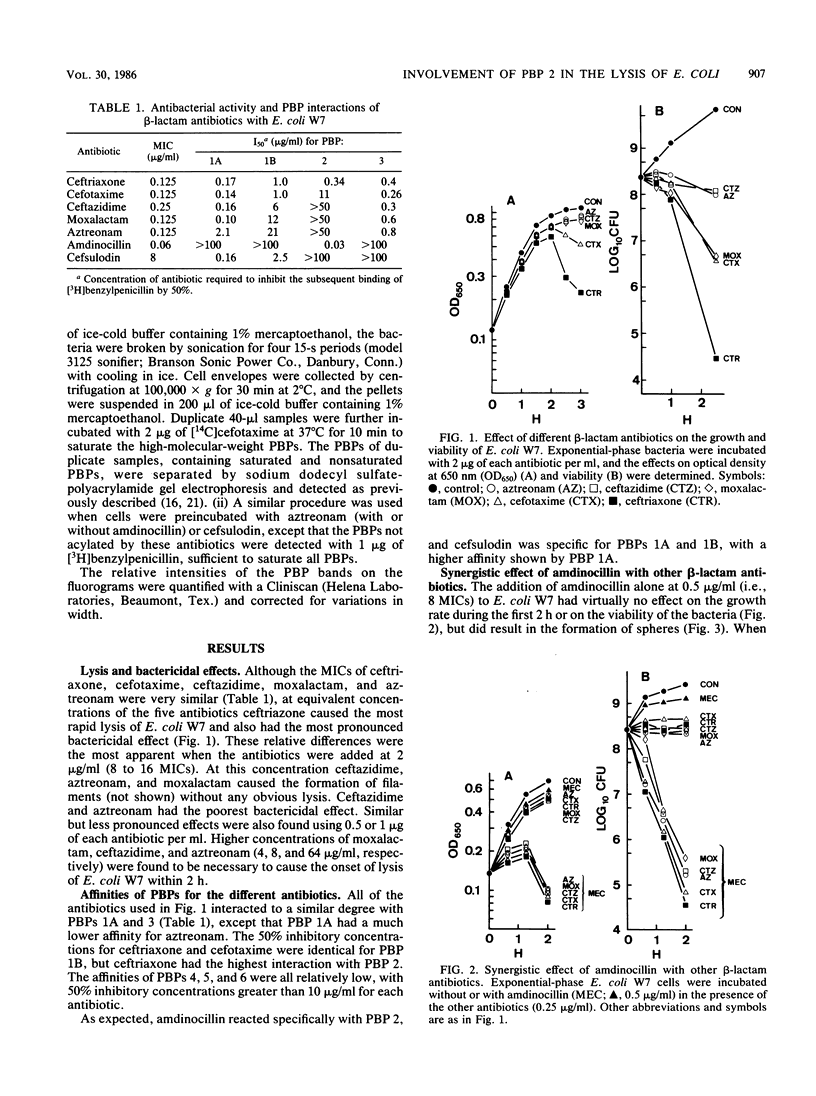
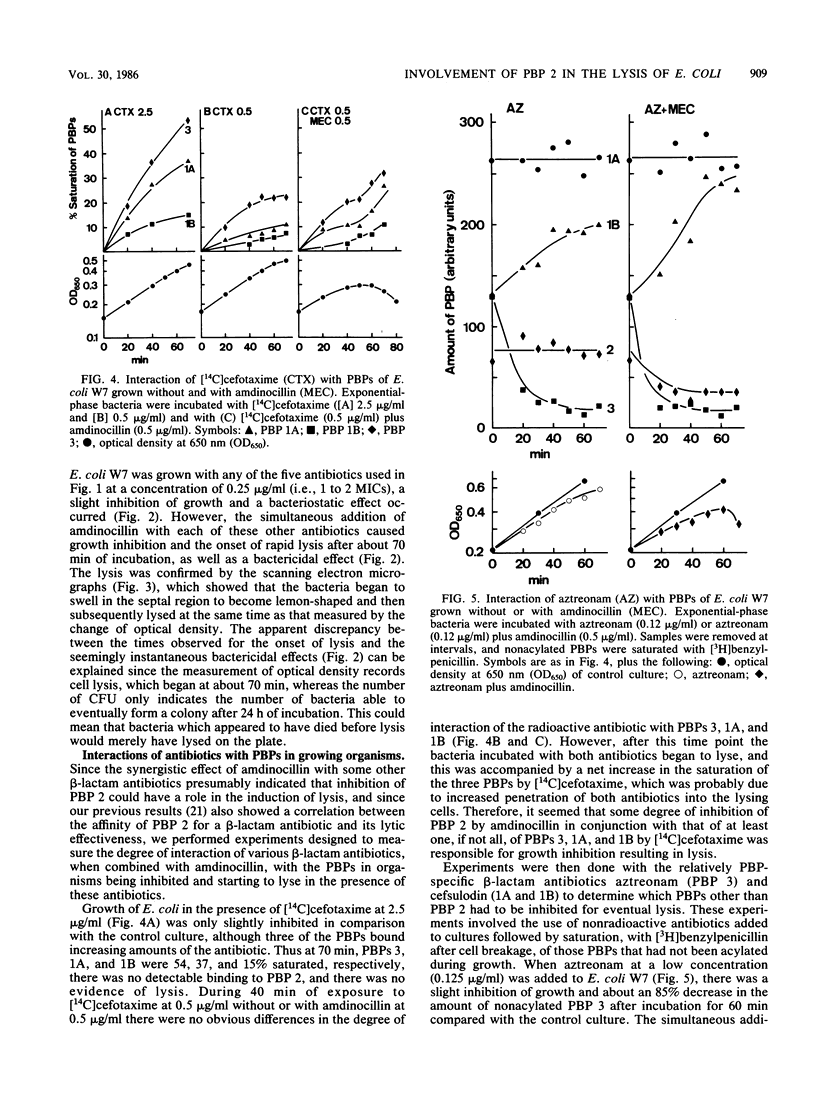
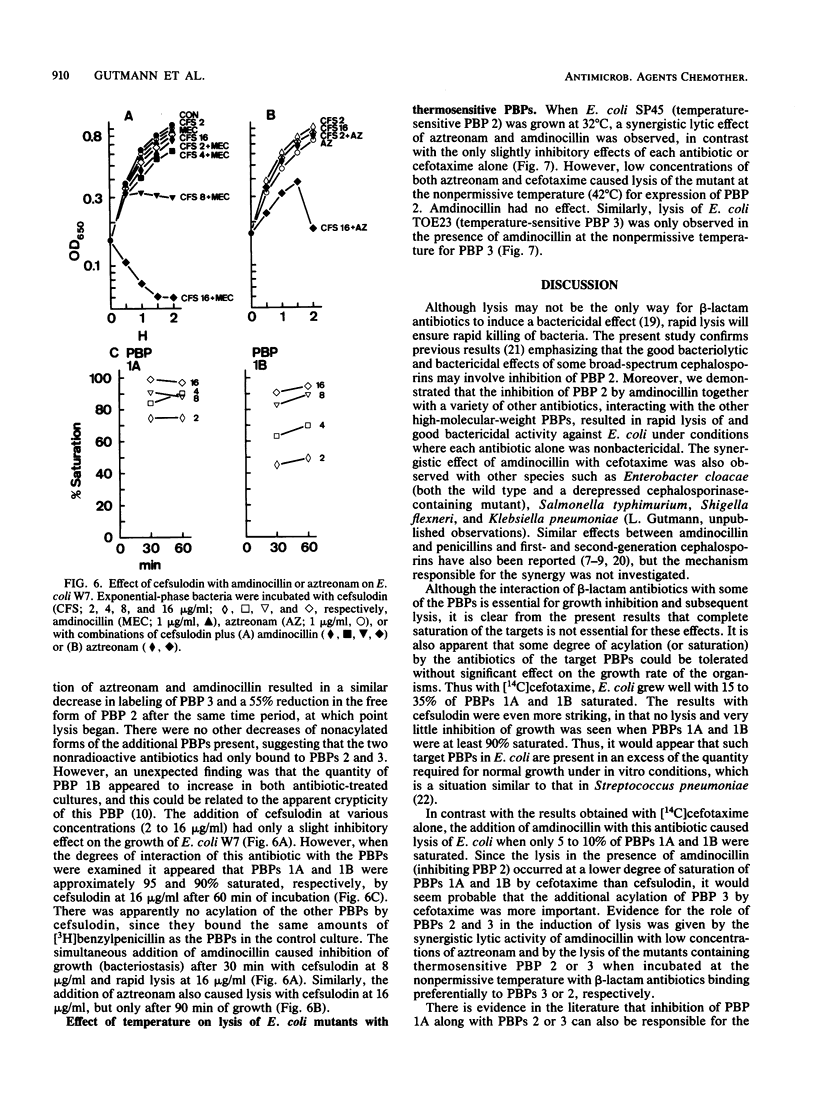
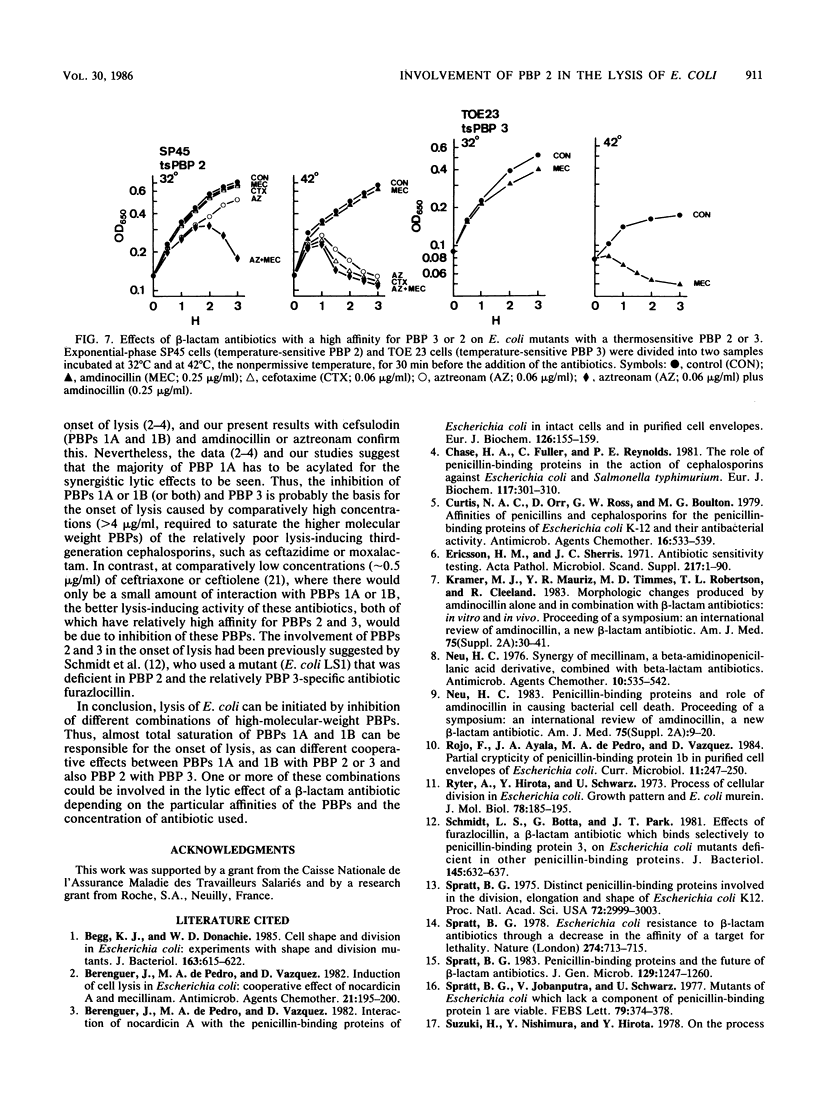
Compared with cefotaxime, ceftazidime, moxalactam, and aztreonam, ceftriaxone produced the best lytic and bactericidal effects when each was added at about 10 times the MIC to Escherichia coli W7. When each of these antibiotics was added at its MIC, only bacteriostasis occurred, but the simultaneous addition of amdinocillin (mecillinam) was synergistic in causing rapid lysis and bactericidal effects. Induction of lysis of two E. coli mutants containing either a thermosensitive penicillin-binding protein (PBP) 2 or 3 by relatively PBP 3-specific (aztreonam) and PBP 2-specific (amdinocillin) antibiotics indicated that inhibition of only PBPs 2 and 3 can cause lysis. Examination of the interactions of cefotaxime, aztreonam, and cefsulodin, with or without amdinocillin, with their targets suggested that other combinations of PBPs could be involved in the onset of lysis. However, inhibition of both PBPs 2 and 3 may explain the better lysis-inducing activity of ceftriaxone (which binds well to both of these PBPs), as well as the synergistic effect of amdinocillin when added together with low concentrations of other beta-lactam antibiotics that interact with PBP 3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg K. J., Donachie W. D. Cell shape and division in Escherichia coli: experiments with shape and division mutants. J Bacteriol. 1985 Aug;163(2):615–622. doi: 10.1128/jb.163.2.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer J., De Pedro M. A., Vázquez D. V. Interaction of nocardicin A with the penicillin-binding proteins of Escherichia coli in intact cells and in purified cell envelopes. Eur J Biochem. 1982 Aug;126(1):155–159. doi: 10.1111/j.1432-1033.1982.tb06760.x. [DOI] [PubMed] [Google Scholar]
- Berenguer J., de Pedro M. A., Vázquez D. Induction of cell lysis in Escherichia coli: cooperative effect of nocardicin A and mecillinam. Antimicrob Agents Chemother. 1982 Feb;21(2):195–200. doi: 10.1128/aac.21.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H. A., Fuller C., Reynolds P. E. The role of penicillin-proteins in the action of cephalosporins against Escherichia coli and Salmonella typhimurium. Eur J Biochem. 1981 Jul;117(2):301–310. doi: 10.1111/j.1432-1033.1981.tb06337.x. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. J., Mauriz Y. R., Timmes M. D., Robertson T. L., Cleeland R. Morphologic changes produced by amdinocillin alone and in combination with beta-lactam antibiotics: in vitro and in vivo. Am J Med. 1983 Aug 29;75(2A):30–41. doi: 10.1016/0002-9343(83)90091-8. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Penicillin-binding proteins and role of amdinocillin in causing bacterial cell death. Am J Med. 1983 Aug 29;75(2A):9–20. doi: 10.1016/0002-9343(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Neu J. C. Synergy of mecillinam, a beta-amidinopenicillanic acid derivative, combined with beta-lactam antibiotics. Antimicrob Agents Chemother. 1976 Sep;10(3):535–542. doi: 10.1128/aac.10.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Schmidt L. S., Botta G., Park J. T. Effects of furazlocillin, a beta-lactam antibiotic which binds selectively to penicillin-binding protein 3, on Escherichia coli mutants deficient in other penicillin-binding proteins. J Bacteriol. 1981 Jan;145(1):632–637. doi: 10.1128/jb.145.1.632-637.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Escherichia coli resistance to beta-lactam antibiotics through a decrease in the affinity of a target for lethality. Nature. 1978 Aug 17;274(5672):713–715. doi: 10.1038/274713a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. On the mechanism of the irreversible antimicrobial effects of beta-lactams. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):303–308. doi: 10.1098/rstb.1980.0047. [DOI] [PubMed] [Google Scholar]
- Tybring L., Melchior N. H. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: bactericidal action and synergy in vitro. Antimicrob Agents Chemother. 1975 Sep;8(3):271–276. doi: 10.1128/aac.8.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Gutmann L., Kitzis M. D., Acar J. F. An evaluation of the bacteriolytic and biochemical properties of ceftiolene (42980RP). J Antimicrob Chemother. 1984 Dec;14(6):581–593. doi: 10.1093/jac/14.6.581. [DOI] [PubMed] [Google Scholar]
- Williamson R., Tomasz A. Inhibition of cell wall synthesis and acylation of the penicillin binding proteins during prolonged exposure of growing Streptococcus pneumoniae to benzylpenicillin. Eur J Biochem. 1985 Sep 16;151(3):475–483. doi: 10.1111/j.1432-1033.1985.tb09126.x. [DOI] [PubMed] [Google Scholar]
- Yousif S. Y., Broome-Smith J. K., Spratt B. G. Lysis of Escherichia coli by beta-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985 Oct;131(10):2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]