Abstract
Although there is a substantial literature on the role of parenting on adolescent substance use, most parenting effects have been small in magnitude and studied outside the context of genetically informative designs, raising debate and controversy about the influence that parents have on their children (D. C. Rowe, 1994). Using a genetically informative twin-family design, we studied the role of parental monitoring on adolescent smoking at age 14. Although monitoring had only small main effects, consistent with the literature, there were dramatic moderation effects associated with parental monitoring: at high levels of parental monitoring environmental influences were predominant in the etiology of adolescent smoking, but at low levels of parental monitoring, genetic influences assumed far greater importance. These analyses demonstrate that the etiology of adolescent smoking varies dramatically as a function of parenting.
Keywords: smoking, genetics, interaction, parenting, monitoring
Parental Monitoring Moderates the Importance of Genetic and Environmental Influences on Adolescent Smoking
Cigarette smoking is a major preventable cause of premature death (Center for Disease Control, 2006). The significant morbidity and mortality associated with smoking underscores the need to understand what factors are involved in the establishment of smoking patterns. The vast majority of tobacco users initiate use during adolescence (Marshall et al., 2006). One factor thought to contribute to adolescent smoking is parenting. Smoking is known to be transmitted within families intergenerationally (Bierut et al., 1998), and to the extent that parents have an influence on their children’s smoking practices, this may provide an important avenue for prevention efforts. Several aspects of parenting have been related to adolescent smoking, including poor parent-child relations, low parental involvement, and poor parental monitoring (Harakeh, Scholte, Vermulst, de Vries, & Engels, 2004). However, the effects of these general parenting variables do not appear to be mediated directly through associations with smoking-specific messages that parents communicate to their children (Chassin et al., 2005). Instead, these aspects of parenting have been related more broadly to a spectrum of adolescent problem behavior that includes externalizing behavior and other forms of substance use in addition to smoking. Parental monitoring has received particular attention in relation to adolescent substance use, with a substantial body of literature consistently demonstrating that higher parental monitoring is associated with reduced risk of smoking and alcohol use, as well as other deviant and risky behaviors among adolescents (Barnes & Farrell, 1992; Chilcoat & Anthony, 1996; Steinberg, Fletcher, & Darling, 1994). In studies that have examined multiple dimensions of parenting, monitoring often has the strongest effect (Barnes & Farrell, 1992; Griffin, Botvin, Scheier, Diaz, & Miller, 2000).
Despite the fairly consistent association between parenting and adolescent substance use, the effect sizes associated with measures of parenting and outcome are generally small in magnitude, particularly in comparison with the strong historical and theoretical belief in the importance of parenting (Watson, 1924). Furthermore, most of these findings are based on traditional parent-child correlations, which are unable to tease apart genetic and environment influences. In recognition of this complexity, there has been a growing movement to study parenting effects in the context of genetically informative designs (Caspi et al., 2002; Heath et al., 2002; Jaffee, Moffitt, Caspi, & Taylor, 2003; Moffitt, 2005; Rose, Dick, Viken, & Kaprio, 2001). This has been made possible, in part, by the development of more sophisticated methods of analysis and study designs (Dick, Rose, Viken, Kaprio, & Koskenvuo, 2001; D’Onofrio et al., 2003; Purcell, 2002). In this paper, we use data from a longitudinal, population-based Finnish twin study of adolescent behavior (FinnTwin12), to study the influence of parental monitoring measured at age 12, on the subsequent development of smoking patterns at age 14. We first extend the traditional twin design to incorporate measured information about parental monitoring, enabling us to estimate the amount of variance attributable to parental monitoring on adolescent smoking within our population-based sample, when studied as a simple main effect. This model is comparable to what has routinely been done in the parenting literature and serves as a reference for comparison with the more complex, genetically informative models that we have developed that allow us to go beyond main effects and study how parental monitoring may moderate the expression of genetic predispositions and the importance of other environmental variables.
Methods
Participants and Procedures
FinnTwin12 (FT12) is a population-based, developmental twin study of health-related behaviors and correlated risk factors (Kaprio, Pulkkinen, & Rose, 2002). It consists of five consecutive birth cohorts (1983–87) of twins identified in Finland’s Population Registry Center (PRC), permitting exhaustive and unbiased ascertainment of all twins living and resident in the country. Questionnaires were mailed to all eligible families, of which 87% completed the initial family questionnaire. Immediately on receipt of the completed family questionnaire, individual questionnaires were mailed to both co-twins and both their parents (including parents not residing with either twin child). The twins’ self-report questionnaires were mailed in the late autumn of the year in which the consecutive birth cohorts reached age 11, and most twins returned their questionnaires in the first month(s) of the year they turned age 12 (mean age at response 11.4 years, SD=0.3) . All twins were sent a follow-up questionnaire within three months of the date they reached age 14 (mean age at response 14.1 years, SD=0.1). Response rates were ~90% across both waves of questionnaire assessment. Assessments of non-responders at each stage uncovered no evidence of biased response.
Zygosity was determined using a well-validated questionnaire completed by both co-twins at the baseline, containing items regarding similarity and confusability (Kaprio et al., 2002). This was supplemented by parental information and comparisons of school photographs for twins whose zygosity could not be determined definitively from information in the questionnaires. The sample used in the analyses presented here consisted of 812 monozygotic (MZ) twin pairs (411 female pairs, 401 male pairs), and 830 same-sex dizygotic (DZ) twin pairs (391 female and 439 male pairs). Preliminary power analyses suggested that there was low power to discriminate sex effects, due to the large sample sizes necessary for adequate power to detect moderating effects with ordinal outcomes. Accordingly female and male twins were collapsed by zygosity in modeling.
Measures
Parental monitoring
Monitoring was assessed with three questions included in the twins’ questionnaire administered in late autumn, at the age 11–12 baseline. The questions, created by Chassin et al. (Chassin, Pillow, Curran, Molina, & Barrera, 1993), asked the adolescents to report on the degree to which their parent(s) discuss with them their daily plans, know of their interests and activities, and know their whereabouts and the identity of their associates when they are not at home; responses were made on a 4-point scale from “almost always” to “almost never”. For evaluation of the psychometric properties of the three parental monitoring questions in our Finnish data, we randomly chose one twin from each twin pair for a subset of the data; coefficient alpha was .72, comparable to that reported by Chassin et al. (1993), and the item-total correlations for the three items ranged from .77 to .83. The correlation of monitoring scores between co-twins of all same-sex twin pairs was 0.53, reflecting substantial agreement, but also substantial variation, in perceived monitoring by twin siblings. We note that although we refer to this measure as “parental monitoring” it likely reflects both solicited information and spontaneous information provided by the child (Kerr & Stattin, 2000; Stattin & Kerr, 2000).
Adolescent smoking
Adolescent smoking at age 14 was assessed with a multi-part question that first asked “Have you ever smoked (or tried smoking)?” to which adolescents responded yes or no. Adolescents who responded yes subsequently answered a question that asked “How many cigarettes have you smoked altogether up to now?” with four response options: only one, about 2–10, about 11–50, over 50. These items were collapsed to form one variable with 5 alternative responses, in which 0 represented the individuals who reported never trying smoking, 1 represented individuals who had only smoked 1 cigarette, 2=individuals who reported smoking 2–10 cigarettes, etc. In the FinnTwin12 sample of individuals from same-sex twin pairs, 41% of children reported that they had tried smoking. Of those individuals, 35% reported that they had smoked only 1 cigarette by age 14, 32% reported that they had smoked 2–10 cigarettes; 15% had smoked 11–50 cigarettes, and 19% reported that they had smoked >50 cigarettes. Males were more likely to report increased experimentation with smoking compared to females (see Table 1). Accordingly, thresholds were modeled separately for males and females in all moderation models.
Table 1.
Percentage of Adolescents Reporting Various Smoking Quantities at Age 14
| Cigarettes Smoked | Males | Females |
|---|---|---|
| None | 56.8 | 61.3 |
| Only 1 | 16.2 | 12.4 |
| 2–10 | 14.4 | 11.6 |
| 11–50 | 5.0 | 7.0 |
| >50 | 7.6 | 7.6 |
Data Analyses
The basic twin model incorporating main effects of parenting
Allowing for certain assumptions, comparisons of MZ and DZ twins yield information about the degree of influence that can be attributed to genetic and environmental factors for a particular outcome (Plomin, DeFries, McClearn, & McGuffin, 2001). The basic genetically informative twin model partitions variance in a behavior into additive genetic influences (A), common environmental influences (C) [or genetic influences due to dominance (D)], and unique environmental influences (E). Genetic influences correlate 1.0 between monozygotic (MZ) twins, who share all of their genes identical-by-descent, and 0.5 between dizygotic (DZ) twins, who share, on average, 50% of their segregating genes, as do ordinary siblings. Common environmental effects, as defined in biometrical twin modeling, refer to all environmental influences that make siblings more similar to one another. By definition, these influences correlate 1.0 between both MZ and DZ twins. If common environmental effects are important, we expect the DZ correlation to exceed half the MZ correlation (because rDZ= ½ rMZ is the degree of similarity expected based solely on additive genetic similarity). When the DZ correlation is less than half the MZ correlation, it suggests that genetic effects due to dominance (D) are acting. When only twins are available, C and D cannot be estimated simultaneously in the twin model. For our adolescent smoking variable, the DZ correlation exceeded ½ the MZ correlation, suggesting that C effects were important. Accordingly, ACE models (rather than ADE models) were fit to the data. Unique environmental influences (E) are uncorrelated between co-twins and have the effect of decreasing the covariance between siblings. The E term also includes error variance. When information on specific measured variables is obtained, this basic model can be extended to estimate the amount of variance that can be attributed to the specific measure (for another example of this model, see (Caspi, Taylor, Moffitt, & Plomin, 2000)). Parental monitoring was entered separately according to each twin’s report.
Twin models incorporating moderation effects
Figure 1 shows a classic twin model (for only one twin in the pair) that has been modified to include a moderation component. The standard paths a, c, and e, indicating the magnitude of effect of additive genetic influences, common environmental influences, and unique environmental influences, now each include a β term, which indicates the significance of a potential moderator variable M on each of these genetic and environmental influences. The value of M changes from subject to subject, taking on the value of the measured variable for that subject (i.e. parental monitoring scores in our models). In the moderation model, the additive genetic value is a linear function of the moderator M, represented by the equation a + βXM, where βX is an unknown parameter to be estimated from the data, representing the magnitude of the moderating effect. If βX is significantly different from zero, there is evidence for a moderating effect. A similar logic follows for the βY and βZ pathways, which represent the extent to which a specific moderator variable alters the importance of common and unique environmental influences, respectively. In other words, the moderation model allows us to test whether the importance of additive genetic effects (a), common environmental effects (c), and unique environmental effects (e) are changing as a function of the measured variable. The pathway μ + βMM models main effects of the moderator variable on the outcome. Also included in this pathway are any gene-environment correlation effects between the moderator variable and outcome. Thus, any covariance between the moderator and the outcome is incorporated into the means model (Purcell, 2002; Turkheimer, Haley, Waldron, D’Onofrio, & Gottesman, 2003); accordingly, any interactions detected will be associated with the variance components unique to the outcome (i.e. genetic influences on smoking that are not shared with genetic influences on twins’ reports of parenting).
Figure 1.
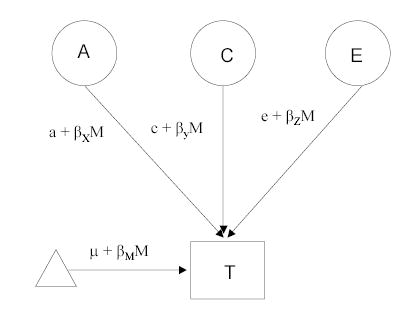
Moderation model. The latent variable A, represented in a circle, indicates additive genetic influences on the trait (T) of interest. C represents common (shared) environmental influences on the trait, and latent E represents unique environmental influences, which are uncorrelated between the twins. The triangle indicates the mean/thresholds for T and is necessary when modeling raw data. The standard paths a, c, and e, indicating the magnitude of effect of each latent variable on the trait, each include a β term, which indicates the significance of a measured moderator variable M on each of these genetic and environmental influences.
All modeling was conducted using the raw ordinal data option in Mx (Neale, Boker, Xie, & Maes, 1999). Mx is a structural equation modeling program developed specifically for the use of twin data. When the outcome is ordinal, the model involves the use of thresholds, rather than means. All moderating variables were standardized for analyses. The first application of the moderation model using quasi-continuous environmental moderators was to the study of socioregional factors on alcohol use among young adults using Finnish twin data (Dick et al., 2001). These models have subsequently been detailed and expanded (Purcell, 2002). The significance of each of the parameters in the model can be tested by dropping a parameter and evaluating the change in –2 log likelihood between the initial model and the nested submodel. This difference is evaluated using a chi square distribution. A significant change in fit between the models (p < .05) for the difference in degrees of freedom indicates that dropping the parameter caused a significant decrease in fit of the model, indicating that pathway significantly contributes to the outcome trait and should be retained in the model.
Results
Main Effects
Parental monitoring had a significant main effect on adolescent smoking, accounting for 2% of the total variance in adolescent smoking (95% CI: .01 – .03). Genetic influences accounted for 21% of the variance (95% Confidence Interval (CI): .13 – .30), common environmental effects accounted for 67% of the variance (95% CI: .58 – .74), and unique environmental effects accounted for the remaining 10% of the variance (95% CI: .08 – .13). Dropping parental monitoring from the model caused a highly significant decrease in fit (Δχ2 = 39.003, 1df, p<0.001).
Moderating Effects
Parental monitoring significantly moderated the effects of genetic factors, common environmental factors, and unique environmental factors on adolescent smoking. Dropping the moderating effect associated with each of these variance components caused a significant decrease in fit of the model. See Table 2 for the fit statistics associated with each submodel. Figure 2 shows the changing variance components across different levels of parental monitoring. As parental monitoring increased, common environmental effects increased in importance in the development of smoking behavior, whereas genetic effects significantly decreased in importance, and unique environmental effects also showed a decrease in importance. Genetic factors (a2) accounted for more than 60% of the variance at the extreme low end of parental monitoring, while accounting for less than 15% of the variance at extremely high levels of monitoring. Conversely, common environmental effects accounted for only about 20% of the variance at the extreme low end of monitoring, but more than 80% of the variance at the extremely high end of monitoring. Unique environmental effects decreased from approximately 20% to <10% of the total variance with increasing levels of monitoring.
Table 2.
Fit Statistics for the Full Moderation Model, as well as the Submodels Testing the Significance of Dropping the Moderation Effect of Parental Monitoring on Each Variance Component (A=Additive Genetic Effects, C=Common Environmental Effects, E=Unique Environmental Effects) Influencing Adolescent Smoking
| -2LogLikelihood | df | Δχ2 | Δdf | p-value | |
|---|---|---|---|---|---|
| Full Model | 5799.166 | 2829 | |||
| No A moderation | 5804.278 | 2830 | 5.113 | 1 | 0.024 |
| No C moderation | 5807.923 | 2830 | 8.757 | 1 | 0.003 |
| No E moderation | 5806.038 | 2830 | 6.872 | 1 | 0.009 |
Figure 2.
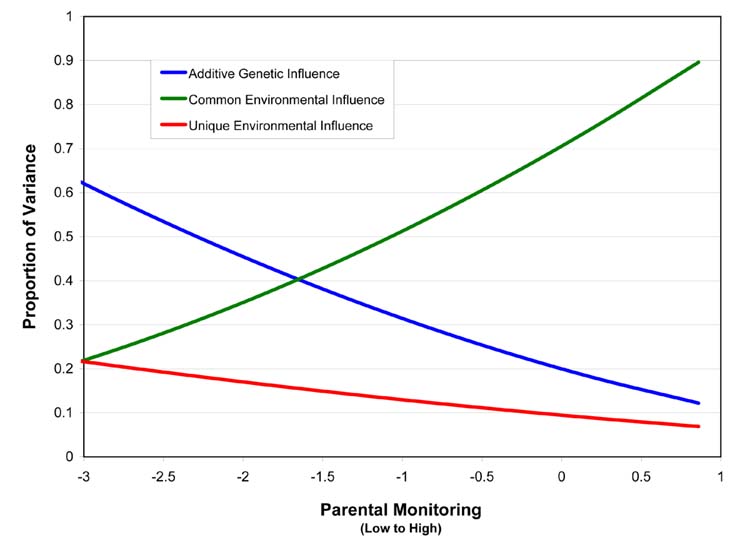
Changing variance in additive genetic effects, common environmental effects, and unique environmental effects on adolescent smoking across increasing levels of standardized parental monitoring scores.
Discussion
The importance of parenting and its effect on children’s outcomes has been a controversial and highly debated topic (Maccoby, 2000; Scarr & Riccuitti, 1991), due in part to the small effect sizes often associated with parenting variables. Nonetheless, parenting remains of important theoretical interest in the etiology of many problem behaviors that have their onset in adolescence, ranging from externalizing psychopathology to substance use. Consistent with many previous studies, we found that parental monitoring had a highly significant main effect on adolescent smoking in our population-based, epidemiological sample; however, monitoring accounted for only 2% of the variance in smoking. More interestingly, when testing for more complex effects associated with parenting, we found that the relative importance of genetic and environmental influences changed dramatically as a function of parental monitoring. The importance of adolescent’s genetic predispositions decreased, while the importance of common environmental influences increased, with increasing levels of parental monitoring. These effects were dramatic, with more than a 3-fold difference in genetic effects, and a 4-fold difference in common environmental effects, from one extreme in parental monitoring to the other. These analyses suggest that when adolescents receive little parental monitoring, it creates an environment that allows for greater opportunity to express genetic predispositions. These results are in line with previous findings from the Finnish twin studies, which indicated that in neighborhoods where there is less stability, presumably engendering less community monitoring, there was greater evidence of genetic influence (Dick et al., 2001). Conversely, in more supervised and restricted environments, there was less opportunity to express genetic predispositions and greater influence of environmental effects (Dick et al., 2001; Rose et al., 2001). Finally, we found that the influence of unique environmental effects decreased with increasing levels of parental monitoring. This indicates that at higher levels of monitoring, adolescents’ smoking patterns are less likely to be influenced by unique environmental events.
The results of this study should be interpreted in the context of several limitations. As stated previously, our measure of parental monitoring does not distinguish between the extent to which parents are soliciting information about the whereabouts and activities of their children and the extent to which parents have knowledge of their children’s activities due to spontaneous disclosure on the part of the child. We do have information on a related dimension of parenting in the FinnTwin sample, a 10 item scale measuring parental restrictiveness (Metsapelto & Pulkkinen, 2003). Restrictiveness was reported by the parents, rather than the adolescents, and it contains items that may be viewed as more closely related to attempts at parental control. We note that the direction of moderating effects for this variable paralleled the results for parental monitoring.
Secondly, we were unable to test whether parenting effects on substance use differed between adolescent boys and girls. The complexity of these moderation models requires large sample sizes to detect significant interaction, particularly when both A and C effects are involved in the outcome, as is the case with adolescent smoking, and when the outcome measure is ordinal. To achieve sufficient power to detect these effects, we collapsed across sex. However, we did fit the model separately to male and female data to examine the pattern of results for suggestion of possible sex differences. The results obtained from these sex-specific analyses looked very similar for males and females to the overall results obtained from the full sample.
Thirdly, it is uncertain to what extent these results are unique to the Finnish culture. Finnish smoking laws are very similar to those in the United States, with tobacco sales forbidden to minors under the age of 18. Smoking is forbidden at all places of work and on school grounds, in public transportation and public indoor spaces, and there are non-smoking sections in all restaurants (with a total ban of smoking in restaurants to begin in 2007). Rates of smoking in Finland are comparable to other Western countries, including the Unites States (Center for Disease Control, 2006; Marshall et al., 2006). However, Finnish children are traditionally given a great deal of independence at an early age. Both mothers and fathers of adolescent children have the same rates of workforce participation. Thus, it is not clear whether the same moderating effects associated with parental monitoring will be observed in other cultures where parental control is much greater at this age, and mothers are more frequently at home or do only part-time work. This possibility will be interesting to explore in other datasets.
In conclusion, we have studied the effects of parental monitoring, measured at age 12, on smoking patterns among adolescents at age 14, using a genetically informative design. We find that parental monitoring had a small, significant effect on adolescent smoking when studied in a main effect framework. However, applying more complex interactive models to the data, we find strong moderating effects associated with parental monitoring, whereby the importance of genetic influences dramatically decreased, and the importance of common environmental influences significantly increased, with increasing levels of parental monitoring. The changing importance of genetic effects as a function of parental monitoring might be considered an example of gene-environment interaction. However, we have avoided this terminology in the paper, as parental monitoring is not truly an environmental variable in the sense that it is influenced by both genetic and environmental factors itself, reflecting both genetically-influenced characteristics of the parents (Perusse, Neale, Heath, & Eaves, 1994; Wade & Kendler, 2000), as well as elicited responses based on the children’s genetically-influenced temperaments (O’Connor, Deater-Deckard, Fulker, Rutter, & Plomin, 1998; Plomin & Bergeman, 1991; Plomin, McClearn, Pedersen, Nesselroade, & Bergeman, 1989; Reiss, 1995; D. Rowe, 1981). Understanding the factors that impact parental monitoring is an important area of study itself. Particularly since our analyses powerfully demonstrate that the etiology of adolescent smoking varies dramatically as a function of parental monitoring.
Acknowledgments
Preparation of this manuscript was supported by AA015416 to DMD. FinnTwin12 has been supported by the National Institute of Alcoholism and Alcohol Abuse (grants AA-12502, AA-00145, and AA-09203 to RJR), and by the Academy of Finland (to JK), the Finnish Centre of Excellence Programme (to LP and JK) and grants from the Yrjö Jahnsson Foundation (to JK).
Contributor Information
Danielle M. Dick, Washington University, St. Louis.
Richard Viken, Indiana University, Bloomington.
Shaun Purcell, Whitehead Institute, MIT.
Jaakko Kaprio, University of Helsinki & National Public Health Institute, Finland.
Lea Pulkkinen, University of Jyväskylä, Finland.
Richard J. Rose, Indiana University, Bloomington
References
- Barnes GM, Farrell MP. Parental support and control as predictors of adolescent drinking, delinquency, and related problem behaviors. Journal of Marriage and the Family. 1992;54:763–776. [Google Scholar]
- Bierut L, Dinwiddle SH, Begleiter H, Crowe R, Hesselbrock V, Nurnberger JI, Jr, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: A report from the Collaborative Study on the Genetics of Alcoholism. Archives of General Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Taylor A, Moffitt TE, Plomin R. Neighborhood deprivation affects children’s mental health: Environmental risks idenitified in a genetic design. Psychological Science. 2000;11:338–342. doi: 10.1111/1467-9280.00267. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. Use of cigarettes and other tobacco products among students aged 13–15 years worldwide, 1999 – 2005. MMWR Weekly. 2006;55(20):553–556. [PubMed] [Google Scholar]
- Chassin L, Pillow D, Curran P, Molina B, Barrera M. Relation of parental alcoholism to early adolescent substance use: A test of three mediating mechanisms. Journal of Abnormal Psychology. 1993;102:3–19. doi: 10.1037//0021-843x.102.1.3. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose J, Sherman SJ, Davis MJ, Gonzalez JL. Parenting style and smoking specific parenting practices as predictors of adolescent smoking onset. Journal of Pediatric Psychology. 2005;30:334–344. doi: 10.1093/jpepsy/jsi028. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Anthony JC. Impact of parental monitoring on initiation of drug use through late childhood. Journal of the American Acedemy of Child and Adolescent Psychiatry. 1996;35:91–100. doi: 10.1097/00004583-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene-environment interactions: Socioregional moderation of alcohol use. Journal of Abnormal Psychology. 2001;110:625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer EN, Eaves LJ, Corey LA, Berg K, Solaas MH, et al. The role of the Children of Twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- Griffin KW, Botvin GJ, Scheier LM, Diaz T, Miller NL. Parenting practices as predictors of substance use, delinquency, and agression among urband minority youth: Moderating effects of family structure and gender. Psychology of Addictive Behaviors. 2000;14:174–184. doi: 10.1037//0893-164x.14.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh Z, Scholte RHJ, Vermulst AA, de Vries H, Engels RCME. Parental factors and adolescents’ smoking behavior: an extension of The theory of planned behavior. Preventive Medicine. 2004;39:951–961. doi: 10.1016/j.ypmed.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Heath A, Todorov AA, Nelson EC, Madden PA, Bucholz KK, Martin NG. Gene-environment interaction effects on behavioral variation and risk of complex disorders: The example of alcoholism and other psychiatric disorders. Twin Research. 2002;5:30–37. doi: 10.1375/1369052022875. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Taylor A. Life with (or without) father: The benefits of living with two biological parents depend on the father’s antisocial behavior. Child Development. 2003;74:109–126. doi: 10.1111/1467-8624.t01-1-00524. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Research. 2002;5:358–365. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kerr M, Stattin H. What parents know, how they know it, and several forms of adolescent adjustment: Further support for a reinterpretation of monitoring. Developmental Psychology. 2000;36:366–380. [PubMed] [Google Scholar]
- Maccoby EE. Parenting and is effects on children: On reading and misreading behavior genetics. Annual Review of Psychology. 2000;51:1–27. doi: 10.1146/annurev.psych.51.1.1. [DOI] [PubMed] [Google Scholar]
- Marshall LM, Schooley M, Ryan H, Cox P, Easton A, Healton C, et al. Youth Tobacco Surveillance -- United States, 2001–2002. CDC surveillance summaries. 2006;55(SS03):1–56. [PubMed] [Google Scholar]
- Metsapelto RL, Pulkkinen L. Personality traits and parenting: Neuroticism, extraversion, and openness to experience as discriminative factors. European Journal of Personality. 2003;17:59–78. [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: Gene-environment interplay in antisocial behavior. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Neale, M. C., Boker, S. M., Xie, G., & Maes, H. H. (1999). Mx: Statistical Modeling (Version 5th Edition). Box 126 MCV, Richmond, VA 23298: Department of Psychiatry.
- O’Connor TG, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype-environment correlations in late childhood and early adolescence: Antisocial behavioral problems and coercive parenting. Developmental Psychology. 1998;34:970–981. doi: 10.1037//0012-1649.34.5.970. [DOI] [PubMed] [Google Scholar]
- Perusse D, Neale MC, Heath A, Eaves LJ. Human parental behavior: Evidence for genetic influence and potential implication for gene-culture transmission. Behavior Genetics. 1994;24:327–335. doi: 10.1007/BF01067533. [DOI] [PubMed] [Google Scholar]
- Plomin R, Bergeman CS. The nature of nurture: Genetic influence on “environmental” measures. Behavioral and Brain Sciences. 1991;14:373–427. [Google Scholar]
- Plomin, R., DeFries, J. C., McClearn, G. E., & McGuffin, P. (2001). Behavioral genetics (Vol. 4th edition). London: Worth.
- Plomin R, McClearn GE, Pedersen NL, Nesselroade JR, Bergeman CS. Genetic influences on adults’ ratings of their current family environment. Journal of Marriage and the Family. 1989;51:791–803. [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Reiss D. Genetic influence on family systems: Implications for development. Journal of Marriage and the Family. 1995;57:543–560. [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25:637–643. [PubMed] [Google Scholar]
- Rowe D. Environmental and genetic influences on dimensions of perceived parenting: A twin study. Developmental Psychology. 1981;17:209–214. [Google Scholar]
- Rowe, D. C. (1994). The limits of family influence: Genes, experience, and behavior New York: Guilford Press.
- Scarr, S., & Riccuitti, A. (1991). What effects do parents have on their children? In L. Okagaki & R. J. Sternberg (Eds.), Directors of development (pp. 3–23). Hillsdale, NJ: Erlbaum.
- Stattin H, Kerr M. Parental monitoring: A reinterpretation. Child Development. 2000;71:1072–1085. doi: 10.1111/1467-8624.00210. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Fletcher A, Darling N. Parental monitoring and peer influences on adolescent substance use. Pediatrics. 1994;93:1060–1064. [PubMed] [Google Scholar]
- Turkheimer EN, Haley A, Waldron M, D’Onofrio BM, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Wade TD, Kendler KS. The genetic epidemiology of parental discipline. Psychological Medicine. 2000;30:1303–1313. doi: 10.1017/s0033291799003013. [DOI] [PubMed] [Google Scholar]
- Watson, J. B. (1924). Behaviorism New York: Norton.


