Abstract
The goal of this study was to interrogate the role of inducible NO synthase (iNOS) in the late phase of ischemic preconditioning (PC) in vivo. A total of 321 mice were used. Wild-type mice preconditioned 24 h earlier with six cycles of 4-min coronary occlusion/4-min reperfusion exhibited a significant (P < 0.05) increase in myocardial iNOS protein content, iNOS activity (assessed as calcium-independent l-citrulline formation), and nitrite + nitrate tissue levels. In contrast, endothelial NOS protein content and calcium-dependent NOS activity remained unchanged. No immunoreactive neuronal NOS was detected. When wild-type mice were preconditioned 24 h earlier with six 4-min occlusion/4-min reperfusion cycles, the size of the infarcts produced by a 30-min coronary occlusion followed by 24 h of reperfusion was reduced markedly (by 67%; P < 0.05) compared with sham-preconditioned controls, indicating a late PC effect. In contrast, when mice homozygous for a null iNOS allele were preconditioned 24 h earlier with the same protocol, infarct size was not reduced. Disruption of the iNOS gene had no effect on early PC or on infarct size in the absence of PC. These results demonstrate that (i) the late phase of ischemic PC is associated with selective up-regulation of iNOS, and (ii) targeted disruption of the iNOS gene completely abrogates the infarct-sparing effect of late PC (but not of early PC), providing unequivocal molecular genetic evidence for an obligatory role of iNOS in the cardioprotection afforded by the late phase of ischemic PC. Thus, this study identifies a specific protein that mediates late PC in vivo.
Ischemic preconditioning (PC) is the phenomenon whereby a mild ischemic stress enhances the tolerance of the heart to a subsequent ischemic stress (1, 2). The time course of ischemic PC consists of an initial, short-lived (2–3 h) wave of protection (early phase of PC) followed, 12–24 h later, by a second, sustained window of protection that lasts at least 72 h (late phase of PC) (1–9). The early phase of PC is an immediate response caused by the activation of G-coupled membrane receptors and downstream kinases (1). The late phase, on the other hand, is a delayed adaptation that requires the synthesis of new proteins (10). Because of its sustained and powerful protective effects, the mechanism of the late phase of PC has became the focus of considerable interest (2).
Recent evidence suggests that the cardioprotection afforded by late PC is mediated by up-regulation of nitric oxide synthase (NOS) activity and, specifically, by the inducible isoform (iNOS). This concept is based on the finding that, in conscious rabbits, the beneficial effects of late PC are abrogated by the nonselective NOS inhibitor Nω-nitro-l-arginine as well as by two relatively selective iNOS inhibitors [aminoguanidine (AG) and S-methylisothiourea (SMT)], administered 24 h after the PC stimulus (7, 9). The results of these pharmacologic studies (7, 9), however, are inherently limited by several factors. First, the effects of NOS inhibitors provide only indirect evidence for the involvement of iNOS. Direct documentation that ischemic PC up-regulates the expression and activity of iNOS in cardiac tissue has never been reported. Second, AG and SMT are only 10–40 times selective for iNOS vs. endothelial NOS (eNOS) (11–13). Thus, it is possible that the doses used in those studies (7, 9) may have inhibited not only iNOS, but also eNOS and/or neuronal NOS (nNOS). Finally, both AG and SMT have numerous nonspecific actions unrelated to NOS inhibition (reviewed in ref. 12). As is apparent from these considerations, the concept that late PC is mediated by NOS, and that iNOS is the specific isoform involved, remains to be firmly established.
The availability of genetically engineered mice in which the iNOS gene is selectively disrupted (14, 15) offers an opportunity to conclusively establish whether iNOS is causally involved in late PC. We recently have developed a murine model of late PC in which fundamental physiologic variables that may impact on infarct size (body temperature, arterial oxygenation, acid-base balance, heart rate, and arterial blood pressure) are carefully controlled and kept within normal limits (16). In the present study, we used this model to interrogate the role of iNOS in the late phase of ischemic PC in vivo. The results show that targeted ablation of the iNOS gene abrogates late PC, demonstrating that iNOS is an essential mediator of this cardioprotective response.
METHODS
Wild-Type (WT) and iNOS−/− Mice.
The study was performed in male mice weighing 30.5 ± 0.6 g (age, 18.4 ± 0.8 wk). Breeding pairs of iNOS−/− mice were kindly provided by V. E. Laubach, University of Virginia (14), C. Nathan, Cornell University (15), and T. Hintze, New York Medical College, and maintained in the homozygous state. The iNOS−/− mice were of hybrid genetic background (C57BL/6J and 129/Sv [B6129/J]). WT mice with a genetic background (B6129F2/J) as close as possible to the iNOS−/− mice (14, 15) were used as controls. All mice were maintained in sterile microisolator cages under pathogen-free conditions. Food and water were autoclaved, and all handling was done under a laminar flow hood following standard procedures for maintaining pathogen-free transgenic mice. The iNOS−/− mice were genotyped by PCR and Southern blot hybridization as described (14, 15). Mice were typed as they were bred, and then retyped by using DNA prepared from tissue samples taken at the end of the experiments.
Experimental Preparation.
The experimental preparation has been described in detail (16). Briefly, mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and ventilated by using carefully selected parameters. After administration of antibiotics, the chest was opened through a midline sternotomy, and a nontraumatic balloon occluder was implanted around the mid-left anterior descending coronary artery by using an 8–0 nylon suture. To prevent hypotension, blood from a donor mouse was given during surgery. Rectal temperature was maintained close to 37°C throughout the experiment.
The study consisted of two successive phases. The objective of phase A was to determine whether ischemic PC up-regulates NOS and, if so which isoform(s). The aim of phase B was to determine whether the infarct-sparing effects of ischemic PC occur in iNOS−/− mice.
Phase A: Experimental Protocol.
Mice were assigned to four groups. In groups II (WT) and IV (iNOS−/−) (late PC groups), ischemic PC was produced with a sequence of six cycles of 4-min coronary occlusion/4-min reperfusion (16). The animals then were allowed to recover. Twenty-four hours later, the mice were euthanized, the heart was excised, and myocardial samples (≈20 mg) were rapidly removed from the ischemic-reperfused region (whose boundaries had been marked with 10–0 nylon sutures at the time of coronary occlusion) and the nonischemic region [posterior left ventricular (LV) wall], frozen in liquid nitrogen, and stored at −70°C until used. Groups I (WT) and III (iNOS−/−) (control groups) were subjected to the same protocol as groups II and IV (including 60 min of open-chest state and placement of the occluder and sutures) but did not undergo coronary occlusion; 24 h later, the mice were euthanized and myocardial samples were obtained from the anterior and posterior LV walls. To obtain sufficient tissue for analysis, samples from two hearts were combined into one sample (the measurements on the pooled sample were regarded as one observation, n = 1).
Western Immunoblotting Analysis.
Tissue samples were homogenized in buffer A (25 mM Tris⋅HCl, pH 7.5/0.5 mM EDTA/0.5 mM EGTA/1 mM PMSF/2 μM leupeptin/1 μM pepstatin/1 μM aprotinin/10 mM NaF/100 μM dephostatin) and centrifuged at 14,000 g for 15 min, and the resulting supernatants were collected as cytosolic fractions. The pellets were incubated in a lysis buffer (buffer A + 1% NP-40) for 4 h and centrifuged, and the resulting supernatants were used as membrane proteins. The protein content in the cytosolic and membranous fractions was determined by using the Bradford technique (Bio-Rad). The expression of iNOS, eNOS, and nNOS was assessed by using standard SDS/PAGE Western immunoblotting techniques. Briefly, proteins (80–120 μg) were electrophoresed on an 8% denaturing gel at 25 mA per gel for 2–3 h and then electrophoretically transferred onto nitrocellulose membranes (Bio-Rad) overnight at 4°C. Gel transfer efficiency was recorded carefully by making photocopies of membranes dyed with reversible Ponceau staining (17); gel retention was determined by Coomassie blue staining as described (17). The membranes were incubated in 5% nonfat dry milk in a washing buffer (10 mM Tris⋅HCl, pH 7.2/0.15 M NaCl/0.05% Tween-20) followed by incubation with specific polyclonal anti-iNOS, monoclonal anti-eNOS, and monoclonal or polyclonal anti-nNOS antibodies (Transduction Laboratories, Lexington, KY). After rinsing with washing buffer, the blots were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (depending on the primary antibody used) and developed with the use of an enhanced chemiluminescence system (ECL kit, Amersham Pharmacia). The NOS signals detected by immunoblotting and the corresponding records of Ponceau stains of nitrocellulose membranes were quantitated by using an image scanning densitometer (Personal PI, Molecular Dynamics). To quantitate NOS as accurately as possible, each NOS signal was normalized to the corresponding Ponceau stain signal (17). In all samples, the content of NOS protein was expressed as a percentage of the corresponding NOS protein in the anterior LV wall of group I (WT control mice).
Measurement of NOS Activity.
NOS activity was determined by measuring the conversion of [14C] l-arginine to [14C] l-citrulline by using a modification of the procedure of Bredt and Snyder (18). Briefly, isolated cytosolic or membrane proteins (80–120 μg) were incubated in assay buffer (total volume 80 μl) containing 50 mM Tris⋅HCl (pH 7.4), 1 mM NADPH, 5 μM FAD, 5 μM FMN, 10 μM tetrahydrobiopterin, 10 μM l-arginine, and purified [14C] l-arginine [≈220,000 cpm (≈0.1 μCi)/tube; NEN] at 30°C for 60 min. To determine calcium-dependent NOS (cNOS) activity, 2 mM CaCl2 and 100 nM calmodulin were included in the assay. To determine calcium-independent NOS (iNOS) activity, the assay was conducted in the presence of 1 mM EGTA without calcium and calmodulin. In both cases, duplicate assays were performed in the presence or absence of 1 mM Nω-nitro-l-arginine methyl ester, and the differences in cpm were used to calculate NOS activity. NOS activity was expressed as pmol of l-citrulline/min per mg protein.
Measurement of Nitrite and Nitrate (NOx).
Tissue samples were homogenized in a buffer containing 25 mM Tris⋅HCl (pH 7.5), 0.5 mM EDTA, and 0.5 mM EGTA and centrifuged at 14,000 g for 15 min, and the resulting supernatants were collected as cytosolic fractions. The supernatants were loaded to a Centricon-30 filtrator and centrifuged to remove substances larger than 30 kDa. Nitrite was assayed by using the Griess reaction as modified by Gilliam et al. (19). Nitrate content was determined after conversion of nitrate to nitrite with Aspergillus nitrate reductase (19). All assays were performed in duplicate. Tissue NOx levels were expressed as nmol/mg protein.
Phase B: Experimental Protocol.
The coronary occlusion/reperfusion protocols have been described in detail (16). In all groups, myocardial infarction was produced by a 30-min coronary occlusion followed by 24 h of reperfusion (16). Mice were assigned to eight groups. Groups V (WT) and VI (iNOS−/−) (control groups) underwent the 30-min occlusion with no prior PC or sham surgery. Groups IX (WT) and X (iNOS−/−) (late PC groups) underwent a sequence of six 4-min occlusion/4-min reperfusion cycles on day 1; 24 h later (day 2), the 8–0 nylon suture (which had been left in place after the first surgery) was used to produce the 30-min coronary occlusion (16). On day 1, groups VII (WT) and VIII (iNOS−/−) (late PC sham groups) were subjected to the same protocol as groups IX and X (including 60 min of open-chest state and placement of the 8–0 nylon suture) but did not undergo coronary occlusion; 24 h later (day 2), the mice underwent the 30-min occlusion. Groups XI (WT) and XII (iNOS−/−) (early PC groups) underwent the 30-min occlusion 10 min after six cycles of 4-min occlusion/4-min reperfusion.
Postmortem Tissue Analysis.
At the conclusion of the study, the occluded/reperfused vascular bed and the infarct were identified by postmortem perfusion of the heart with triphenyltetrazolium chloride and phthalo blue dye (16). Infarct size was calculated by using computerized videoplanimetry (16).
Statistical Analysis.
Data are reported as means ± SEM. Measurements were analyzed with a one-way or a two-way repeated-measures ANOVA, as appropriate, followed by unpaired Student’s t tests with the Bonferroni correction.
RESULTS
A total of 321 mice were used. Of the 299 mice used for phases A and B, 58 (19%) died or were excluded for technical reasons. In six open-chest mice ventilated at a rate of 105 breaths/min and a tidal volume of 2.0 ml (the settings used in phases A and B), arterial pH was found to average 7.44, pCO2 32.5 mmHg, pO2 204 mmHg, and HCO3− 20.6 mmol/liter. These results confirm our previous findings (16) and indicate that the respiratory settings selected in this study resulted in normal acid-base balance and adequate oxygenation. In 16 mice, mean arterial blood pressure (measured by cannulating the right carotid artery) remained above 80 mmHg throughout the six 4-min occlusion/4-min reperfusion cycles and thereafter, confirming our previous observations (16) and indicating that our blood replacement protocol (16) was effective in preventing hypotension.
Phase A: NOS Protein Expression.
In WT control mice (group I), 67% of total tissue iNOS protein was found in the cytosolic fraction and 33% in the membranous fraction. A representative Western blot analysis of iNOS in cytosolic fractions prepared from WT and iNOS−/− mice is illustrated in Fig. 1A. In WT mice, the total (cytosolic + membranous fractions) tissue content of iNOS in the ischemic-reperfused region increased 41 ± 6% above control levels (P < 0.05) 24 h after ischemic PC. The increase was more pronounced in the cytosolic fraction of the homogenate (+47 ± 12%; Fig. 2A). In iNOS−/− mice, iNOS protein signals were undetectable under control conditions as well as after ischemic PC (Figs. 1A and 2A). In contrast to iNOS, the expression of eNOS did not increase 24 h after ischemic PC, either in WT or in iNOS−/− mice (Figs. 1B and 3A). No nNOS was detected in control or preconditioned heart samples by using commercially available nNOS monoclonal or polyclonal antibodies (Transduction Laboratories) (Fig. 1C). For comparison, in a separate group of seven WT mice given lipopolysaccharide (12.5 mg/kg i.p.) 6 h earlier, the iNOS protein content in the cytosolic fraction of LV samples increased 853 ± 131% above control levels, i.e., ≈18 times more than after ischemic PC.
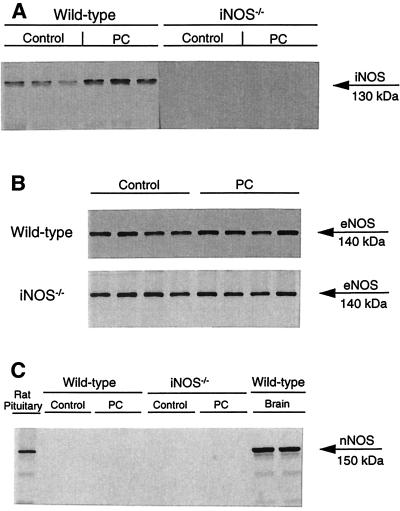
Figure 1.
Western immunoblots showing the effects of ischemic PC on iNOS (A), eNOS (B), and nNOS (C) expression in WT and iNOS−/− mice. Myocardial samples were obtained from the anterior LV wall of WT control mice and iNOS−/− control mice that underwent a sham operation (1 h of open-chest state without coronary occlusion/reperfusion; groups I and II, respectively) and from the ischemic-reperfused region of WT and iNOS−/− mice that underwent an ischemic PC protocol consisting of six 4-min occlusion/4-min reperfusion cycles (groups III and IV, respectively). Tissue samples were taken 24 h after sham surgery or ischemic PC. (A) iNOS immunoreactivity (cytosolic fraction) increased after ischemic PC in WT mice but was undetectable in iNOS−/− mice, either after sham operation or after ischemic PC. (B) eNOS immunoreactivity in total homogenates (cytosolic + membranous fractions) remained unchanged after ischemic PC both in WT and iNOS−/− mice. (C) No nNOS immunoreactivity was detected in total homogenates (cytosolic + membranous fractions) of myocardial samples, either after sham operation or after ischemic PC. In contrast, a robust expression of nNOS was detected in mouse brain and rat pituitary tissue (used as positive controls). This blot was performed by using mAbs (Transduction Laboratories). Similar results were obtained by using polyclonal anti-nNOS antibodies (Transduction Laboratories) (data not shown). Rat pituitary extracts were obtained from Transduction Laboratories.
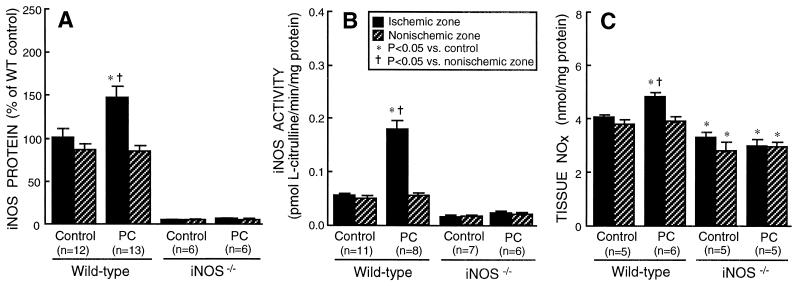
Figure 2.
Effect of ischemic PC on iNOS expression and activity in WT and iNOS−/− mice. Tissue samples were obtained as described in the legend to Fig. 1. (A) Myocardial content of iNOS (cytosolic fraction). In WT mice, the iNOS protein content in the ischemic-reperfused region was increased by 47 ± 12% 24 h after ischemic PC. In iNOS−/− mice, immunoreactive iNOS protein was undetectable, either after sham surgery or after ischemic PC. (B) Calcium-independent NOS (iNOS) activity in the cytosolic fraction. In WT mice, iNOS activity in the ischemic-reperfused region increased by 227% 24 h after ischemic PC. In iNOS−/− mice, low levels of calcium-independent NOS activity were noted in iNOS−/− mice, either after sham operation or after ischemic PC. These levels probably represent cNOS activity (20, 21). (C) Total myocardial content of nitrite and nitrate (NOx). In WT mice, NOx levels in the ischemic-reperfused region increased significantly 24 h after ischemic PC. In iNOS−/− mice, ischemic PC did not bring about any increase in NOx levels; furthermore, NOx levels were significantly lower in iNOS−/− mice compared with WT mice. Data are means ± SEM.
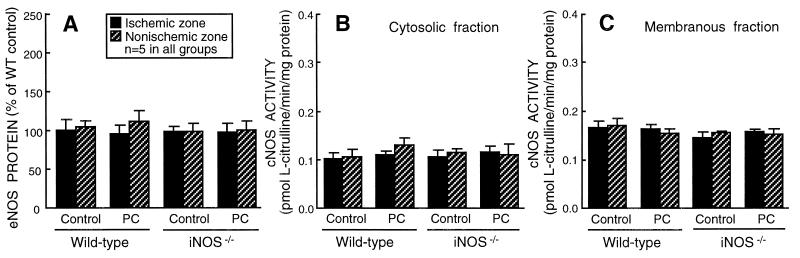
Figure 3.
Effect of ischemic PC on eNOS expression and cNOS (eNOS and/or nNOS) activity in WT and iNOS−/− mice. Tissue samples were obtained as described in the legend to Fig. 1. (A) Total (cytosolic + membranous fractions) myocardial content of eNOS. (B) cNOS (eNOS and/or nNOS) activity in the cytosolic fraction. (C) cNOS activity in the membranous fraction. eNOS protein and cNOS activity did not increase after ischemic PC, either in WT or iNOS−/− mice. Data are means ± SEM.
NOS Activity.
NOS activity was measured as Nω-nitro-l-arginine methyl ester-inhibitable l-citrulline production (Methods). In WT mice, total (cytosolic + membranous fractions) calcium-independent NOS activity (iNOS activity) in the ischemic-reperfused region increased by 131% above control levels 24 h after ischemic PC (0.259 ± 0.020 vs. 0.112 ± 0.006 pmol l-citrulline/min per mg protein; P < 0.05). As was the case for iNOS protein, the increase in iNOS activity was more pronounced in the cytosolic fraction (+227%; Fig. 2B) than in the membranous fraction (+39%; P < 0.05). Very low levels of calcium-independent NOS activity were noted in iNOS−/− mice, either under control conditions or 24 h after ischemic PC (Fig. 2B). The presence of calcium-independent NOS activity in iNOS−/− mice is likely the result of the persistence of residual eNOS and/or nNOS activity in the absence of calcium (20, 21). In contrast to iNOS activity, cNOS (eNOS and/or nNOS) activity in the ischemic-reperfused region did not change appreciably 24 h after ischemic PC (Fig. 3 B and C). The tissue levels of nitrite + nitrate (NOx), the stable oxidation products of NO, increased significantly (P < 0.05) 24 h after ischemic PC in WT mice but did not change in iNOS−/− mice (Fig. 2C). Because both nitrite and nitrate diffuse rapidly out of the cell, tissue NOx levels would not be expected to increase in proportion to tissue NOS activity (Fig. 2B) and protein (Fig. 2A).
Phase B: Body Temperature and Heart Rate.
By experimental design (16), rectal temperature remained within a narrow, physiologic range (36.8–37.3°C) in all groups. Five minutes before the 30-min coronary occlusion, the average heart rate in groups V-XII ranged from 472 to 600 beats per min (P = not significant). Heart rate did not differ significantly among the eight groups at any time during the 30-min occlusion or the ensuing reperfusion (data not shown).
Infarct Size.
There were no significant differences among the eight groups with respect to body weight, LV weight, or weight of the region at risk (Table 1). Groups V and VI (control groups) underwent a 30-min coronary occlusion without any prior manipulation (no PC protocol and no sham surgery). In control WT mice (group V), infarct size averaged 58.8 ± 2.1% of the region at risk (Fig. 4). In control iNOS−/− mice (group VI), infarct size was similar (Fig. 4), indicating that iNOS does not modulate the extent of cell death in the absence of PC. Groups VII and VIII (late PC sham groups) were studied to determine whether the stress of surgery would, in itself, induce a late PC effect. On day 1, these groups underwent a protocol that was identical to that used in the late PC groups (groups IX and X) except that no coronary occlusion was performed; on day 2 (24 h later), they underwent a 30-min occlusion. As shown in Table 1 and Fig. 4, infarct size in groups VII and VIII was similar to that observed in groups V and VI, indicating that a sternotomy with a 60-min period of open-chest state without coronary occlusion did not affect the extent of cell death induced by a 30-min coronary occlusion 24 h later.
Table 1.
Size of LV, risk region, and infarct
| Body weight, g | LV, mg | RR, mg | Infarct, mg | RR, % of LV | Infarct, % of RR | Infarct, % of LV | |
|---|---|---|---|---|---|---|---|
| Group V | 28.3 ± 1.1 | 114.1 ± 5.6 | 39.5 ± 3.0 | 23.3 ± 2.0 | 34.9 ± 2.3 | 58.8 ± 2.1 | 20.6 ± 1.6 |
| Group VI | 30.8 ± 2.4 | 120.7 ± 9.4 | 44.3 ± 2.4 | 23.5 ± 1.9 | 38.0 ± 2.8 | 53.4 ± 3.5 | 20.3 ± 2.2 |
| Group VII | 28.3 ± 1.0 | 122.1 ± 7.3 | 40.3 ± 3.2 | 23.2 ± 2.1 | 33.0 ± 1.6 | 58.9 ± 4.3 | 19.2 ± 1.4 |
| Group VIII | 32.2 ± 1.4 | 121.4 ± 7.3 | 47.1 ± 3.9 | 23.6 ± 1.9 | 38.6 ± 1.8 | 50.4 ± 2.2 | 19.5 ± 1.3 |
| Group IX | 31.7 ± 1.5 | 109.4 ± 6.1 | 37.7 ± 4.4 | 6.3 ± 0.8* | 34.2 ± 2.8 | 19.3 ± 3.4* | 6.2 ± 1.0* |
| Group X | 31.8 ± 1.2 | 129.0 ± 6.6 | 46.9 ± 2.9 | 27.8 ± 1.9 | 37.3 ± 3.2 | 59.7 ± 2.7 | 22.0 ± 2.0 |
| Group XI | 31.7 ± 1.6 | 110.5 ± 10.1 | 37.1 ± 3.5 | 3.1 ± 0.8† | 33.9 ± 2.0 | 8.3 ± 1.7† | 2.9 ± 0.7† |
| Group XII | 28.0 ± 1.6 | 105.7 ± 6.2 | 38.1 ± 2.4 | 2.8 ± 1.0‡ | 36.6 ± 2.6 | 8.3 ± 3.1‡ | 2.5 ± 0.9‡ |
RR, region at risk. Data are means ± SEM. *, P < 0.05 vs. group VII;
, P < 0.05 vs. group V;
, P < 0.05 vs. group VI.
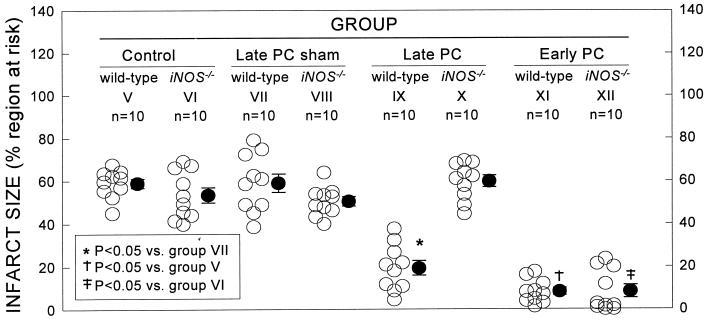
Figure 4.
Myocardial infarct size in groups V (WT, control), VI (iNOS−/−, control), VII (WT, late PC sham), VIII (iNOS−/−, late PC sham), IX (WT, late PC), X (iNOS−/−, late PC), XI (WT, early PC), and XII (iNOS−/−, early PC). ○, individual mice; ●, means ± SEM.
Groups IX and X (late PC groups) were studied to determine the role of iNOS in the late phase of ischemic PC. When WT mice were preconditioned with six 4-min occlusion/4-min reperfusion cycles on day 1 (group IX), the size of the infarct produced by a 30-min coronary occlusion 24 h later (day 2) was 67% smaller compared with group VII (WT, late PC sham group) (P < 0.05; Table 1; Fig. 4), indicating the development of a late PC effect. In contrast, when iNOS−/− mice were preconditioned with six 4-min occlusion/4-min reperfusion cycles on day 1 (group X), the size of the infarct produced by a 30-min occlusion on day 2 was not decreased compared with group VIII (iNOS−/−, late PC sham group) (Table 1; Fig. 4), indicating that the late PC effect was completely abrogated.
Groups XI and XII (early PC groups) were studied to determine whether iNOS plays a role in the early phase of ischemic PC. In these mice, the PC protocol was the same as that used in groups IX and X (six 4-min occlusion/reperfusion cycles) but the interval between the PC protocol and the 30-min occlusion was 10 min instead of 24 h. In WT mice (group XI), infarct size was reduced by 86% compared with control animals (group V) (P < 0.05; Table 1 and Fig. 4), indicating an early PC effect against infarction. A similar reduction in infarct size (84%) was observed in iNOS−/− mice (group XII) compared with the corresponding control group (group VI) (P < 0.05; Table 1 and Fig. 4), indicating that the protective effect of the early phase of PC was fully manifest in these animals.
DISCUSSION
Perhaps the most critical unresolved issue pertaining to the mechanism of late PC is the nature of the cellular mediator that is responsible for conferring increased tolerance to lethal ischemic injury 24–72 h after a brief ischemic challenge. The search for this mediator (presumed to be a gene product) has been intense, and many hypotheses have been formulated (reviewed in ref. 8). The implications of this issue are potentially vast, because identification of the key cytoprotective protein(s) is essential not only for understanding the pathophysiology of the delayed myocardial adaptations to stress, but also for formulating therapeutic strategies aimed at mimicking these adaptations with pharmacological or genetic manipulations capable of inducing a sustained cardioprotective effect similar to that afforded by the late phase of ischemic PC. Although pharmacologic studies have implicated iNOS as the mediator of late PC (7, 9), these data are far from conclusive because of the poor specificity and isoform-selectivity of the iNOS inhibitors examined (11–13) and also because of the lack of direct evidence for iNOS up-regulation.
The present study gives insights into this issue. The results of phase A indicate that the late phase of ischemic PC is associated with an increase in the protein expression and activity of iNOS, but not eNOS or nNOS, demonstrating selective up-regulation of this isoform. The results of phase B indicate that targeted disruption of the iNOS gene completely abrogates the infarct-sparing effect of late PC, demonstrating that the activity of iNOS is indispensable for this cardioprotective phenomenon to occur. Taken together, the results presented herein provide unequivocal molecular genetic evidence for an obligatory role of iNOS in the cardioprotection afforded by the late phase of ischemic PC and therefore identify a specific protein that mediates this phenomenon in vivo.
Although eNOS and nNOS are constitutively expressed, it is now clear that they can be up-regulated by a variety of stimuli (22). For example, an increase in eNOS transcript levels, associated with increased NOx and cGMP production, has been observed in immature rabbit hearts subjected to chronic hypoxia (23), and an augmented vascular response to endothelium-dependent dilators (presumably mediated by eNOS) has been found 24 h after brief ischemia in conscious dogs (24). Accordingly, assessment of eNOS and nNOS was important to examine the possibility that either of them may participate in the cardioprotective effects of late PC. The results of phase A demonstrate that the expression of eNOS proteins was virtually unchanged 24 h after ischemic PC (Fig. 3A). nNOS was not detectable, either in the absence or in the presence of ischemic PC. cNOS activity (a cumulative measure of eNOS and nNOS activities) was also virtually unchanged 24 h after ischemic PC (Fig. 3 B and C). Thus, we found no evidence that either the protein expression or the enzymatic activity of eNOS or nNOS increases during the late phase of ischemic PC, indicating that among the three NOS isoforms, iNOS is selectively up-regulated.
The up-regulation of iNOS protein expression after ischemic PC was mild, ≈18-fold less than after a lethal dose of lipopolysaccharide (47% vs. 853% increase, respectively, in iNOS protein content in the cytosolic fraction of LV samples). This may be an important reason why the activity of iNOS in preconditioned myocardium is protective rather than deleterious. The precise cellular mechanism responsible for the synthesis of new iNOS proteins during late PC remains to be defined, but activation of protein kinase C (17) and NF-κB (25) is likely to play an important role. Further studies will be necessary to identify the specific cell type(s) in which iNOS is up-regulated by ischemic PC. Interestingly, we found evidence of iNOS protein expression and activity in all of the nonpreconditioned tissue samples examined in WT mice, including the anterior and posterior LV wall from control mice (group I) and the nonischemic zone (posterior LV wall) from preconditioned mice (group III) (Figs. 1A and 2). The presence of iNOS in these mice was not the result of the trauma of surgery 24 h earlier, because similar levels of iNOS protein and activity were found in a separate group of five WT mice that were not subjected to surgery or any other manipulation (iNOS protein: 94 ± 8% of the levels found in the anterior LV wall of group I, WT controls; iNOS activity: 0.099 ± 0.005 pmol/min per mg protein). Apparently unstimulated expression of iNOS in cardiac tissue has also been reported by others (26, 27). Our data, however, indicate that this “basal” iNOS expression is insufficient to confer protection against lethal ischemia/reperfusion injury, because in the two nonpreconditioned WT groups (groups V and VII), infarct size was not less than that measured in the corresponding iNOS−/− groups (groups VI and VIII) (Fig. 4).
The finding that iNOS is expressed in nonpreconditioned hearts prompted us to investigate whether this enzyme is involved in the early phase of ischemic PC as well. Because ischemic PC is associated with rapid activation of tyrosine kinases (28, 29), and because iNOS is known to undergo posttranslational modification via tyrosine phosphorylation (resulting in enzymatic activation) (30), it is theoretically possible that the iNOS tonically expressed in the heart may contribute to the early phase of protection. The finding that early PC was not affected by disruption of the iNOS gene (group XII, Fig. 4), however, demonstrates that this is not the case. In the present investigation, a cardioprotective role of iNOS was observed only in the setting of late PC.
In conclusion, this study demonstrates that the late (but not the early) phase of ischemic PC is mediated by iNOS. The observations reported herein reveal the existence of a cardioprotective mechanism that is based on the up-regulation of an enzyme that traditionally has been thought to serve as a mediator of tissue injury rather than protection (31, 32). Our results challenge the view that induction of iNOS necessarily leads to cytotoxicity and imply that the pathophysiological role of this enzyme in vivo is considerably more complex than heretofore appreciated. The finding that iNOS mediates late PC impels a critical reassessment of current paradigms regarding the functional significance of iNOS induction in disease states. We propose that iNOS activity can play either a beneficial or a detrimental role depending on the intensity of iNOS induction and on the specific pathophysiologic setting. We suggest that induction of iNOS after ischemic PC is protective because the magnitude of this up-regulation is relatively modest, in contrast to other situations (such as inflammation or septic shock) in which iNOS induction is massive. The identification of iNOS as the specific protein responsible for late PC provides a target for therapeutic manipulations aimed at mimicking the infarct-sparing effects of ischemic PC with pharmacologic or genetic strategies.
Acknowledgments
We thank Dr. Thomas Hintze for kindly providing breeding pairs of iNOS−/− mice and Michael Flaherty for technical assistance. This study was supported in part by National Institutes of Health Grants R01 HL-43151 and HL-55757 (R.B.) and HL-63034 (W.K.J.), American Heart Association Kentucky Affiliate Grants KY-9804557 and KY-9920595V (Y.G.), and the Jewish Hospital Research Foundation, Louisville, KY.
ABBREVIATIONS
- cNOS
calcium-dependent NOS
- eNOS
endothelial NOS
- iNOS
inducible NOS
- iNOS−/−
iNOS gene knockout
- LV
left ventricle or left ventricular
- nNOS
neuronal NOS
- NOS
nitric oxide synthase
- NOx
nitrate/nitrite
- PC
preconditioning
- WT
wild type
Footnotes
A Commentary on this article begins on page 10953.
References
- 1.Cohen M V, Downey J M. Cardiol Rev. 1995;3:137–149. [Google Scholar]
- 2.Marber M S, Yellon D M. Ann NY Acad Sci. 1996;793:123–141. doi: 10.1111/j.1749-6632.1996.tb33510.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Circ Res. 1993;72:1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 4.Marber M S, Latchman D S, Walker J M, Yellon D M. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 5.Yang X M, Baxter G F, Heads R J, Yellon D M, Downey J M, Cohen M V. Cardiovasc Res. 1996;31:777–783. doi: 10.1016/0008-6363(96)00026-0. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R, Bhatti Z A, Tang X L, Qiu Y, Zhang Q, Guo Y, Jadoon A K. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Bolli R, Manchikalapudi S, Tang X L, Takano H, Qiu Y, Guo Y, Zhang Q, Jadoon A K. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 8.Qiu Y, Rizvi A, Tang X L, Machikalapudi S, Takano H, Jadoon A K, Wu W J, Bolli R. Am J Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 9.Takano H, Manchikalapudi S, Tang X L, Qiu Y, Rizvi A, Jadoon A K, Zhang Q, Bolli R. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi A, Tang X L, Qiu Y, Xuan Y T, Takano H, Jadoon A K, Bolli R. Am J Physiol. 1999;277:H874–H884. doi: 10.1152/ajpheart.1999.277.3.H874. [DOI] [PubMed] [Google Scholar]
- 11.Misko T P, Moore W M, Kasten T P, Nickols G A, Corbett J A, Tilton R G, McDaniel M L, Williamson J R, Currie M G. Eur J Pharmacol. 1993;233:119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 12.Southan G J, Szabo C. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 13.Szabo C, Southan G J, Thiemermann C. Proc Natl Acad Sci USA. 1994;91:12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laubach V E, Shesely E G, Smithies O, Sherman P A. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, et al. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Wu W J, Qiu Y, Tang X L, Yang Z, Bolli R. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ping P, Zhang J, Qiu Y, Tang X L, Manchikalapudi S, Cao X, Bolli R. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 18.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilliam M B, Sherman M P, Griscavage J M, Ignarro L J. Anal Biochem. 1993;212:359–365. doi: 10.1006/abio.1993.1341. [DOI] [PubMed] [Google Scholar]
- 20.Mulsch A, Bassenge E, Busse R. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill W C. Am J Physiol. 1995;269:C863–C869. doi: 10.1152/ajpcell.1995.269.4.C863. [DOI] [PubMed] [Google Scholar]
- 22.Sase K, Michel T. Trends Cardiovasc Med. 1997;7:28–37. doi: 10.1016/S1050-1738(96)00121-1. [DOI] [PubMed] [Google Scholar]
- 23.Baker J E, Holman P, Kalyanaraman B, Pritchard K A., Jr Adv Exp Med Biol. 1998;454:203–217. doi: 10.1007/978-1-4615-4863-8_25. [DOI] [PubMed] [Google Scholar]
- 24.Kim S J, Ghaleh B, Kudej R K, Huang C H, Hintze T H, Vatner S F. Circ Res. 1997;81:53–59. doi: 10.1161/01.res.81.1.53. [DOI] [PubMed] [Google Scholar]
- 25.Xuan Y T, Tang X L, Banerjee S, Takano H, Li R C, Han H, Qiu Y, Li J J, Bolli R. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 26.Freeman G L, Colston J T, Zabalgoitia M, Chandrasekar B. Am J Physiol. 1998;274:H249–H258. doi: 10.1152/ajpheart.1998.274.1.H249. [DOI] [PubMed] [Google Scholar]
- 27.Wildhirt S M, Suzuki H, Horstman D, Weismuller S, Dudek R R, Akiyama K, Reichart B. Circulation. 1997;96:1616–1623. doi: 10.1161/01.cir.96.5.1616. [DOI] [PubMed] [Google Scholar]
- 28.Baines C P, Wang L, Cohen M V, Downey J M. J Mol Cell Cardiol. 1998;30:383–392. doi: 10.1006/jmcc.1997.0601. [DOI] [PubMed] [Google Scholar]
- 29.Ping P, Zhang J, Zheng Y T, Li R C, Dawn B, Tang X L, Takano H, Balafanova Z, Bolli R. Circ Res. 1999;85:542–550. doi: 10.1161/01.res.85.6.542. [DOI] [PubMed] [Google Scholar]
- 30.Pan J, Burgher K L, Szczepanik A M, Ringheim G E. Biochem J. 1996;314:889–894. doi: 10.1042/bj3140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Belder A J, Radomski M W, Martin J F, Moncada S. Eur J Clin Invest. 1995;25:1–8. doi: 10.1111/j.1365-2362.1995.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 32.Kelly R A, Balligand J L, Smith T W. Circ Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]