Abstract
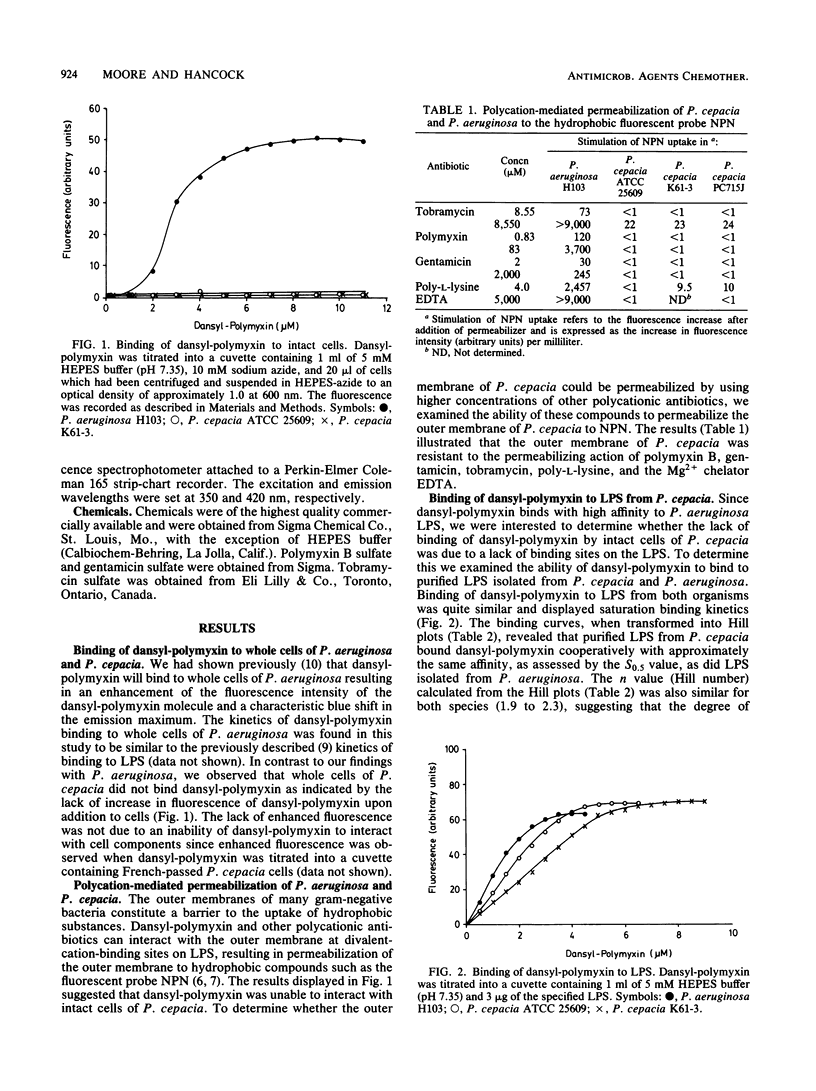
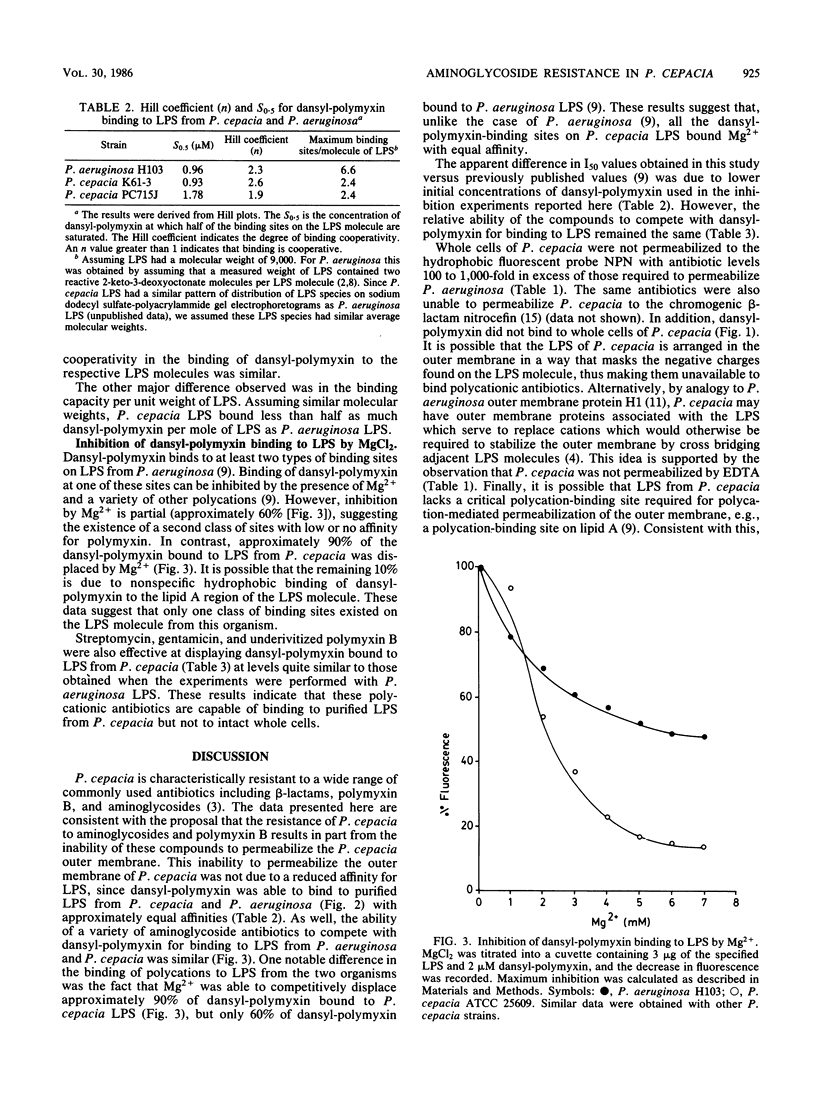
Pseudomonas cepacia was found to be resistant to the outer membrane-permeabilizing effects of aminoglycoside antibiotics, polymyxin B, and EDTA. Permeabilization of P. cepacia to the fluorescent probe 1-N-phenylnaphthylamine was not achieved at concentrations 100- to 1,000-fold above those required to permeabilize Pseudomonas aeruginosa. Furthermore, in contrast to P. aeruginosa cells, intact cells of P. cepacia did not bind the fluorescent probe dansyl-polymyxin. However, purified lipopolysaccharide (LPS) from P. cepacia bound dansyl-polymyxin with approximately the same affinity as did LPS from P. aeruginosa. Also, binding of dansyl-polymyxin to P. cepacia (and P. aeruginosa) LPS was inhibited by polymyxin B, streptomycin, gentamicin, and Mg2+. These data suggest that P. cepacia does not utilize the self-promoted pathway for aminoglycoside uptake and that the outer membrane is arranged in a way that conceals or protects cation-binding sites on LPS which are capable of binding polycations such as aminoglycosides or polymxyin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J., Teuber M. Action of polymyxin B on bacterial membranes. 1. Binding to the O-antigenic lipopolysaccharide of Salmonella typhimurium. Z Naturforsch C. 1973 Jul-Aug;28(7):422–430. [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Barnishan J. In vitro susceptibilities of nonfermentative gram-negative bacilli other than Pseudomonas aeruginosa to 32 antimicrobial agents. Rev Infect Dis. 1980 Nov-Dec;2(6):841–853. doi: 10.1093/clinids/2.6.841. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wong P. G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984 Jul;26(1):48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh B., Grant C., Hancock R. E. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Oct;26(4):546–551. doi: 10.1128/aac.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manniello J. M., Heymann H., Adair F. W. Isolation of atypical lipopolysaccharides from purified cell walls of Pseudomonas cepacia. J Gen Microbiol. 1979 Jun;112(2):397–400. doi: 10.1099/00221287-112-2-397. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Bates N. C., Hancock R. E. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother. 1986 Mar;29(3):496–500. doi: 10.1128/aac.29.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. A., Chan L., Hancock R. E. Evidence for two distinct mechanisms of resistance to polymyxin B in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Oct;26(4):539–545. doi: 10.1128/aac.26.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. M., Cavill R. H. Electron microscope study of the effect of benzalkonium, chlorhexidine and polymyxin on Pseudomonas cepacia. Microbios. 1981;29(115):23–31. [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]



